Abstract
Objective
Cyclosporin-A (CsA) has been reported to reduce myocardial infarct size in both the experimental and clinical settings. This protective effect is dependent on its ability to prevent the opening of the mitochondrial permeability transition pore, a critical determinant of cell death in the setting of acute ischaemia-reperfusion injury. Whether CsA can reduce the extent of peri-operative myocardial injury (PMI) in patients undergoing coronary artery bypass graft (CABG) surgery is unknown, and is investigated in this randomised controlled clinical trial.
Methods
78 adult patients undergoing elective CABG surgery were randomised to receive either an intravenous bolus of CsA (2.5 mg/kg) or placebo administered after induction of anaesthesia and prior to sternotomy. PMI was assessed by measuring serum cardiac enzymes, troponin T (cTnT) and CK-MB at 0, 6, 12, 24, 48 and 72 h after surgery.
Results
There was no significant difference in mean peak cTnT levels between control (n=43) and CsA treatment (n=40) patients (0.56±0.06 ng/mL with control vs 0.35±0.05 ng/mL with CsA; p=0.07). However, in higher-risk patients with longer cardiopulmonary bypass times, there was a significant reduction in PMI with CsA therapy (p=0.049), with a reduced postoperative cTnT rise by 0.03 ng/mL for every 10 min, when compared with control.
Conclusions
In patients with longer cardiopulmonary bypass times, a single intravenous bolus of CsA administered prior to CABG surgery reduced the extent of PMI.
Keywords: Cardiac Surgery, Myocardial Ischaemia and Infarction (IHD)
Introduction
For patients with multi-vessel coronary artery disease, the treatment of choice is coronary revascularisation by coronary artery bypass graft (CABG) surgery. In most patients undergoing routine CABG surgery, the peri-operative risk of surgery remains low. However, in recent years, much higher-risk patients are undergoing CABG surgery. The reasons for this include the ageing population, the increased prevalence of comorbidities such as diabetes and hypertension, and result in a higher peri-operative risk, increased risk of peri-operative myocardial injury (PMI), and worse clinical outcomes. Crucially, the magnitude of PMI (as measured by serum cardiac enzymes such as CK-MB, troponin-T (cTnT) and troponin-I) has been reported to be associated with worse short and long term prognosis in patients undergoing CABG surgery.1–3 This increased risk of PMI occurs despite current strategies for myocardial preservation including cross-clamp fibrillation and blood cardioplegia. As such, novel therapeutic interventions are required to further protect the heart during CABG surgery in these high-risk patients in terms of limiting the extent of PMI and preserving left ventricular systolic function so as to improve clinical outcomes.4
In patients undergoing CABG surgery, the heart is subjected to acute myocardial injury for a number of different reasons including acute global ischaemia-reperfusion injury (IRI, as the heart is put on and taken off cardiopulmonary bypass, CPB), inflammatory injury from CPB, direct myocardial injury from handling of the heart and coronary micro-embolisation.4 5 Mitochondrial dysfunction induced by the opening of the mitochondrial permeability transition pore (MPTP) has been demonstrated to be a critical determinant of cardiomyocyte death in the heart subjected to acute IRI.6 7 A number of experimental studies,8–10 and one recent clinical study,11 have found that administering cyclosporin-A (CsA) to prevent MPTP opening at the onset of reperfusion can limit myocardial infarct (MI) size (reviewed in12). Whether, targeting the MPTP with CsA in adult patients undergoing elective CABG surgery can reduce the extent of PMI is not known and is investigated in the current study.
Methods
This study received institutional ethical approval from University College London and King's College London. It was registered with the Multicentre Research Ethical Committee, reference number 06/Q0502/83 and with the ISRCTN Register, reference number 49989273. Written informed consent was obtained from all patients entering the study.
Between August 2010 and September 2012 consecutive adult patients referred for elective CABG surgery only were recruited. We excluded patients older than 85 years, with unstable angina, moderate or severe renal impairment (estimated glomerulo-filtration rate of less than 45 mL/min/m2), cirrhotic liver disease and immuno-compromised conditions. We also excluded patients taking oral glibenclamide or nicorandil, as these drugs may interfere with preconditioning. Computerised-generated random number sequences were used for randomisation, and blinded treatment allocation was achieved using opaque numbered envelopes.
