The authors reveal a mechanism underlying jasmonate (JA) and ethylene (ET) antagonism: Interaction between the JA-activated transcription factor MYC2 and the ET-stabilized transcription factor EIN3, reciprocally repressing their transcriptional activity, modulates the antagonistic actions of JA and ET in regulating apical hook curvature, wound-responsive gene expression, and defense against insect attack.
Abstract
Plants have evolved sophisticated mechanisms for integration of endogenous and exogenous signals to adapt to the changing environment. Both the phytohormones jasmonate (JA) and ethylene (ET) regulate plant growth, development, and defense. In addition to synergistic regulation of root hair development and resistance to necrotrophic fungi, JA and ET act antagonistically to regulate gene expression, apical hook curvature, and plant defense against insect attack. However, the molecular mechanism for such antagonism between JA and ET signaling remains unclear. Here, we demonstrate that interaction between the JA-activated transcription factor MYC2 and the ET-stabilized transcription factor ETHYLENE-INSENSITIVE3 (EIN3) modulates JA and ET signaling antagonism in Arabidopsis thaliana. MYC2 interacts with EIN3 to attenuate the transcriptional activity of EIN3 and repress ET-enhanced apical hook curvature. Conversely, EIN3 interacts with and represses MYC2 to inhibit JA-induced expression of wound-responsive genes and herbivory-inducible genes and to attenuate JA-regulated plant defense against generalist herbivores. Coordinated regulation of plant responses in both antagonistic and synergistic manners would help plants adapt to fluctuating environments.
INTRODUCTION
Sessile plants have evolved sophisticated mechanisms for integration of endogenous and exogenous signals to regulate their growth, development, and defense responses, which benefits their survival in the changing environment. Both ethylene (ET) and jasmonate (JA) are essential plant hormones that regulate various plant developmental processes and diverse defense responses (Kieber, 1997; Bleecker and Kende, 2000; Guo and Ecker, 2004; Broekaert et al., 2006; Howe and Jander, 2008; Browse, 2009; Shan et al., 2012; Wasternack and Hause, 2013). ET signal is perceived by its receptors ETHYLENE RESPONSE1 (ETR1), ETR2, ETHYLENE RESPONSE SENSOR1 (ERS1), ERS2, and ETHYLENE INSENSITIVE4 (EIN4) (Hua and Meyerowitz, 1998) to repress CONSTITUTIVE TRIPLE RESPONSE1 (CTR1) (Kieber et al., 1993), which activates EIN2 (Alonso et al., 1999; Ju et al., 2012; Qiao et al., 2012; Wen et al., 2012) and subsequently stabilizes EIN3 and EIN3-LIKE1 (EIL1) (Chao et al., 1997; Guo and Ecker, 2003; Potuschak et al., 2003; Gagne et al., 2004) to mediate various ET responses, including hypocotyl growth (Zhong et al., 2012), apical hook formation (Knight et al., 1910; An et al., 2012), root growth (Ortega-Martínez et al., 2007; Růzicka et al., 2007), flowering (Ogawara et al., 2003; Achard et al., 2007), fruit ripening (Burg and Burg, 1962; Theologis et al., 1992), leaf senescence (Gepstein and Thimann, 1981; Li et al., 2013), freezing tolerance (Shi et al., 2012), and resistance against pathogen infection (Alonso et al., 2003; Chen et al., 2009).
JA plays essential roles in the regulation of plant development and defense. Upon perception of JA signal (Fonseca et al., 2009; Yan et al., 2009; Sheard et al., 2010), the F-box protein CORONATINE INSENSITIVE1 (COI1) (Xie et al., 1998; Yan et al., 2009) recruits the JASMONATE ZIM-DOMAIN (JAZ) proteins (Chini et al., 2007; Thines et al., 2007; Yan et al., 2007) for degradation, which leads to the release of various downstream factors, including MYC2/JASMONATE INSENSITIVE1 (JIN1), MYC3, and MYC4 (Cheng et al., 2011; Fernández-Calvo et al., 2011; Niu et al., 2011), as well as WD-repeat/bHLH/MYB complex (Qi et al., 2011), MYB21, MYB24, and MYB57 (Mandaokar et al., 2006; Song et al., 2011) and the IIId bHLH factors (Nakata et al., 2013; Song et al., 2013b), which regulate diverse JA-mediated functions. These functions include root growth (Dathe et al., 1981; Chen et al., 2011), apical hook formation (Turner et al., 2002), flowering (Robson et al., 2010), stamen development (McConn and Browse, 1996; Song et al., 2011, 2013a), leaf senescence (Ueda and Kato, 1980; Shan et al., 2011), secondary metabolism (De Geyter et al., 2012; Schweizer et al., 2013), drought responses (Seo et al., 2011), wounding responses (Mason and Mullet, 1990; Acosta et al., 2013; Mousavi et al., 2013), and defense against pathogen infection (Thomma et al., 1998; Vijayan et al., 1998; Melotto et al., 2006; Rowe et al., 2010; Yang et al., 2012; Zheng et al., 2012) and insect attack (McConn et al., 1997; Hu et al., 2013a).
Previous studies showed that both JA and ET concomitantly and synergistically regulate plant defense against necrotrophic fungi (Penninckx et al., 1998; Thomma et al., 1998; Thomma et al., 1999) and root hair development (Zhu et al., 2006). It is so far reported that such JA-ET signaling synergy is mediated by derepression of ET-stabilized EIN3 and EIL1: JAZ proteins directly interact with and repress EIN3/EIL1, while JA induces JAZ degradation to derepress EIN3 and EIL1 (Zhu et al., 2011). JA-induced EIN3 and EIL1 activation (Zhu et al., 2011) and ET-induced EIN3 and EIL1 stabilization (Guo and Ecker, 2003; Potuschak et al., 2003; Gagne et al., 2004) mediate JA and ET signaling synergy in the regulation of root hair development and resistance against necrotrophic fungal infection.
In addition to their synergistic regulation, JA and ET also act antagonistically in regulating expression of wound-responsive genes (Rojo et al., 1999; Lorenzo et al., 2004) and metabolite biosynthetic genes (Mikkelsen et al., 2003). JA represses apical hook formation (Turner et al., 2002) and positively regulates plant defense against insect attack (Fernández-Calvo et al., 2011; Schweizer et al., 2013), while ET functions oppositely (Guzmán and Ecker, 1990; Mewis et al., 2005, 2006; Bodenhausen and Reymond, 2007). However, the molecular mechanism for such antagonism between JA and ET signaling remains unclear. In this study, we show that MYC2 interacts with EIN3 and EIL1 to repress the transcriptional activity of EIN3 and EIL1 in Arabidopsis thaliana and consequently to inhibit ET-regulated apical hook formation; similarly, we found that EIN3 and EIL1 interact with and repress MYC2, further attenuate JA-induced expression of wound-responsive genes and herbivory-inducible genes, and inhibit plant defense against insect attack. This molecular, biochemical, and genetic evidence reveals that interactions of the JA-activated transcription factor MYC2 with the ET-stabilized transcription factors (EIN3 and EIL1) repress their respective transcriptional activities to modulate JA and ET signaling antagonism, which provides insights into how plants integrate various phytohormone signals to coordinately regulate plant development, growth, and defense.
RESULTS
MYC2, MYC3, and MYC4 Function Redundantly to Mediate JA-Inhibited Apical Hook Curvature
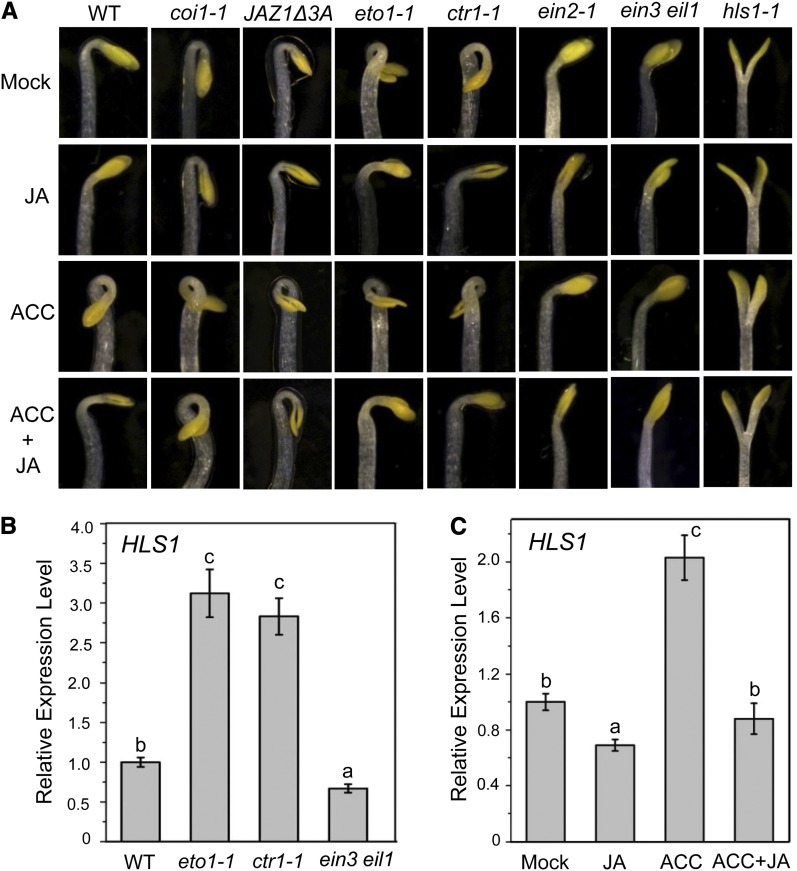
The formation of the apical hook helps cotyledons and meristem tissues protrude from the soil without being damaged. Previous studies showed that ET induces hook curvature (Guzmán and Ecker, 1990), whereas JA antagonizes the ET pathway that functions in apical hook formation in etiolated Arabidopsis seedlings (Turner et al., 2002). Consistent with previous observations (Turner et al., 2002), 1-aminocyclopropane-1-carboxylic acid (ACC), the ET biosynthesis precursor, enhanced the apical hook curvature, while JA obviously suppressed the ET-enhanced hook curvature of the dark-grown Arabidopsis wild-type seedlings (Figure 1A); the coi1-1 mutant exhibited an exaggerated apical hook curvature (Figure 1A). As expected, the mutants with ET overproduction (ethylene overproducer1 [eto1-1]) or constitutive ET responses (ctr1-1) exhibited constitutive exaggerated hook curvature, while the mutants deficient in ET signaling (ein2-1 and ein3-1 eil1-3) displayed obvious reduction in hook curvature (Figure 1A). We further found that the exaggerated hook curvature in eto1-1 and ctr1-1 was clearly inhibited by JA (Figure 1A), which indicates that JA functions downstream of ETO1 and CTR1 to repress ET-regulated hook curvature.
