Abstract
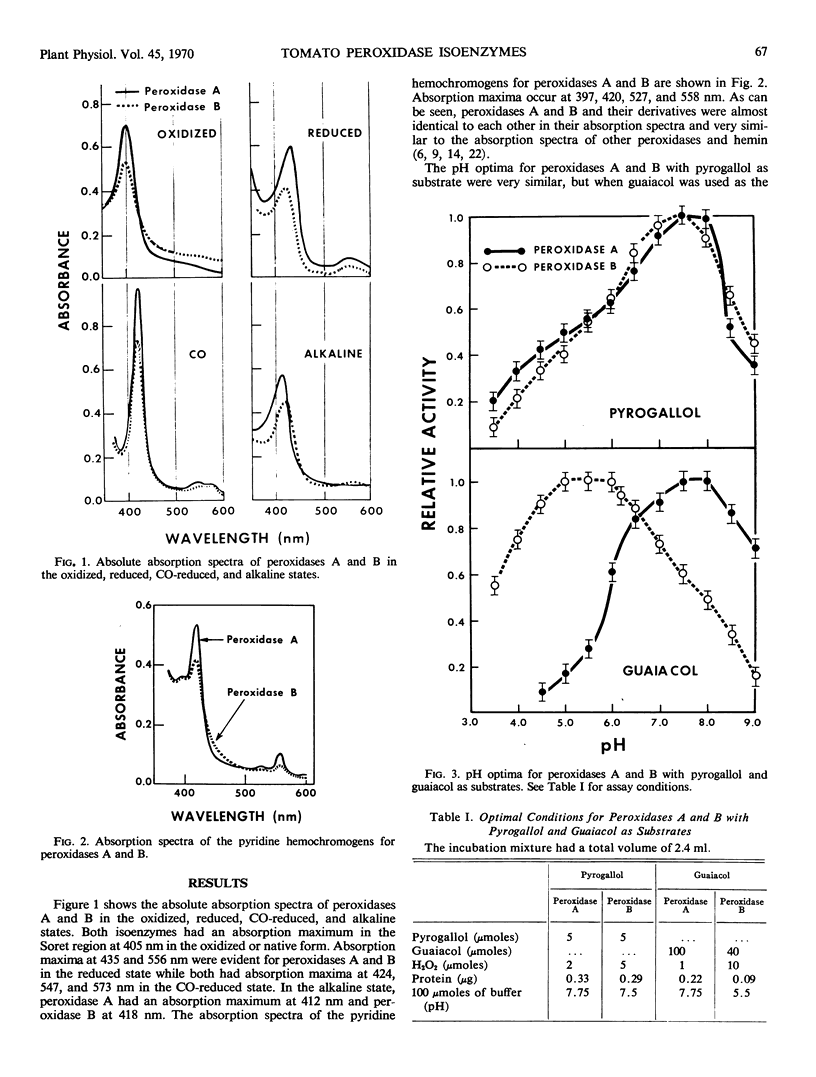
The kinetic and spectral properties of peroxidases A and B from the dwarf tomato plant were compared. The absolute absorption spectra were essentially the same for peroxidases A and B and their derivatives. Peroxidases A and B had different pH optima with guaiacol as the hydrogen donor but essentially the same optimum when pyrogallol was the substrate. The substrate concentrations required for optimum activity were different not only for the different substrates but also for each isoenzyme. When pyrogallol was used as the substrate, peroxidases A and B were 80% active when assayed under conditions optimal for the other isoenzyme. When guaiacol was used as the substrate, peroxidase A was completely inactive when assayed under conditions optimal for peroxidase B. In this case the pH was not optimum and the H2O2 concentration was inhibitory. Similarly, peroxidase B retained only 9% of its peroxidase activity toward guaiacol when assayed under conditions optimum for peroxidase A. In this case the pH was not optimum and the H2O2 was limiting. A possible role for peroxidase isoenzymes is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans J. J. Increased buffer density as a means of separating peroxidase isoenzymes by preparative continuous-flow carrier-free electrophoresis. Anal Biochem. 1969 Oct 15;32(1):101–105. doi: 10.1016/0003-2697(69)90108-0. [DOI] [PubMed] [Google Scholar]
- Evans J. J. Peroxidases from the extreme dwarf tomato plant. Identification, isolation, and partial purification. Plant Physiol. 1968 Jul;43(7):1037–1041. doi: 10.1104/pp.43.7.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher W. A., Elliott W. B. The formation of pyridine haemochromogen. Biochem J. 1965 Oct;97(1):187–193. doi: 10.1042/bj0970187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay E., Shannon L. M., Lew J. Y. Peroxidase isozymes from horseradish roots. II. Catalytic properties. J Biol Chem. 1967 May 25;242(10):2470–2473. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAEHLY A. C., CHANCE B. The assay of catalases and peroxidases. Methods Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Morrison M., Horie S. Determination of heme a concentration in cytochrome preparations by hemochromogen method. Anal Biochem. 1965 Jul;12(1):77–82. doi: 10.1016/0003-2697(65)90144-2. [DOI] [PubMed] [Google Scholar]
- SHIN M., NAKAMURA W. Indoleacetic acid oxidase activity of peroxidase. J Biochem. 1962 Dec;52:444–451. doi: 10.1093/oxfordjournals.jbchem.a127642. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Ray G. S. Studies on cytochrome c peroxidase. I. Purification and some properties. J Biol Chem. 1965 Nov;240(11):4503–4508. [PubMed] [Google Scholar]