After induction of anaesthesia but prior to sternotomy, patients were randomly allocated to receive either an intravenous bolus of CsA (2.5 mg/kg) in 100 mL NaCl 0.9% over 10 min or an intravenous bolus of 100 mL NaCl 0.9% over 10 min. We used a dose of 2.5 mg/kg, as this was shown to limit MI size in ST-segment elevation myocardial infarction (STEMI) patients treated by percutaneous coronary intervention (PPCI).11 Patients, surgeons, theatre staff, ITU staff and investigators collecting the data were all blinded to the treatment allocation.
All the following methods were part of the normal induction and maintenance of general anaesthesia. On arrival in theatre, all patients received 0.2–0.3 mg/kg midazolam before induction, and peripheral venous and arterial cannulae were inserted. Induction of anaesthesia included analgesia with fentanyl, hypnosis with propofol 1–2 mg/kg, and muscle relaxation with atracurium 0.5–0.7 mg/kg or rocuronium 0.6 mg/kg. The trachea was intubated and mechanical ventilation was started thereafter. The central line was placed in the right internal jugular vein. All patients received 3 mg/kg of tranexamic acid and 2.5 g of MgSO4 at the beginning of surgery. General anaesthesia was maintained with isoflurane 1 minimum alveolar concentration (MAC) and isoflurane or propofol infusion during bypass. Intraoperative analgesics used were remifentanil infusion and morphine after bypass. Monitoring included continuous arterial blood pressure, central venous pressure, leads I and V of the ECG, nasopharyngeal temperature, and trans-oesophageal echocardiography if available.
CPB was started after heparin administration with a non-pulsatile flow and a membrane oxygenator. Cardioprotection was provided by cold-blood cardioplegia (1:4), which was administered anterogradely, after cross-clamping of the aorta, into the coronary arteries, or by cross-clamp fibrillation, in both groups. After grafting, the CPB was discontinued and protamine was given to reverse the heparin.
Blood samples for measurements of troponin-T and CK-MB were taken before surgery, and at 6, 12, 24, 48 and 72 h after surgery. Routine peri-operative blood results included measurements of haemoglobin, white cell count, estimated glomerular filtration rate, liver transaminases and bilirubin.
Commercially available methods were used to measure CKMB (IMMULITE 2000, Siemens Healthcare Diagnostics) and cTnT (Elecsys 1010 System, Roche Diagnostics). The minimal detectable concentration of CKMB was 0.6 ng/mL and the reference range was larger than 5 ng/mL. The minimal detection limit for cTnT was 0.01 ng/mL and the reference range was larger than 0.1 ng/mL.
Statistical analysis
The primary outcome measure was postoperative peak cTnT released. For comparison of continuous variables, the t test was used and for categorical variables the χ2 test. The aim of the study was to investigate the protective effect of cyclosporine on myocardial injury as observed with increasing bypass times. The significance of any effect was investigated with model fitting. Thus, no multiple testing was undertaken as would be the case for post hoc testing. Multi-level model fitting (MLwiN 2.15) was used to analyse changes of biochemical markers over time. Changes in values over time (level 1) in each individual (level 2) were modelled. An indicator variable was introduced for designation of the placebo and CsA treatment groups. An interaction term was introduced to investigate the a priori question of any effect modification in the rise of troponin by CsA from the length of bypass time. Significance was tested using likelihood ratio testing. Significant differences were defined as p<0.05. Data are presented as mean± SD or SEM. The analysis was carried out on the basis of intention to treat.
A sample size of at least 72 patients in total was determined based on the following assumptions from a previous study:13 (a) a 46% reduction in postoperative peak cTnT level from 0.69 to 0.37 ng/mL with cardioprotective treatment; (b) a SD of 0.48 ng/mL; (c) a power of 80%; and (d) significance declared at the two-sided 5% level.