Figure 1.
JA Suppresses the ET-induced Apical Hook Formation.
(A) The hook phenotypes of 4-d-old etiolated Arabidopsis seedlings Columbia-0 (Col-0; WT), coi1-1, JAZ1Δ3A, eto1-1, ctr1-1, ein2-1, ein3-1 ein1-3 (ein3 eil1), and hls1-1 grown in the dark on MS medium supplied without (Mock) or with 5 μM MeJA (JA), 10 μM ACC, or 10 μM ACC plus 5 μM MeJA (ACC+JA).
(B) Real-time PCR analysis for HLS1 in 4-d-old etiolated Col-0 (WT), eto1-1, ctr1-1, and ein3 eil1. Actin8 was used as the internal control. Data are means (±sd) of three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
(C) Real-time PCR analysis for HLS1 in 4-d-old etiolated Col-0 (WT) treated with mock, 100 μM MeJA (JA), 100 μM ACC, or 100 μM ACC plus 100 μM MeJA (ACC+JA) for 6 h. Actin8 was used as the internal control. Data are means (±sd) of three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
HOOKLESS1 (HLS1) is a central positive regulator of apical hook development (Lehman et al., 1996; An et al., 2012) (Figures 1A and 1B). The mutants eto1-1 and ctr1-1, with high level of HLS1 expression, exhibited exaggerated hook curvature, while the mutant ein3-1 eil1-3, with low levels of HLS1 expression, displayed reduced hook curvature (Figures 1A and 1B). ACC treatment induced HLS1 expression and exaggerated apical hook curvature in the wild type, and ACC-induced HLS1 expression and hook formation were obviously repressed by JA treatment (Figures 1A and 1C). These results suggest that JA represses HLS1 expression to inhibit ET-enhanced hook formation and imply that JA acts upstream of HLS1 to repress hook curvature.
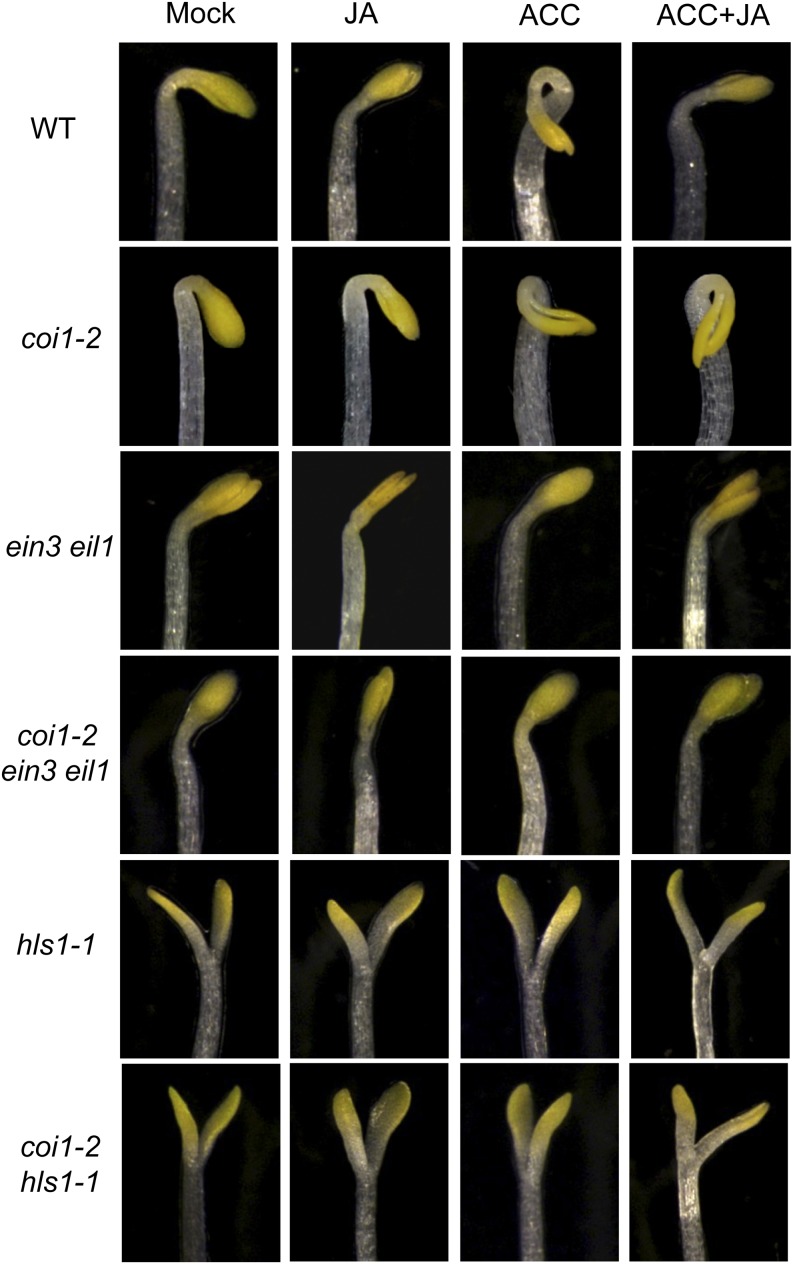
To genetically verify whether JA acts upstream of HLS1, we further generated the double mutant coi1-2 hls1-1 and the triple mutant coi1-2 ein3 eil1 via genetic cross of coi1-2 with hls1-1 or ein3 eil1. The results in Figure 2 showed that the coi1-2 exhibited an exaggerated hook curvature, while no hook was formed in coi1-2 hls1-1 and hls1-1. Similar data were also observed for coi1-2 ein3 eil1 (Figure 2). Suppression of the exaggerated hook curvature in coi1-2 by the hls1-1 and ein3 eil1 mutations suggests that COI1 acts upstream of the EIN3/EIL1-HLS1 cascade to regulate apical hook formation.
Figure 2.
COI1 Acts Upstream of EIN3/EIL1 and HLS1 in Regulation of Apical Hook Formation.
The hook phenotypes of 4-d-old etiolated Arabidopsis seedlings Col-0 (WT), coi1-2, ein3 eil1, coi1-2 ein3 eil1, hls1-1, and coi1-2 hls1-1 grown in the dark on MS medium supplied without (Mock) or with 5 μM MeJA (JA), 10 μM ACC, or 10 μM ACC plus 5 μM MeJA (ACC+JA).
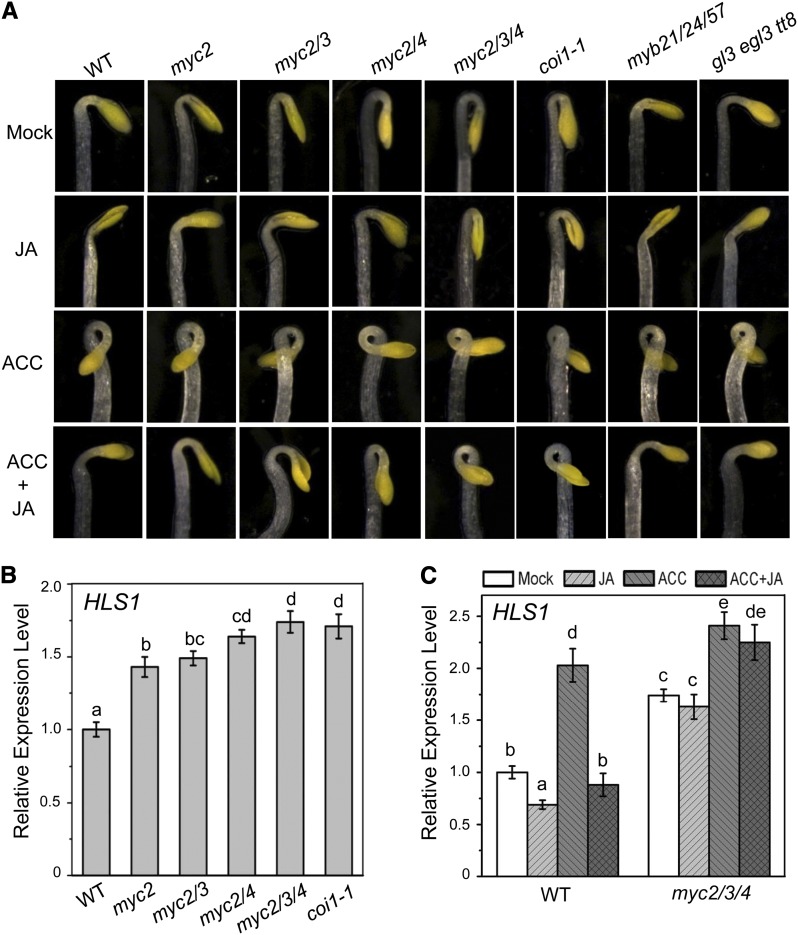
To identify the key components responsible for repression of hook curvature in JA signaling pathway, we examined apical hook phenotypes in JA signaling mutants. As expected, the coi1-1 mutant exhibited an exaggerated apical hook curvature (Figure 3A) (Turner et al., 2002). JAZ1Δ3A transgenic plants, with high levels of JAZ proteins (Thines et al., 2007), also displayed an exaggerated apical hook curvature (Figure 1A). Among the key transcription factors targeted by JAZ proteins, MYB21/MYB24/MYB57 (Song et al., 2011) and WD-repeat/bHLH/MYB complex (Qi et al., 2011) are not involved in the suppression of hook formation, as the myb21 myb24 myb57 and gl3 egl3 tt8 mutants exhibited wild-type-like hook curvature (Figure 3A).