Results
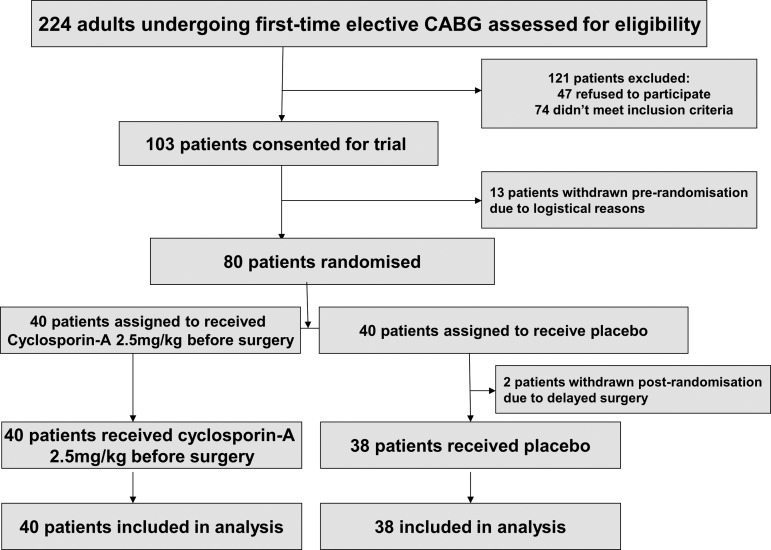
A total of 224 patients were screened as being eligible for patient recruitment, of whom 103 agreed to give informed consent, 80 were randomised and 78 studied with 40 patients in the CsA and 38 patients in the control groups (see figure 1).
Figure 1.
Consort diagram.
There were no significant differences between the groups in terms of baseline patient characteristics, intraoperative variables or routine preoperative blood results (tables 1–3). Serum CsA levels taken from 10 patients just after the release of the aortic cross-clamp were found to be within the therapeutic range for CsA (551±173ng/ml (mean±SD)), confirming that, at the time of acute global reperfusion, CsA was present at therapeutic levels.
Table 1.
Patient baseline characteristics
| Preoperative variables | CsA (n=40) | Control (n=38) | p Value |
|---|---|---|---|
| Age (years) | 65.8 (10.7) | 67.1 (8.7) | 0.20 |
| Gender | 0.54 | ||
| Female | 13 (32.5) | 9 (24) | |
| Male | 27 (67.5) | 29 (76) | |
| Diabetes | 0.89 | ||
| None | 30 (75) | 28 (74) | |
| Non-IDDM | 9 (22.5) | 8 (21) | |
| IDDM | 1 (2.5) | 2 (5) | |
| Previous stroke | 2 (5) | 2 (5) | 0.96 |
| Hypertension | 29 (72.5) | 29 (76) | 0.90 |
| Peripheral vascular disease | 0 (0) | 0 (0) | |
| Hypercholesterolaemia | 29 (72.5) | 29 (76) | 0.90 |
| Previous MI | 21 (52.5) | 21 (55) | 0.99 |
| Smoker | 0.26 | ||
| Never | 15 (37.5) | 20 (53) | |
| Ex | 17 (42.5) | 11 (29) | |
| Current | 8 (20) | 7 (18) | |
| Family history of IHD | 19 (47.5) | 13 (34) | 0.36 |
| BMI | 28.8 (4.3) | 28.2 (3.5) | 0.55 |
| LVEF | 0.10 | ||
| >55% | 20 (50) | 27 (71) | |
| 35%–55% | 17 (42.5) | 10 (26) | |
| <35% | 3 (7.5) | 1 (2) | |
| Drug history | |||
| Aspirin | 34 (85) | 34 (89) | 0.80 |
| β-Blocker | 25 (62.5) | 31 (82) | 0.11 |
| Cholesterol-lowering drug | 31 (77.5) | 32 (84) | 0.64 |
| ACE inhibitor/AT2RB | 27 (67.5) | 27 (71) | 0.92 |
| Insulin | 3 (7.5) | 3 (8) | 0.95 |
| Metformin | 4 (10) | 6 (16) | 0.67 |
| EuroSCORE | 4.8 (2.9) | 4.6 (2.7) | 0.86 |
Data are mean (SD) or number (%).
BMI, body mass index; CsA, cyclosporin A; MI, myocardial infarct; IDDM, insulin-dependant diabetes mellitus; IHD, ischaemic heart disease.
Table 2.
Patient intraoperative variables
| Intraoperative variables | CsA (n=40) | Control (n=38) | p Value |
|---|---|---|---|
| Number of grafts | |||
| One | 1 (2.5) | 1 (3) | 0.97 |
| Two | 3 (7.5) | 5 (13) | 0.65 |
| Three | 18 (45) | 19 (50) | 0.83 |
| Four | 18 (45) | 13 (34) | 0.46 |
| Cross-clamp time (min) | 54.7 (22.7) | 53.9 (23.4) | 0.89 |
| Bypass time (min) | 92.6 (29.4) | 89.2 (28.9) | 0.62 |
| Type of cardiac arrest | |||
| Cardioplegia | 24 (60) | 28 (74) | 0.48 |
| Cross-clamp fibrillation | 16 (40) | 10 (26) | 0.30 |
Data are mean (SD) or number (%). CsA, cyclosporin-A.
Table 3.
Patient peri-operative blood results
| CsA (n=40) | Control (n=38) | p Value | |
|---|---|---|---|
| Haemoglobin (g/dL) | |||
| Preoperative | 12.9 (2.3) | 13.6 (2.0) | 0.17 |
| Postoperative day 3 | 9.2 (1.1) | 9.7 (1.3) | 0.09 |
| White cell count (×109/L) | |||
| Preoperative | 8.4 (2.3) | 7.8 (1.9) | 0.22 |
| Postoperative day 3 | 10.0 (2.8) | 9.2 (3.1) | 0.24 |
| Urea (mmol/L) | |||
| Preoperative | 5.9 (1.5) | 6.4 (1.8) | 0.27 |
| Postoperative day 1 | 6.2 (1.7) | 6.4 (2.1) | 0.51 |
| Postoperative day 2 | 6.7 (2.7) | 7.1 (3.2) | 0.58 |
| Postoperative day 3 | 7.0 (3.7) | 7.0 (3.1) | 0.99 |
| Creatine (µmol/L) | |||
| Preoperative | 86.9 (16.5) | 84.8 (21.7) | 0.66 |
| Postoperative day 1 | 85.5 (21.2) | 83.4 (18.9) | 0.67 |
| Postoperative day 2 | 87.1 (33.6) | 85.7 (23.3) | 0.83 |
| Postoperative day 3 | 82.5 (27.5) | 87.5 (31.3) | 0.42 |
| Bilirubin (µmol/L) | |||
| Preoperative | 9.7 (7.4) | 9.6 (5.4) | 0.94 |
| Postoperative day 3 | 11.3 (5.6) | 10.1 (3.9) | 0.31 |
| ALP (IU/L) | |||
| Preoperative | 76.3 (32.1) | 74.6 (29.6) | 0.82 |
| Postoperative day 3 | 67.1 (29.0) | 58.7 (27.3) | 0.22 |
| AST (IU/L) | |||
| Preoperative | 27.7 (8.2) | 27.8 (11.7) | 0.98 |
| Postoperative day 3 | 27.4 (10.1) | 24.2 (9.0) | 0.18 |
Data are mean (SD) or number (%).
ALP, alkaline phosphatase; AST, aspartate transaminase; CsA, cyclosporin-A.