Figure 3.
MYC2, MYC3, and MYC4 Function Redundantly to Mediate the JA-Inhibited Apical Hook Formation.
(A) The hook phenotypes of 4-d-old etiolated Arabidopsis seedlings Col-0 (WT), myc2-2 (myc2), myc2-2 myc3 (myc2/3), myc2-2 myc4 (myc2/4), myc2-2 myc3 myc4 (myc2/3/4), myb21 myb24 myb57 (myb21/24/57), and gl3 egl3 tt8 grown in the dark on MS medium supplied without (Mock) or with 5 μM MeJA (JA), 10 μM ACC, or 10 μM ACC plus 5 μM MeJA (ACC+JA).
(B) Real-time PCR analysis for HLS1 in the indicated 4-d-old etiolated seedlings. Actin8 was used as the internal control. Data are means (±sd) of three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
(C) Real-time PCR analysis for HLS1 in the 4-d-old etiolated Col-0 (WT) and myc2-2 myc3 myc4 (myc2/3/4) treated with mock, 100 μM MeJA (JA), 100 μM ACC, or 100 μM ACC plus 100 μM MeJA (ACC+JA) for 6 h. Actin8 was used as the internal control. Data are means (±sd) of three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
Interestingly, the myc2 single mutant exhibited a mildly exaggerated apical hook curvature compared with the wild type (Figure 3A); the apical hook curvature was clearly enhanced in the double mutants myc2 myc3 and myc2 myc4 (Figure 3A), while the triple mutant myc2 myc3 myc4 displayed the strongest apical hook curvature (Figure 3A), which is similar to that observed in the coi1 mutant (Figure 3A). The hook curvature of the single or double mutants (myc2, myc2 myc3, and myc2 myc4) could be further inhibited by JA treatment, whereas the triple mutant (myc2 myc3 myc4) was completely insensitive to JA-inhibited hook curvature (Figure 3A). Furthermore, JA was unable to repress ACC-enhanced hook curvature in myc2 myc3 myc4 (Figure 3A). Consistent with the exaggerated hook curvature, the expression of HLS1 was upregulated in the mutants myc2, myc2 myc3, myc2 myc4, and myc2 myc3 myc4 (Figures 3B). Furthermore, ACC-enhanced HLS1 expression in myc2 myc3 myc4 was not repressed by JA treatment (Figures 3C).
Taken together, the results in Figure 3 suggest that MYC2, MYC3, and MYC4 function redundantly to mediate JA-inhibited hook curvature.
MYC2, MYC3, and MYC4 Interact with EIN3 and EIL1
Having shown that MYC2, MYC3, and MYC4 function redundantly to repress HLS1 expression and mediate JA inhibition of ET-enhanced hook curvature (Figure 3), we further found that MYC2, MYC3, and MYC4 were able to interact with EIN3 and EIL1 (Figure 4), activators of HLS1 (An et al., 2012).
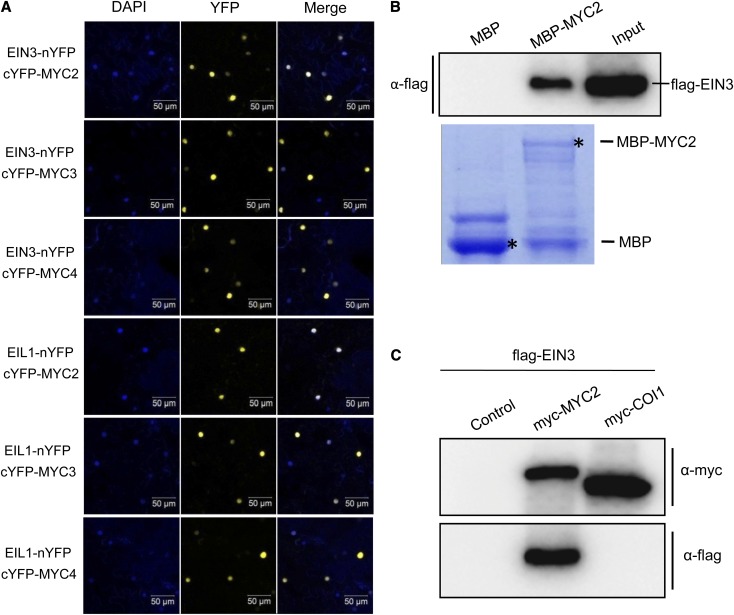
Figure 4.
MYC2, MYC3, and MYC4 Interact with EIN3 and EIL1.
(A) BiFC assay to detect the interactions of MYC2, MYC3, and MYC4 with EIN3 and EIL1. EIN3 and EIL1 were fused with the N-terminal fragment of YFP (nYFP) to form EIN3-nYFP and EIL1-nYFP, respectively. MYC2, MYC3, and MYC4 were fused with the C-terminal fragment of YFP (cYFP) to generate cYFP-MYC2, cYFP-MYC3, and cYFP-MYC4. YFP fluorescence was detected in N. benthamiana leaves coinfiltrated with the combination of indicated constructs. The positions of nuclei were shown by 4′,6-diamidino-2-phenylindole (DAPI) staining.
(B) In vitro pull-down assay to verify the interaction of MYC2 with EIN3. The purified MBP and MBP-MYC2 fusion protein were incubated with the total protein from N. benthamiana leaves with transient expression of flag-EIN3. Bound proteins were washed, separated on SDS-PAGE, and immunoblotted with the anti-flag antibody (α-flag; top panel). The input lane shows the protein level of flag-EIN3 expressed in leaves of N. benthamiana. The positions of purified MBP and MBP-MYC2 separated on SDS-PAGE are marked with asterisks (bottom panel; stained by Coomassie blue).
(C) Co-IP assay to verify the interaction of MYC2 with EIN3 in planta. The flag-EIN3 was coexpressed without (Control) or with myc-MYC2 or myc-COI1 in the N. benthamiana leaves. The total protein extracts from the N. benthamiana leaves with transient expression of flag-EIN3, flag-EIN3 plus myc-MYC2, or flag-EIN3 plus myc-COI1 were immunoprecipitated with the anti-c-myc antibody-conjugated agarose and were further detected by immunoblot using anti-flag antibody and anti-c-myc antibody.
The yellow fluorescent protein (YFP)–based bimolecular fluorescence complementation (BiFC) assays showed that coexpression of EIN3-nYFP (fusion of EIN3 with N-terminal fragment of YFP) or EIL1-nYFP with cYFP-MYC2 (fusion of MYC2 with C-terminal fragment of YFP), cYFP-MYC3, or cYFP-MYC4 produced strong YFP signals in the nuclei (Figure 4A), while the negative controls did not (Supplemental Figure 1), demonstrating that MYC2, MYC3, and MYC4 interact with EIN3 and EIL1.
We also used pull-down assays to representatively examine the interaction of MYC2 with EIN3 (Figure 4B). Purified maltose binding protein (MBP)–fused MYC2 (MBP-MYC2) resin was incubated with total protein from Nicotiana benthamiana leaves with transient expression of flag-tagged EIN3 (flag-EIN3) and separated by SDS-PAGE for immunoblotting with anti-flag antibody. As shown in Figure 4B, the MBP-MYC2 resin could pull down flag-EIN3, suggesting that MYC2 interacts with EIN3.
Furthermore, we performed coimmunoprecipitation (Co-IP) assays to examine the interaction between MYC2 and EIN3 in planta. The flag-EIN3 was coexpressed with myc-tagged MYC2 (myc-MYC2) or myc-COI1, respectively, in leaves of N. benthamiana, and the total proteins were then used for coimmunoprecipitation. The results showed that flag-EIN3 was indeed coimmunoprecipitated with myc-MYC2 (Figure 4C), but not with the control protein myc-COI1 (Figure 4C). Taken together, the BiFC assay, pull-down assay, and Co-IP assay consistently demonstrate that MYC2, MYC3, and MYC4 interact with EIN3 and EIL1 (Figure 4).
MYC2 Inhibits Transcriptional Activity of EIN3 and EIL1
Having shown that MYC2, MYC3, and MYC4 interact with EIN3 and EIL1, we then investigated whether such interactions affect the transcriptional activity of EIN3 and EIL1 using an Arabidopsis mesophyll protoplast transfection-based transcriptional activity assay (Hellens et al., 2005).
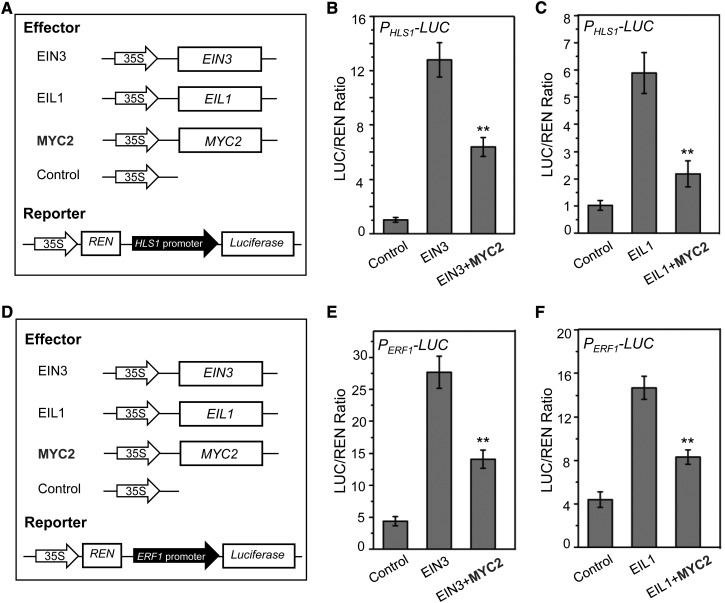
A previous study showed that EIN3 could bind to the promoter of HLS1 to activate its expression, leading to hook curvature (An et al., 2012). We first examined whether MYC2 affects the influence of EIN3 on HLS1 transcription. As expected (An et al., 2012), expression of EIN3 dramatically activated the expression of LUC driven by the HLS1 promoter (Figures 5A and 5B). However, coexpression of MYC2 with EIN3 significantly repressed EIN3-activated PHLS1-LUC activity (Figure 5B). Similarly, expression of EIL1 activated PHLS1-LUC activity, whereas coexpression of MYC2 repressed EIL1-activated PHLS1-LUC activity (Figures 5A and 5C). The results in Figures 4 and 5A to 5C demonstrate that MYC2 interacts with EIN3 and EIL1 to interfere with their effect on the transcription of HLS1.