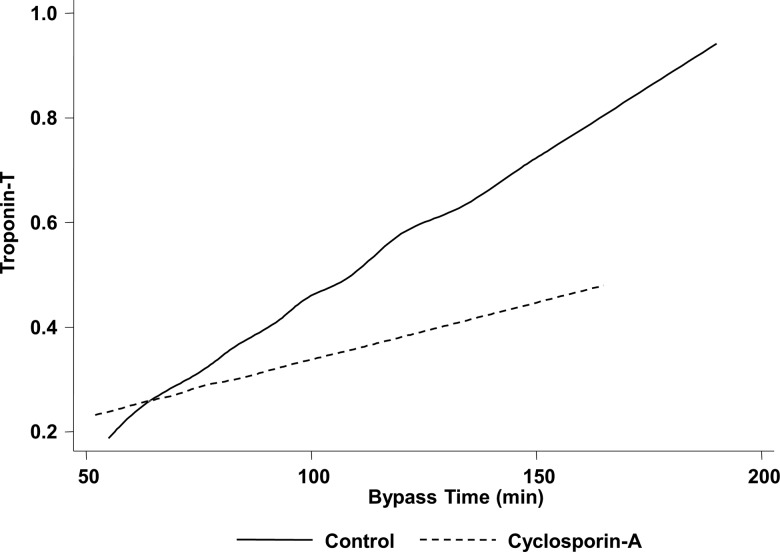
Table 4 demonstrates a rise in serum CKMB and cTnT postoperatively in both patient groups indicating the presence of PMI with surgery. There was no significant difference in peak cTnT at 6 h in CsA treated patients when compared with control (0.35±0.05 ng/mL with CsA vs 0.56±0.06 ng/mL with control; p=0.07; table 4). Nevertheless, when the results were analysed according to the CPB time with multi-level model fitting, the protective effect of CsA was found to be greater and the cTnT release during the postoperative time period was lower in those patients with longer CPB times (p=0.049). The average cTNT rise was 0.05 ng/mL with every 10 min increase in the CPB time. However, in those patients who had received CsA, this increase in cTNT was less with an average rise of only 0.02 ng/mL of cTnT. Therefore, patients who had received CsA had an attenuated increase in their average cTnT. Figure 2 demonstrates that the slope of the average cTnT rise is significantly less in the group of patients who had been given CsA as compared with the control group.
Table 4.
Patient peri-operative serum CK-MB and troponin-T (cTnT) levels
| CsA (n=40) | Control (n=38) | p Value | |
|---|---|---|---|
| CK-MB (ng/mL) | |||
| Preoperative | 2.2 (0.3) | 2.3 (1.5) | 0.79 |
| 6 h postoperative | 21.1 (2.2) | 26.5 (16.3) | 0.15 |
| 12 h postoperative | 19.2 (2.3) | 22.9 (14.3) | 0.30 |
| 24 h postoperative | 18.9 (2.8) | 18.2 (11.9) | 0.86 |
| 48 h postoperative | 8.6 (1.0) | 7.3 (4.2) | 0.33 |
| 72 h postoperative | 5.3 (0.6) | 4.0 (3.9) | 0.20 |
| TnT (ng/mL) | |||
| Preoperative | 0 | 0 | |
| 6 h postoperative | 0.35 (0.05) | 0.56 (0.06) | 0.07 |
| 12 h postoperative | 0.33 (0.04) | 0.44 (0.05) | 0.07 |
| 24 h postoperative | 0.31 (0.03) | 0.33 (0.04) | 0.87 |
| 48 h postoperative | 0.17 (0.04) | 0.21 (0.03) | 0.11 |
| 72 h postoperative | 0.12 (0.04) | 0.12 (0.02) | 0.97 |
Data are mean (SEM).
Figure 2.
Effect of CsA on the slope of the average cTnT rise analysed by multi-level model fitting. For every 10 min increase in the cardiopulmonary bypass time, patients who had received CsA had an attenuated increase in their average cTnT rise by 0.03 ng/mL. CsA; cyclosporin-A.