Figure 5.
MYC2 Represses Transcriptional Activity of EIN3 and EIL1.
(A) The schematic diagram shows the constructs used in the transient transcriptional activity assays of (B) and (C).
(B) and (C) Transient transcriptional activity assays show that activation of HLS1 promoter by EIN3 (B) and EIL1 (C) is repressed by MYC2. The PHLS1-LUC reporter was cotransformed with the indicated constructs. The LUC/REN ratio represents the PHLS1-LUC activity relative to the internal control (REN driven by 35S promoter). Data are means (±sd) of three biological replicates. Asterisks represent Student’s t test significance between EIN3 and EIN3+MYC2 or EIL1 and EIL1+MYC2 samples (**P < 0.01).
(D) The schematic diagram shows the constructs used in the transient transcriptional activity assays of (E) and (F).
(E) and (F) Transient transcriptional activity assays show that activation of ERF1 promoter by EIN3 (E) and EIL1 (F) is repressed by MYC2. The PERF1-LUC reporter was cotransformed with the indicated constructs. Data are means (±sd) of three biological replicates. Asterisks represent Student’s t test significance between EIN3 and EIN3+MYC2 or EIL1 and EIL1+MYC2 samples (**P < 0.01).
Having shown that MYC2 suppresses the effect of EIN3 and EIL1 on HLS1 transcription, we further examined whether MYC2 could repress the effects of EIN3 and EIL1 on the transcription of another target gene, ETHYLENE RESPONSE FACTOR1 (ERF1) (Solano et al., 1998), a key transcription factor that activates the expression of PDF1.2 to induce resistance against necrotrophic pathogens (Pré et al., 2008; Zarei et al., 2011). As shown in Figures 5D and 5E, overexpression of EIN3 activated the ERF1 promoter that controlled expression of the LUC gene (PERF1-LUC), whereas such EIN3-activated PERF1-LUC expression was obviously repressed by coexpression of MYC2 (Figures 5D and 5E). Furthermore, we found that expression of EIL1 also activated PERF1-LUC activity, while coexpression of MYC2 repressed the EIL1-activated PERF1-LUC activity (Figures 5D and 5F). Taken together (Figures 4 and 5), these results demonstrate that MYC2 interacts with EIN3 and EIL1 to attenuate their effect on the transcription of their target genes HLS1 and ERF1.
Disruption of EIN3 and EIL1 Suppresses Exaggerated Apical Hook Formation and Resistance against a Necrotrophic Pathogen in myc2
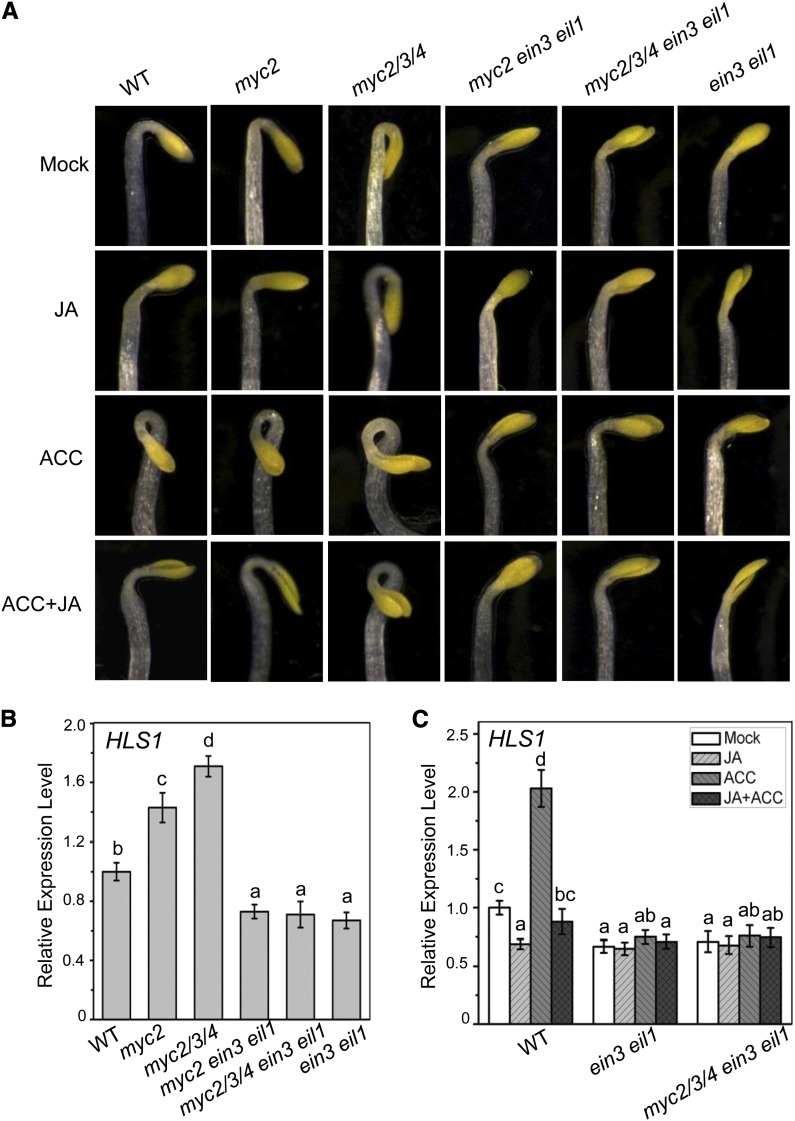
In agreement with the observation that MYC2 represses the transcriptional activity of EIN3 and EIL1, abolishment of MYC2 in planta is expected to derepress EIN3 and EIL1, which would further activate the expression of HLS1 (essential for hook curvature) and ERF1 (vital for resistance against Botrytis cinerea). Indeed, the myc2 mutants (e.g., myc2 myc3, myc2 myc4, and myc2 myc3 myc4) showed increased expression of HLS1 (Figures 3B, 3C, and 6B), and the myc2 mutant exhibited elevated expression of defensive genes, such as ERF1, OCTADECANOID-RESPONSIVE ARABIDOPSIS AP2/ERF59 (ORA59) (ERF1 homolog), and their target gene PLANT DEFENSIN1.2 (PDF1.2) (Figure 7C). Consistent with their gene expression patterns, the myc2-related mutants showed exaggerated hook formation (Figures 3A and 6A) and the myc2 mutant displayed increased resistance against B. cinerea (Figures 7A and 7B) (Lorenzo et al., 2004). These results suggest that mutation in MYC2 releases EIN3 and EIL1 to further activate the expression of HLS1 and ERF1, which are vital for hook curvature and disease resistance.
Figure 6.
Mutations in EIN3 and EIL1 Block the Exaggerated Hook Curvature of myc2 and myc2 myc3 myc4.
(A) The hook phenotypes of 4-d-old etiolated Arabidopsis Col-0 (WT), myc2-2 (myc2), jin1-2 myc3 myc4 (myc2/3/4), myc2-2 ein3 eil1 (myc2 ein3 eil1), jin1-2 myc3 myc4 ein3 eil1 (myc2/3/4 ein3 eil1), and ein3 eil1 grown in the dark on MS medium supplied without (Mock) or with 5 μM MeJA (JA), 10 μM ACC, or 10 μM ACC plus 5 μM MeJA (ACC+JA).
(B) Real-time PCR analysis for HLS1 in the indicated 4-d-old etiolated seedlings. Actin8 was used as the internal control. Data are means (±sd) of three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
(C) Real-time PCR analysis for HLS1 in the indicated 4-d-old etiolated seedlings treated with mock, 100 μM MeJA (JA), 100 μM ACC, or 100 μM ACC plus 100 μM MeJA (JA+ACC) for 6 h. Actin8 was used as the internal control. Data are means (±sd) of three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
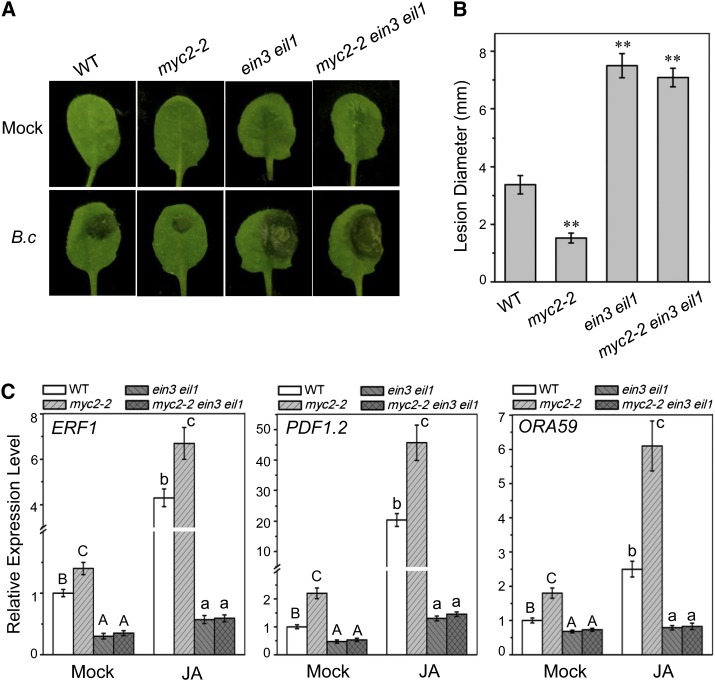
Figure 7.
Mutations in EIN3 and EIL1 Repress the Enhanced Resistance against Necrotrophic Pathogen Botrytis cinerea in myc2.
(A) Symptoms on detached rosette leaves from 3-week-old plants of Col-0 (WT), myc2-2, ein3 eil1, and myc2-2 ein3 eil1 at day 2 after inoculation with mock or B. cinerea (B.c) spores.
(B) The lesion sizes on rosette leaves at day 2 after inoculation with B. cinerea spores. Data are means (±sd) of three biological replicates. Asterisks represent Student’s t test significance compared with the wild type (**P < 0.01).