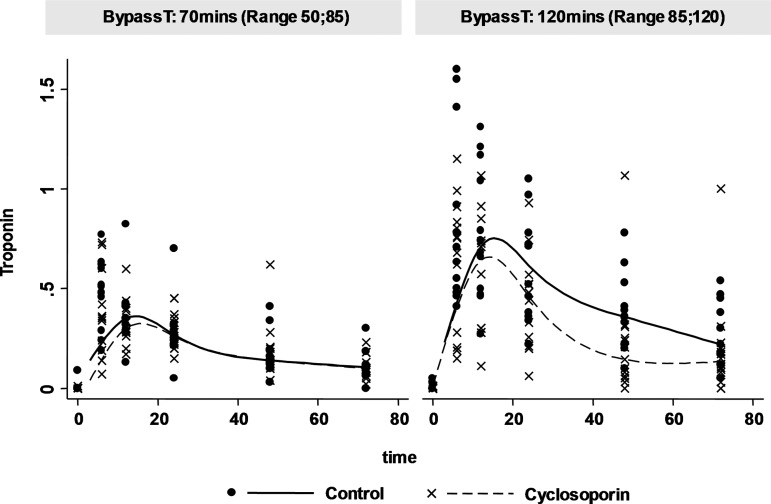
The reduced cTnT release in patients with longer CPB times after a single intravenous bolus of CsA is also illustrated in figure 3 where the cTnT rise profile is demonstrated for patients in our cohort with shorter and longer bypass times. The potential beneficial effect of CsA in reducing the cTnT rise profile with increasing bypass times was observed in the group with longer bypass times of 120 min as compared with those with a shorter bypass time of 70 min. Thus, the preoperative administration of CsA appears to demonstrate a cardioprotective effect which is more marked the longer the CPB time.
Figure 3.
Effect of CsA on peri-operative myocardial injury assessed by serum cTnT levels over the 72 h peri-operative period analysed with respect to the median cardiopulmonary bypass time (BypassT). This figure illustrates the interaction between bypass time and cyclosporine using a linear mixed model of the interaction between cyclosporine and bypass time. The graph plots the individual data (points) and the average profile change (lines) in troponin with and without cyclosporine at two arbitrary bypass times, namely 70 and 120 min. The graphs demonstrate a larger difference between the troponin profiles of control patients and cyclosporine treated patients at the longer bypass time. For visual clarity further CIs were not included. CsA; cyclosporin-A.
A classical linear mixed model was fitted with random intercept and slopes model was fitted. The main effects of time were fitted with higher order polynomials of time allowing for a parsimonious fit. Given the small sample size, we endeavoured to control for Type I error by fitting random intercepts and slopes for time. The further randomness introduced with the inclusion of random slopes to the model reduces type I error.14 Along with the main effects of time, the main effects of cyclosporine, bypass time and their interaction were included to infer any protective effect of cyclosporine on myocardial injury with longer exposure to bypass.
Postoperative outcome variables were analysed from prospectively collected data in a subgroup of patients with n=30 in the control arm and n=32 in the treatment arm. There were no significant differences the following outcome measures: length of hospital stay (7.9±4.2 days with control vs 8.6±3.6 days with CsA; p=0.53); length of stay in the postoperative critical care unit (25.7±9.0 h with control vs 27.4±9.8 h with CsA; p=0.53); and inotrope use (eight patients (27%) in the control versus eight patients (25%) in the CsA group). The balloon pump was not needed for inotropic support in any of the patients postoperatively. Two patients (6%) in the CsA group and none of the patients in the control group presented with a postoperative wound infection.
Discussion
In this proof-of-concept clinical study, we investigated for the first time the effects of CsA in adult patients undergoing CABG surgery. For those patients with longer cardiac bypass times (85–120 min), we have demonstrated that administering a single dose of CsA (2.5 mg/kg) prior to CABG surgery reduced the extent of PMI when compared with control. Importantly, the administration of CsA as a single intravenous bolus (2.5 mg/kg) was found to be safe with no related adverse effects, and with no difference in peri-operative serum markers for renal and liver function. It is probably not surprising that the patients with the longer CPB times benefited most from CsA cardioprotection, as patients with shorter CPB times probably receive adequate myocardial protection from cross-clamp fibrillation and cold-blood cardioplegia.