(C) Quantitative real-time PCR analysis of ERF1, PDF1.2, and ORA59 in 12-d-old wild type, myc2-2, ein3 eil1, and myc2-2 ein3 eil1 treated with mock or 100 μM MeJA (JA) for 6 h. Actin8 was used as the internal control. Data are means (±sd) of three biological replicates. Different letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05). Capital letters correspond with each other, and lowercase letters correspond with each other.
To examine whether ein3 eil1 is able to suppress the exaggerated hooks in the myc2-related mutants, we generated the myc2 ein3 eil1 and myc2 myc3 myc4 ein3 eil1 mutants via crossing myc2-related mutants with the ein3 eil1 mutant. The results in Figure 6A show that the exaggerated hook curvature in myc2-related mutants was repressed by ein3 eil1 (Figure 6A). Consistently, the elevated expression of HLS1 in myc2 and myc2 myc3 myc4 was abolished in myc2 ein3 eil1 and myc2 myc3 myc4 ein3 eil1 (Figure 6B). Furthermore, the expression of HLS1 in the myc2 myc3 myc4 ein3 eil1 mutant was not affected by JA and/or ACC treatment (Figure 6C). Taken together (Figures 3 to 6), these results show that MYC2, MYC3, and MYC4 interact with and attenuate EIN3 and EIL1 to repress hook curvature.
We further investigated whether ein3 eil1 could suppress the increased disease resistance against necrotrophic pathogen B. cinerea in myc2. As shown in Figures 7A and 7B, after inoculation with spores of B. cinerea, the myc2 mutant exhibited disease resistance, as indicated by the smaller lesion size compared with the wild type, which is similar with previous studies demonstrating that MYC2 negatively regulates resistance against B. cinerea (Lorenzo et al., 2004; Zhai et al., 2013). The ein3 eil1 double mutant displayed susceptibility, as indicated by the larger lesion size compared with the wild type (Figures 7A and 7B), confirming that EIN3 and EIL1 are required for resistance against B. cinerea (Alonso et al., 2003; Zhu et al., 2011). Similar to ein3 eil1, the myc2 ein3 eil1 triple mutant also exhibited larger lesion size (Figures 7A and 7B), demonstrating that ein3 eil1 blocked the elevated resistance against B. cinerea in myc2. Consistently, the upregulated expression of defense genes ERF1, ORA59, and PDF1.2 (Zarei et al., 2011) in myc2 was blocked by the ein3 eil1 mutations (Figure 7C). Taken together (Figures 4, 5, and 7), these results showed that MYC2 interacts with and attenuates EIN3 and EIL1 to repress resistance against the necrotrophic pathogen B. cinerea.
In summary, the results in Figures 3 to 7 collectively demonstrate that MYC2 interacts with and represses EIN3 and EIL1 to regulate apical hook formation and resistance against the necrotrophic pathogen B. cinerea.
EIN3 and EIL1 Attenuate the Transcriptional Activation Function of MYC2 to Repress Plant Defense against Insect Attack
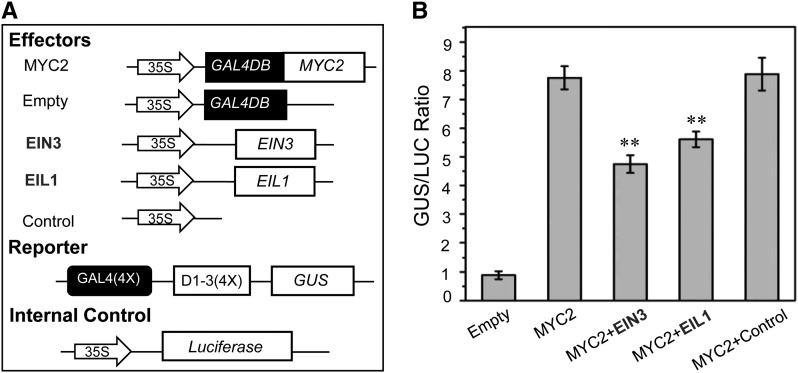
Having shown MYC2 interacts with and represses EIN3 and EIL1 to attenuate hook formation and disease resistance (Figures 3 to 7), we next explored whether EIN3 and EIL1 conversely affect the transcriptional function of MYC2 using the GAL4 DNA binding domain (GAL4DB) and its binding site [GAL4(4X)-D1-3(4X)-GUS]–based Arabidopsis protoplast transient expression system (Tiwari et al., 2001).
The MYC2 gene was fused with GAL4DB under the control of 35S promoter to generate the effector GAL4DB-MYC2. The β-glucuronidase (GUS) gene driven by four copies of GAL4 DNA binding sites [GAL4(4x)-D1-3(4x)] was used as a reporter, whereas the LUC gene under the control of 35S promoter was used as the internal control (Figure 8A). Similar with previous observations (Pauwels et al., 2010; Song et al., 2013b), expression of GAL4DB-MYC2 clearly increased the GUS/LUC ratio (Figure 8B). However, coexpression of EIN3 or EIL1 with GAL4BD-MYC2 obviously reduced the GUS/LUC ratio (Figure 8B), suggesting that EIN3 and EIL1 attenuate the transcriptional activation function of MYC2.
Figure 8.
EIN3 and EIL1 Antagonize the Transcriptional Activation Function of MYC2.
(A) The schematic diagram shows the constructs used in the transient expression assays.
(B) Transient expression assays show that MYC2 acts as a transcriptional activator, while EIN3 and EIL1 attenuate the transcriptional activation function of MYC2. Data are means (±sd) of three biological replicates. Asterisks represent Student’s t test significance between MYC2 and MYC2+EIN3 or MYC2+EIL1 samples (**P < 0.01).
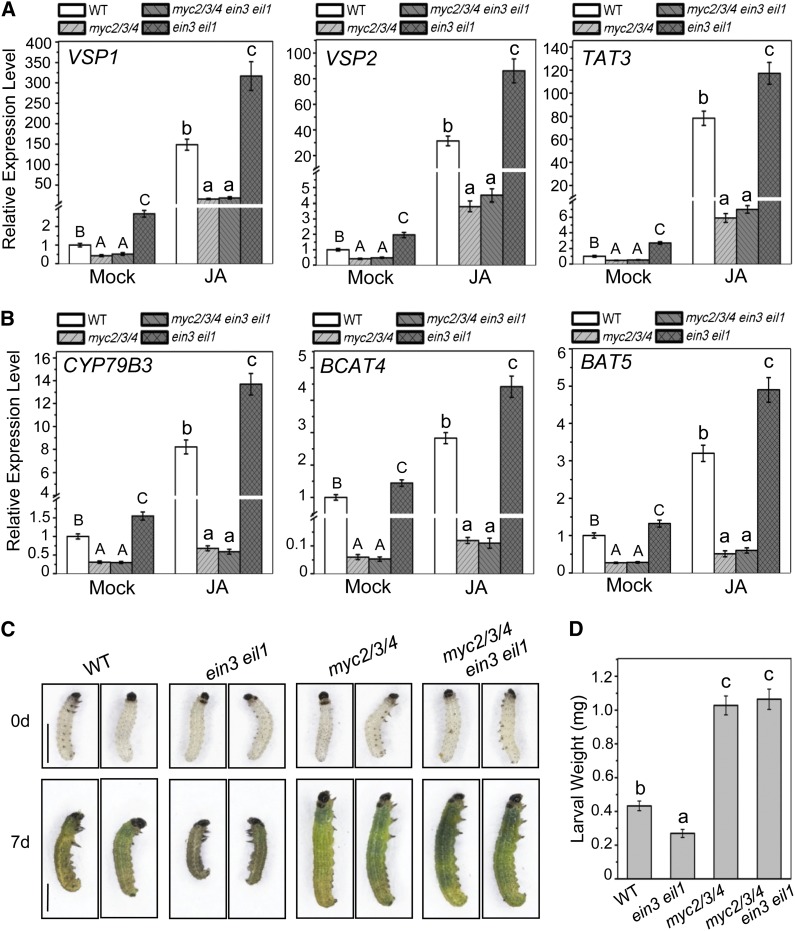
To further verify that the EIN3 and EIL1 repress the transcriptional activation function of MYC2, we investigated whether abolishment of EIN3 and EIL1 in planta would derepress MYC2 to enhance the expression of MYC2-regulated genes. Consistent with previous studies (Lorenzo et al., 2004; Fernández-Calvo et al., 2011; Schweizer et al., 2013), our results showed that MYC2 upregulated JA-induced expression of the wound-responsive genes VEGETATIVE STORAGE PROTEIN1 (VSP1), VSP2, and TYROSINE AMINOTRANSFERASE3 (TAT3) (Figure 9A) and the herbivore-inducible genes CYP79B3, BRANCHED-CHAIN AMINOTRANSFERASE4 (BCAT4), and BILE ACID TRANSPORTER5 (BAT5) (Figure 9B), which are required for the biosynthesis of the secondary metabolites glucosinolates (Zhao et al., 2002; Kliebenstein et al., 2005; Schweizer et al., 2013). Interestingly, the double mutant ein3 eil1 exhibited upregulated expression of these wound-responsive genes (VSP1, VSP2, and TAT3) as well as herbivory-inducible genes (CYP79B3, BCAT4, and BAT5) when treated with (or even without) JA compared with the wild type (Figures 9A and 9B). Consistent with the expression levels of wound-responsive and herbivory-inducible genes (Figures 9A and 9B), the myc2 myc3 myc4 triple mutant, which was almost completely devoid of glucosinolates (Schweizer et al., 2013), exhibited susceptibility to the generalist herbivores Spodoptera littoralis (Schweizer et al., 2013) and Spodoptera exigua (Figures 9C and 9D), while plant defense against these generalist herbivores was enhanced in the ET-signaling mutants ein3 eil1 (Figures 9C and 9D), etr1, and ein2 (Stotz et al., 2000; Mewis et al., 2005, 2006; Bodenhausen and Reymond, 2007). These results demonstrate that the abolishment of EIN3 and EIL1 derepresses MYC2, which enhances the expression of wound-responsive and herbivore-inducible genes and elevates plant defenses against generalist herbivores.