The opening of the MPTP on reperfusion of acutely ischaemic myocardium is a critical determinant of cardiomyocyte death in the setting of acute IRI.6 7 Pharmacological prevention of its opening at this time using CsA has been reported in a number of different animal models to reduce MI size in the region of 30%–40%,8–10 although not all studies have been positive (reviewed in12). We also applied this therapeutic approach to ex vivo human heart tissue and demonstrated cardioprotective effects with CsA following simulated IRI in human atrial trabeculae isolated from right atrial appendage tissue (harvested from patients undergoing CABG surgery).15
Piot et al11 were the first to apply this therapeutic agent in the clinical setting, demonstrating that a single intravenous bolus of CsA (2.5 mg/kg) administered 10 min prior to PPCI could limit MI size in STEMI patients. Whether CsA can actually improve clinical outcomes in STEMI patients treated by PPCI is being investigated in the ongoing 900-patient CIRCUS study (http://www.clinicaltrials.gov NCT01502774). Although CsA has been reported to protect against acute IRI in a number of different experimental settings including stroke, cardiac arrest and other organ IRI (reviewed in16 17), it has not been investigated as a cardioprotective agent in the setting of CABG surgery.
In our study, we administered a single dose of CsA prior to CABG surgery. In patients receiving cold-blood cardioplegia, the patients would have received a proportion of this dose, recirculating during the administration of cardioplegia. Whether CsA would have been even more cardioprotective if it had been added to the cardioplegic solution is not known.
The study limitations include the relatively small size of the study, and the failure to measure other endpoints of cardioprotection such as LVEF and major adverse cardiovascular events. However, it is important to appreciate that this study was not designed or adequately powered to detect the effect of CsA on these other endpoints. It is also important to bear in mind that not all patients will be eligible to receive CsA therapy for cardioprotection given pre-existing medical conditions such as significant renal impairment. In this clinical study, the different methods of myocardial management reflect the different practices by individual surgeons rather than between the two different hospitals. A larger study with functional endpoints is needed to confirm our findings.
Crucially, in those patients who received the CsA therapy, the magnitude of PMI (as measured by the serum cardiac enzymes) was decreased when compared with control, indicating that this therapeutic approach has protected the myocardium from the detrimental effects of acute IRI. The magnitude of PMI has been linked to worse clinical outcomes post-CABG surgery; whether CsA therapy is able to impact on prognosis in this patient group is unknown and needs to be determined in a suitably powered clinical study.
The data from this and the clinical study by Piot et al11 implicate MPTP inhibition as a viable therapeutic strategy for cardioprotection, but it must be appreciated that CsA is a non-specific MPTP inhibitor with potential side-effects. These include immunosuppression (resulting in increased risk of wound infection), renal dysfunction and hypertension in the peri-operative period, although the risk of these side-effects was limited due to only a single dose of CsA being administered. However, in order for this therapeutic approach to be implemented in the clinical setting, more potent and specific MPTP inhibitors will need to be discovered.
We conclude that the administration of a single dose of CsA prior to CABG surgery can reduce PMI in higher-risk patients with longer CPB times. Large multi-centre clinical studies will be required to confirm these results and to investigate whether MPTP inhibition can improve clinical outcomes in patients undergoing CABG surgery.
Key messages.
What is already known on this subject?
Novel therapeutic strategies are required to protect the heart against acute ischaemia-reperfusion injury (IRI) in patients undergoing coronary artery bypass graft (CABG) surgery in order to reduce the extent of peri-operative myocardial injury (PMI) and improve clinical outcomes in this patient group. In this regard, preventing mitochondrial dysfunction by inhibiting the opening of the mitochondrial permeability transition pore (MPTP) during acute IRI, using the drug cyclosporin-A (CsA), has been reported to protect the myocardium. Whether, targeting the MPTP with CsA in patients undergoing CABG surgery can reduce the extent of PMI is unknown.
What this study adds?
In this study, we show for the first time that targeting MPTP opening during CABG surgery with CsA can potentially limit the extent of PMI in those patients with longer cardiopulmonary bypass times, suggesting that this therapeutic approach may benefit those higher-risk patients undergoing CABG surgery.
How might this impact on clinical practice?
By limiting the extent of PMI in those CABG patients with longer cardiopulmonary bypass times, preventing mitochondrial dysfunction by targeting the MPTP with CsA has the potential to improve clinical outcomes in this patient group. However, this will have to be investigated in a large multi-centred adequately powered randomised clinical trial.
Acknowledgments
We would like to thank all the patients and staff at the Heart Hospital and Kings College Hospital.
Footnotes
Contributors: All authors contributed to design, execution, data analysis and writing of paper.
Funding: This work was supported by the British Heart Foundation (grant numbers RG/03/007, FS/10/039/28270 and FS/10/72/28568), the RoseTree Trust and the National Institute for Health Research University College London Hospitals Biomedical Research Centre of which DMY is a Senior Investigator. The trial was also supported by the NIHR CLRN funding stream. GK was supported by a research initiative grant from the Department of Research and Development, King's College Hospital Foundation Trust, London.
Patient consent: Obtained.
Ethics approval: NRES.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Croal BL, Hillis GS, Gibson PH, et al. Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation 2006;114:1468–75 [DOI] [PubMed] [Google Scholar]
- 2.Brener SJ, Lytle BW, Schneider JP, et al. Association between CK-MB elevation after percutaneous or surgical revascularization and three-year mortality. J Am Coll Cardiol 2002;40:1961–7 [DOI] [PubMed] [Google Scholar]
- 3.Fellahi JL, Gue X, Richomme X, et al. Short- and long-term prognostic value of postoperative cardiac troponin I concentration in patients undergoing coronary artery bypass grafting. Anesthesiology 2003;99:270–4 [DOI] [PubMed] [Google Scholar]
- 4.Venugopal V, Ludman A, Yellon DM, et al. ‘Conditioning’ the heart during surgery. Eur J Cardiothorac Surg 2009;35:977–87 [DOI] [PubMed] [Google Scholar]
- 5.Heusch G, Kleinbongard P, Bose D, et al. Coronary microembolization: from bedside to bench and back to bedside. Circulation 2009;120:1822–36 [DOI] [PubMed] [Google Scholar]
- 6.Hausenloy DJ, Yellon DM. The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion. J Mol Cell Cardiol 2003;35:339–41 [DOI] [PubMed] [Google Scholar]
- 7.Leung AW, Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta 2008;1777:946–52 [DOI] [PubMed] [Google Scholar]
- 8.Hausenloy DJ, Maddock HL, Baxter GF, et al. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res 2002;55:534–43 [DOI] [PubMed] [Google Scholar]
- 9.Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res 2003;60:617–25 [DOI] [PubMed] [Google Scholar]
- 10.Argaud L, Gateau-Roesch O, Muntean D, et al. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol 2005;38:367–74 [DOI] [PubMed] [Google Scholar]
- 11.Piot C, Croisille P, Staat P, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 2008;359:473–81 [DOI] [PubMed] [Google Scholar]
- 12.Hausenloy DJ, Boston-Griffiths EA, Yellon DM. Cyclosporin A and cardioprotection: from investigative tool to therapeutic agent. Br J Pharmacol 2012;165:1235–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hausenloy DJ, Mwamure PK, Venugopal V, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 2007;370:575–9 [DOI] [PubMed] [Google Scholar]
- 14.Schielzeth H, Forstmeier W. Conclusions beyond support: overconfident estimates in mixed models. Behav Ecol 2009;20:416–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shanmuganathan S, Hausenloy DJ, Duchen MR, et al. Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol 2005;289:H237–42 [DOI] [PubMed] [Google Scholar]
- 16.Osman MM, Lulic D, Glover L, et al. Cyclosporine-A as a neuroprotective agent against stroke: its translation from laboratory research to clinical application. Neuropeptides 2011;45:359–68 [DOI] [PubMed] [Google Scholar]
- 17.Hausenloy DJ, Boston-Griffiths EA, Yellon DM. Cyclosporin A and cardioprotection: from investigative tool to therapeutic agent. Br J Pharmacol 2012;165:1235–45 [DOI] [PMC free article] [PubMed] [Google Scholar]