Figure 9.
Mutations in MYC2, MYC3, and MYC4 Block the Enhanced Defense against Insect Attack in ein3 eil1.
(A) and (B) Real-time PCR analysis for VSP1, VSP2, TAT3, CYP79B3, BCAT4, and BAT5 in the 12-d-old seedlings Col-0 (WT), jin1-2 myc3 myc4 (myc2/3/4), jin1-2 myc3 myc4 ein3 eil1 (myc2/3/4 ein3 eil1), and ein3 eil1 treated with mock or 100 μM MeJA (JA) for 6 h. Actin8 was used as the internal control. Data are means (±sd) of three biological replicates. Different letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05). Capital letters compare with each other, and lowercase letters compare with each other.
(C) Photograph of S. exigua larvae before feeding (0 d) and 7 d after feeding (7 d) with wild-type, ein3 eil1, jin1-2 myc3 myc4 (myc2/3/4), or jin1-2 myc3 myc4 ein3 eil1 (myc2/3/4ein3 eil1) plants. Bars = 1 mm.
(D) Larval weight of S. exigua reared on wild-type, ein3 eil1, jin1-2 myc3 myc4 (myc2/3/4), or jin1-2 myc3 myc4 ein3 eil1 (myc2/3/4ein3 eil1) plants for 7 d. Ten larvae as one sample were weighed together to obtain one datum for average weight. Fifty larvae (five independent samples) for each genotype in each biological experiment were used. Values are means (±sd) from three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
[See online article for color version of this figure.]
Further comparison of the gene expression pattern among the double mutant ein3 eil1, the pentuple mutant myc2 myc3 myc4 ein3 eil1, and the triple mutant myc2 myc3 myc4 showed that JA-induced expression of VSP1, VSP2, TAT3, CYP79B3, BCAT4, and BAT5 was significantly elevated in ein3 eil1, whereas such elevated gene expression was obviously repressed by the myc2 myc3 myc4 mutations (Figures 9A and 9B). Consistently, plant defense against insect attack was enhanced in ein3 eil1, but disrupted by the myc2 myc3 myc4 mutations (Figures 9C and 9D). These results suggest that mutations in MYC2, MYC3, and MYC4 abolish the enhanced expression of wound/herbivore-inducible genes and plant defense against insect attack in ein3 eil1.
Taken together (Figures 3 to 9), we demonstrated that the interaction between the JA-activated transcription factors (MYC2, MYC3, and MYC4) and the ET-stabilized transcription factors (EIN3 and EIL1) represses their respective transcriptional activities to modulate the JA and ET signaling antagonism. EIN3 and EIL1 interact with and repress MYC2, MYC3, and MYC4 to attenuate JA-induced expression of wound-responsive and herbivore-inducible genes and to repress plant defense against the generalist herbivores S. littoralis and S. exigua (Figures 4, 8, and 9). Conversely, MYC2 interacts with and represses EIN3 and EIL1 to inhibit hook formation and disease resistance against a necrotrophic pathogen (Figures 3 to 7).
DISCUSSION
Land plants live in fixed location, often encounter environmental stresses, and maintain plasticity in growth and development to adapt to the fluctuating environment by integrating multiple signals including the endogenous phytohormone signals JA and ET. JA and ET act synergistically to defend against necrotrophic pathogen infection and to promote root hair development (Penninckx et al., 1996; Zhu et al., 2006) via a synergistic regulatory model in which JA induces degradation of JAZ proteins and derepresses ET-stabilized EIN3 and EIL1, which interact with JAZs (Zhu et al., 2011). However, such a synergistic regulatory model is inconsistent with the antagonistic roles of JA and ET signaling in many important processes. For example, JA antagonizes ET to repress apical hook formation (e.g., exaggerated hook formation in the coi1 mutant) (Figures 1 and 2)(Turner et al., 2002), whereas ET antagonizes JA to repress the expression of wound-responsive genes (VSP1, VSP2, and TAT3) and herbivore-inducible genes (CYP79B3, BCAT4, and BAT5) (Figures 9A and 9B) (Rojo et al., 1999; Mikkelsen et al., 2003) and to attenuate plant defense against generalist herbivores (Figures 9C and 9D) (Stotz et al., 2000; Mewis et al., 2005, 2006; Bodenhausen and Reymond, 2007).
This study reveals a mechanism underlying antagonism between JA and ET signaling. Molecular, biochemical, and genetic evidence suggest an antagonistic regulatory model in which interaction between MYC2, key transcription factor in the JA pathway, and EIN3 and EIL1, master transcription factors in the ET pathway, modulates the JA-ET signaling antagonism (Figure 10). MYC2 interacts with and represses EIN3 and EIL1 to inhibit their effects on the transcription of HLS1 and ERF1, which represses ET-regulated apical hook formation (Figures 10A) and resistance to necrotrophic pathogen (Figures 10B). Conversely, EIN3 and EIL1 interact with and attenuate MYC2, MYC3, and MYC4 to inhibit the expression of wound-responsive and herbivore-inducible genes and to repress JA-regulated plant defense against the generalist herbivores S. littoralis and S. exigua (Figure 10A).
Figure 10.
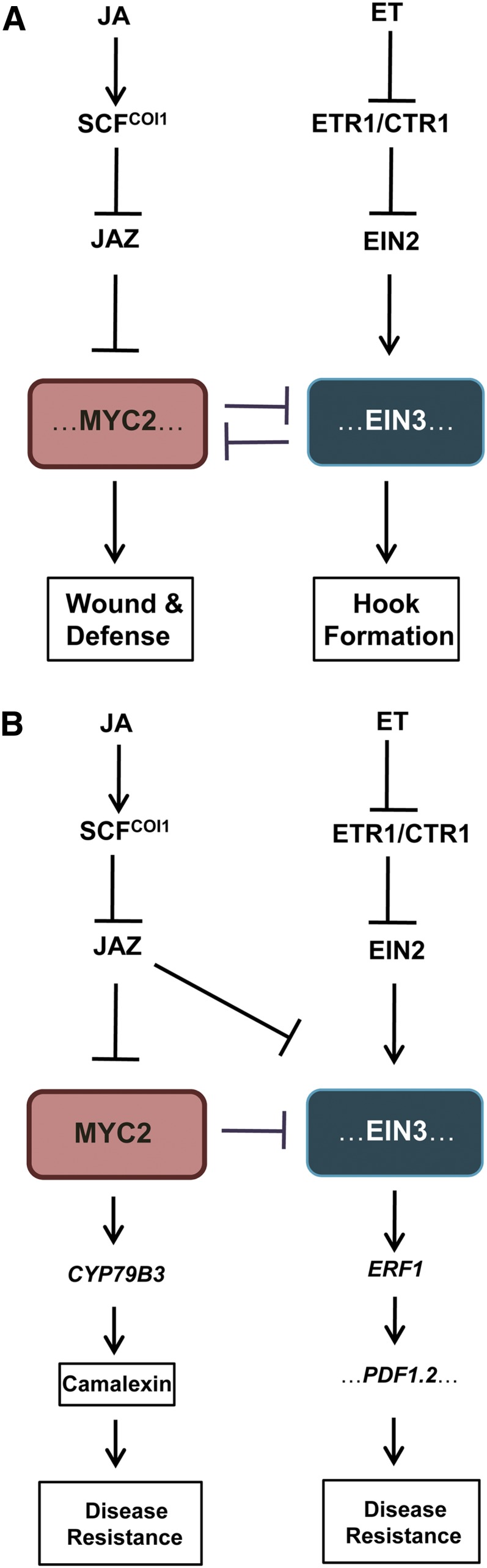
A Simplified Model for JA and ET Signaling Antagonism.
(A) Model for JA and ET antagonistic action in regulating hook curvature, wounding, and defense against insect attack. In response to JA signaling, SCFCOI1 recruits JAZs for ubiquitination and degradation. MYC2, MYC3, and MYC4 (indicated as MYC2) are then released to interact with and repress EIN3 and EIL1 (indicated as EIN3), which leads to attenuation of ET-enhanced hook curvature. ET signal inactivates the ET receptors (indicated as ETR1) and the negative regulator CTR1 to mediate EIN2 translocation into nucleus and to stabilize EIN3 and EIL1. EIN3 and EIL1 then interact with and repress MYC2, MYC3, and MYC4 to inhibit expression of wound responsive genes (e.g., VSP1, VSP2, and TAT3) and herbivory-inducible genes (e.g., CYP79B3, BCAT4, and BAT5) and suppress JA-regulated plant defense against generalist herbivores S. littoralis and S. exigua (indicated as wound and defense).
(B) Model for JA and ET crosstalk in regulating plant resistance against necrotrophic pathogen. JAZs and MYC2 interact with and repress ET-stabilized EIN3 and EIL1 (indicated as EIN3). In response to JA signaling, JAZ proteins are degraded to derepress EIN3/EIL1, leading to the increased disease resistance against necrotrophic pathogen B. cinerea (indicated as disease resistance) (Zhu et al., 2011). Meanwhile, JA-induced JAZ degradation releases MYC2, which counteracts EIN3 and EIL1 to prevent excessive disease resistance responses. In addition, other factors, including CYP79B3, which is required for biosynthesis of camalexin (Glawischnig et al., 2004; Kliebenstein et al., 2005), may be also regulated by MYC2 to modulate disease resistance. Regulation of plant resistance against B. cinerea might be complicated and modulated by the coordinated action of synergistic and antagonistic mechanisms.
[See online article for color version of this figure.]
Consistent with this antagonistic regulatory model, in the myc2 mutant, the absence of MYC2 fails to repress the transcriptional activity of EIN3 and EIL1, leads to the activation of EIN3/EIL1-regulated gene expression (HLS1, ERF1, ORA59, and PDF1.2) and results in enhanced apical hook formation and plant resistance against B. cinerea infection (Figures 3 to 7 and 10). On the other hand, in the ET signaling mutants (e.g., ein3 eil1 and ein2), the absence of EIN3 and EIL1 enables MYC2, MYC3, and MYC4 to induce the expression of wound-responsive genes (VSP1, VSP2, and TAT3) (Figure 9A) (Rojo et al., 1999; Lorenzo et al., 2004) and herbivore-inducible genes (CYP79B3, BCAT4, and BAT5), and enhances plant defense against the herbivores S. littoralis and S. exigua (Figures 4, 8, 9, and 10A) (Stotz et al., 2000; Mewis et al., 2005, 2006; Bodenhausen and Reymond, 2007). In wild-type plants, antagonistic regulation between the ET-stabilized transcription factors (EIN3 and EIL1) and the JA-activated transcription factors (MYC2, MYC3, and MYC4) would lead to suitable expression of MYC2-dependent genes (VSP1, VSP2, TAT3, CYP79B3, BCAT4, and BAT5) and EIN3-regulated genes (HLS1, ERF1, ORA59, and PDF1.2), resulting in proper plant responses, such as hook formation and defense against the herbivores S. littoralis and S. exigua.
MYC2 functions as a key transcription factor to positively regulate diverse JA responses (Kazan and Manners, 2013), including root growth (Boter et al., 2004; Lorenzo et al., 2004), secondary metabolism (Dombrecht et al., 2007; Hong et al., 2012; Schweizer et al., 2013), wound response, and plant defense against insect attack (Zhang and Turner, 2008; Fernández-Calvo et al., 2011; Schweizer et al., 2013). Surprisingly, MYC2 also acts as a negative regulator to repress JA-mediated plant resistance to necrotrophic fungi and pathogenesis-related gene expression (e.g., PDF1.2) (Anderson et al., 2004; Lorenzo et al., 2004; Zhai et al., 2013). Such MYC2-regulated susceptibility to necrotrophic fungi seems incompatible with the previously reported synergistic model (Zhu et al., 2011). Our results provide a mechanistic understanding of the long-standing question of how MYC2 represses JA-regulated plant resistance against necrotrophic fungi: MYC2 interacts with and represses EIN3 and EIL1, which inhibits expression of the EIN3/EIL1-dependent defense genes (ERF1, ORA59, and PDF1.2) and consequently depresses plant resistance against necrotrophic pathogen infection.
Coordinated regulation of plant responses in both antagonistic and synergistic manners would help plants adapt to fluctuating environments. Plant resistance against necrotrophic fungi might be modulated by a balance between the previously described synergistic mechanism (interaction of JAZs with EIN3) (Zhu et al., 2011) (Figure 10B) and our antagonistic regulation model (interaction between MYC2 and EIN3) (Figure 10B). Future research should focus on identifying protein domains essential for the interaction between MYC2 and EIN3 and clarifying whether reciprocal repression between MYC2 and EIN3 occurs on promoters of its target genes or disrupts the binding of MYC2 or EIN3 to its respective target promoters, which would help advance our understanding of the reciprocal regulation of the transcriptional functions of MYC2 and EIN3.
JA and ET exhibit opposite effects on many other plant responses. JA enhances anthocyanin accumulation (Qi et al., 2011) and freezing tolerance (Hu et al., 2013b), inhibits seed germination (Miersch et al., 2008), hypocotyl elongation in the light (Chen et al., 2013) and the ozone-induced spreading of cell death (Rao et al., 2000; Tuominen et al., 2004), and delays flowering (Robson et al., 2010). Conversely, ET suppresses anthocyanin accumulation (Jeong et al., 2010) and freezing tolerance (Shi et al., 2012) and enhances seed germination (Linkies et al., 2009; Linkies and Leubner-Metzger, 2012), hypocotyl elongation in the light (Zhong et al., 2012), the ozone-induced spreading of cell death (Overmyer et al., 2000), and flowering (Ogawara et al., 2003). It would be interesting to investigate whether these ET-JA antagonistic actions are mediated by similar interactions between their respective master transcription factors in the JA and ET pathways.
METHODS
Plant Materials and Growth Conditions
The Arabidopsis thaliana mutants coi1-1 (Xie et al., 1998), coi1-2 (Xu et al., 2002), JAZ1Δ3A (Thines et al., 2007), myc2-2 (Boter et al., 2004), jin1-2 (Lorenzo et al., 2004), myc3 (GK445B11) (Fernández-Calvo et al., 2011), myc4 (GK491E10) (Fernández-Calvo et al., 2011), jin1-2 myc3 myc4 (Fernández-Calvo et al., 2011), eto1-1 (Guzmán and Ecker, 1990), ctr1-1 (Kieber et al., 1993), ein2-1 (Alonso et al., 1999), hls1-1 (Lehman et al., 1996), ein3-1 (Chao et al., 1997), eil1-3/Salk_049679 (Binder et al., 2007), myb21 myb24 myb57 (Cheng et al., 2009), and gl3 egl3 tt8 (Qi et al., 2011) were previously described. The higher order mutants myc2-2 myc3, myc2-2 myc4, myc2-2 myc3 myc4, ein3-1 eil1-3, coi1-2 ein3-1 eil1-3, coi1-2 hls1-1, myc2-2 ein3 eil1-3, and jin1-2 myc3 myc4 ein3-1 eil1-3 were generated by genetic crosses using standard techniques.
The Arabidopsis seeds were sterilized with 20% bleach, plated on Murashige and Skoog (MS) medium, chilled at 4°C for 3 d, and then transferred to a growth room under a 16-h (20 to 24°C)/8-h (16 to 19°C) light/dark photoperiod. For hook phenotype analysis, seeds were sterilized, chilled, and transferred to a growth chamber at 22°C in the dark for 4 d. Nicotiana benthamiana was grown in a growth room under a 16-h (25 to 28°C)/8-h (22 to 25°C) light/dark cycle.
BiFC Assay
For the BiFC assays, the full-length coding sequence (CDS) of Arabidopsis EIN3, EIL1, MYC2, MYC3, and MYC4 were cloned into the binary nYFP or cYFP vector through the Gateway system (Invitrogen) (Qi et al., 2011). Primer pairs used for the generation of constructs are listed in Supplemental Table 1. BiFC assays were performed as previously described (Qi et al., 2011). Equal concentrations and volumes of resuspended Agrobacterium tumefaciens strain GV3101 harboring the indicated nYFP or cYFP vectors in infiltration buffer (0.2 mM acetosyringone, 10 mM MgCl2, and 10 mM MES) were mixed and coinfiltrated into leaves of N. benthamiana using a needleless syringe. Two days after infiltration, the YFP signal was observed using a Zeiss confocal microscope (LSM710). Four hours before observation, 100 μM MG132 was infiltrated into the leaves of N. benthamiana.
Pull-Down Assay
MBP-MYC2 (Chen et al., 2011) and MBP proteins were purified from Escherichia coli using MBP affinity chromatography according to Qi et al. (2011). The full-length CDS of EIN3 was cloned into the modified pCambia1300 vector under the control of 35S promoter for fusion with three flag tags to generate flag-EIN3. Agrobacterium strain harboring flag-EIN3 was infiltrated into N. benthamiana leaves. After 50 h, 5 g of N. benthamiana leaves transiently expressing flag-EIN3 were harvested for total protein extraction in RB buffer (100 mM NaCl, 50 mM Tris-Cl, pH 7.8, 25 mM imidazole, 0.1% [v/v] Tween 20, 10% [v/v] glycerol, EDTA-free complete miniprotease inhibitor cocktail, and 20 mM 2-mercaptoethanol). The extracted total protein was concentrated in centrifugal filter tubes (Millipore) to 400 µL. Coomassie Brilliant Blue was used to confirm the protein amount. About 50 µg of purified MBP and MBP-MYC2 was incubated with 120 μL of amylose resin beads for 2 h at 4°C. These amylose resin beads were then washed five times with 1 mL of RB buffer and incubated with 200 μL of concentrated total proteins containing flag-EIN3 for 2 h at 4°C. After washing five times with 1 mL RB buffer, the mixture was resuspended in SDS loading buffer, boiled for 5 min, separated on 15% SDS-PAGE, and immunoblotted using 1:1000 dilution for anti-flag antibody (Abmart).
Co-IP
N. benthamiana leaves were infiltrated with Agrobacterium strains harboring flag-EIN3, flag-EIN3 with myc-MYC2 (Zhai et al., 2013), or flag-EIN3 with myc-COI1 (Yan et al., 2013). Two days after infiltration, 3 g of agroinfiltrated leaves for each combination was collected and homogenized in Co-IP buffer containing 50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 2 mM DTT, 0.1% Tween 20, 1 mM phenylmethylsulfonyl fluoride, 50 mM MG132, and complete protease inhibitor cocktail (Roche) and centrifuged twice at 16,000g at 4°C. The supernatant was concentrated to 400 μL and incubated with the agarose-conjugated anti-myc matrix (Abmart) for 2 h (4°C, with rotation), then washed three times with 1 mL of immunoprecipitation buffer. After denaturation in 100 μL of SDS loading buffer, the samples were loaded into 15% SDS-PAGE gels, subjected to gel electrophoresis, transferred to polyvinylidene fluoride membranes (Millipore), and immunoblotted with anti-flag antibody (Abmart) and anti-myc antibody, respectively.
Protoplast Transfection Assay
For the transient transcriptional activity assay, constructs harboring the LUC gene under the control of the ∼1500-bp or ∼1523-bp promoter sequences of HLS1 or ERF1, respectively, in the pGreenII 0800-LUC vector were generated as reporters (Hellens et al., 2005). The renilla luciferase (REN) gene under the control of 35S promoter in the pGreenII 0800-LUC vector was used as the internal control. The CDS sequences of EIN3, EIL1, and MYC2 were cloned into the pGreenII 62-SK vector under the control of the 35S promoter and were used as effectors. All primers used for making these constructs are listed in Supplemental Table 1. Arabidopsis mesophyll protoplast was prepared and transfected as previously described (Yoo et al., 2007). The firefly LUC and REN activities were measured using the Dual-Luciferase Reporter Assay System (Promega). LUC/REN ratios were presented.
For transient expression assay, the CDS of MYC2 fused with GAL4DB (GAL4DB-MYC2) under the control of 35S promoter was used as effector. The GUS gene under the control of four copies of upstream GAL4 DNA binding sites [GAL4(4x)-D1-3(4x)]was used as the reporter (Tiwari et al., 2001; Zhu et al., 2008). The firefly LUC gene under the control of 35S promoter was the internal control. Primers used for plasmid construction are shown in Supplemental Table 1. The preparation and subsequent transfection of Arabidopsis mesophyll protoplasts were performed as described previously (Yoo et al., 2007). The GUS/LUC ratios were presented.
Infection with Pathogen
Detached leave from 3-week-old plant were inoculated with 5 μL spores of Botrytis cinerea (SCL2-4, isolated from tomato in 2011, Shanghai) (Song et al., 2013b) (105 spores/mL) suspended in potato dextrose broth (with potato dextrose broth alone as the control), placed in Petri dishes with 0.8% agar, and covered with lids. The lesion diameter from eight leaves for each genotype exhibiting disease symptoms was measured 2 d after inoculation.
Insect Defense Assay with Spodoptera exigua
Newly hatched S. exigua larvae were placed on 3-week-old plants (10-h-light/14-h-dark photoperiod) of each genotype for 7 d of feeding. Ten surviving larvae were weighted as one sample to obtain one datum for average weight. Fifty surviving larvae (five independent samples in total) for each genotype in each biological experiment were used. The experiment was repeated for three biological replicates.
Quantitative Real-Time PCR
For Figures 1B, 3B, and 6B, Arabidopsis seedlings were grown on MS medium at 22°C in the dark for 4 d. For Figures 1C, 3C, and 6C, seedlings were grown on MS medium at 22°C in the dark for 4 d and then were treated with mock, 100 μM methyl jasmonate (MeJA), 100 μM ACC, or 100 μM MeJA plus 100 μM ACC for 6 h. For Figures 7C, 9A, and 9B, seedlings were grown on MS medium for 12 d under a 16-h (20 to 24°C)/8-h (16 to 19°C) light/dark photoperiod and then were treated with mock or 100 μM MeJA for 6 h. These materials were harvested for RNA extraction and subsequent reverse transcription. Real-time PCR analyses were performed using the ABI7500 real-time PCR system with the RealMasterMix (SYBR Green I) (Takara) as described previously (Qi et al., 2011). The primers for real-time PCR analysis are presented in Supplemental Table 2 online. ACTIN8 was used as the internal control. The experiment was repeated for three biological replicates.
Accession Numbers
The Arabidopsis Genome Initiative numbers for genes mentioned in this article are as follows: COI1 (AT2G39940), MYC2 (AT1G32640), MYC3 (AT5G46760), MYC4 (AT4G17880), JAZ1 (AT1G19180), ETO1 (At3g51770), CTR1 (AT5G03730), EIN2 (AT5G03280), EIN3 (AT3G20770), EIL1 (AT2G27050), ERF1 (AT3G23240), HLS1 (AT4G37580), PDF1.2 (AT5G44420), MYB21 (At3g27810), MYB24 (At5g40350), MYB57 (At3g01530), TT8 (AT4G09820), GL3 (AT5G41315), EGL3 (AT1G63650), VSP1 (AT5G24780), VSP2 (AT5G24770), TAT3 (AT2G24850), ORA59 (AT1G06160), CYP79B3 (At2g22330), BCAT4 (At3g19710), BAT5 (At4g12030), and ACTIN8 (AT1G49240).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The Negative Controls for the BiFC Experiments.
Supplemental Table 1. Primers Used for Vector Construction.
Supplemental Table 2. Primers Used for Quantitative Real-Time PCR Analysis.
Supplementary Material
Acknowledgments
We thank Roberto Solano, Joseph Ecker, and John Browse for the mutants and Qilian Qin for S. exigua eggs. The work was supported by the Ministry of Science and Technology (973 Program 2011CB915404) and the National Science Foundation of China (31230008 and 91017012).
AUTHOR CONTRIBUTIONS
S.S., T.Q., and D.X. designed the research. S.S., H.H., H.G., J.W., D.W., Q.Z., and T.Q. performed research. S.S., X.L., S.Y., C.L., T.Q., and D.X. analyzed data. S.S., T.Q., and D.X. wrote the article.
Glossary
- ET
ethylene
- JA
jasmonate
- ACC
1-aminocyclopropane-1-carboxylic acid
- BiFC
bimolecular fluorescence complementation
- Co-IP
coimmunoprecipitation
- MS
Murashige and Skoog
- CDS
coding sequence
- MeJA
methyl jasmonate
- Col-0
Columbia-0
Footnotes
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
References
- Achard P., Baghour M., Chapple A., Hedden P., Van Der Straeten D., Genschik P., Moritz T., Harberd N.P. (2007). The plant stress hormone ethylene controls floral transition via DELLA-dependent regulation of floral meristem-identity genes. Proc. Natl. Acad. Sci. USA 104: 6484–6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta I.F., Gasperini D., Chételat A., Stolz S., Santuari L., Farmer E.E. (2013). Role of NINJA in root jasmonate signaling. Proc. Natl. Acad. Sci. USA 110: 15473–15478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J.M., Hirayama T., Roman G., Nourizadeh S., Ecker J.R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Alonso J.M., Stepanova A.N., Solano R., Wisman E., Ferrari S., Ausubel F.M., Ecker J.R. (2003). Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc. Natl. Acad. Sci. USA 100: 2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An F., Zhang X., Zhu Z., Ji Y., He W., Jiang Z., Li M., Guo H. (2012). Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res. 22: 915–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.P., Badruzsaufari E., Schenk P.M., Manners J.M., Desmond O.J., Ehlert C., Maclean D.J., Ebert P.R., Kazan K. (2004). Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16: 3460–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder B.M., Walker J.M., Gagne J.M., Emborg T.J., Hemmann G., Bleecker A.B., Vierstra R.D. (2007). The Arabidopsis EIN3 binding F-Box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling. Plant Cell 19: 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker A.B., Kende H. (2000). Ethylene: A gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Bodenhausen N., Reymond P. (2007). Signaling pathways controlling induced resistance to insect herbivores in Arabidopsis. Mol. Plant Microbe Interact. 20: 1406–1420 [DOI] [PubMed] [Google Scholar]
- Boter M., Ruíz-Rivero O., Abdeen A., Prat S. (2004). Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev. 18: 1577–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert W.F., Delauré S.L., De Bolle M.F., Cammue B.P. (2006). The role of ethylene in host-pathogen interactions. Annu. Rev. Phytopathol. 44: 393–416 [DOI] [PubMed] [Google Scholar]
- Browse J. (2009). Jasmonate passes muster: A receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 60: 183–205 [DOI] [PubMed] [Google Scholar]
- Burg S.P., Burg E.A. (1962). Role of ethylene in fruit ripening. Plant Physiol. 37: 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Q., Rothenberg M., Solano R., Roman G., Terzaghi W., Ecker J.R. (1997). Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Chen H., Xue L., Chintamanani S., Germain H., Lin H., Cui H., Cai R., Zuo J., Tang X., Li X., Guo H., Zhou J.M. (2009). ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 repress SALICYLIC ACID INDUCTION DEFICIENT2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 21: 2527–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Sonobe K., Ogawa N., Masuda S., Nagatani A., Kobayashi Y., Ohta H. (2013). Inhibition of Arabidopsis hypocotyl elongation by jasmonates is enhanced under red light in phytochrome B dependent manner. J. Plant Res. 126: 161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., et al. (2011). The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell 23: 3335–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Song S., Xiao L., Soo H.M., Cheng Z., Xie D., Peng J. (2009). Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet. 5: e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Sun L., Qi T., Zhang B., Peng W., Liu Y., Xie D. (2011). The bHLH transcription factor MYC3 interacts with the jasmonate ZIM-domain proteins to mediate jasmonate response in Arabidopsis. Mol. Plant 4: 279–288 [DOI] [PubMed] [Google Scholar]
- Chini A., Fonseca S., Fernández G., Adie B., Chico J.M., Lorenzo O., García-Casado G., López-Vidriero I., Lozano F.M., Ponce M.R., Micol J.L., Solano R. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Dathe W., Rönsch H., Preiss A., Schade W., Sembdner, G., and Schreiber, K. (1981). Endogenous plant hormones of the broad bean, Vicia faba L. (2)-Jasmonic acid, a plant growth inhibitor in pericarp. Planta Med. 155: 530–535 [DOI] [PubMed] [Google Scholar]
- De Geyter N., Gholami A., Goormachtig S., Goossens A. (2012). Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 17: 349–359 [DOI] [PubMed] [Google Scholar]
- Dombrecht B., Xue G.P., Sprague S.J., Kirkegaard J.A., Ross J.J., Reid J.B., Fitt G.P., Sewelam N., Schenk P.M., Manners J.M., Kazan K. (2007). MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 19: 2225–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Calvo P., et al. (2011). The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23: 701–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S., Chini A., Hamberg M., Adie B., Porzel A., Kramell R., Miersch O., Wasternack C., Solano R. (2009). (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 5: 344–350 [DOI] [PubMed] [Google Scholar]
- Gagne J.M., Smalle J., Gingerich D.J., Walker J.M., Yoo S.D., Yanagisawa S., Vierstra R.D. (2004). Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc. Natl. Acad. Sci. USA 101: 6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepstein S., Thimann K.V. (1981). The role of ethylene in the senescence of oat leaves. Plant Physiol. 68: 349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glawischnig E., Hansen B.G., Olsen C.E., Halkier B.A. (2004). Camalexin is synthesized from indole-3-acetaldoxime, a key branching point between primary and secondary metabolism in Arabidopsis. Proc. Natl. Acad. Sci. USA 101: 8245–8250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Ecker J.R. (2003). Plant responses to ethylene gas are mediated by SCFEBF1/EBF2-dependent proteolysis of EIN3 transcription factor. Cell 115: 667–677 [DOI] [PubMed] [Google Scholar]
- Guo H., Ecker J.R. (2004). The ethylene signaling pathway: New insights. Curr. Opin. Plant Biol. 7: 40–49 [DOI] [PubMed] [Google Scholar]
- Guzmán P., Ecker J.R. (1990). Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens R.P., Allan A.C., Friel E.N., Bolitho K., Grafton K., Templeton M.D., Karunairetnam S., Gleave A.P., Laing W.A. (2005). Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G.J., Xue X.Y., Mao Y.B., Wang L.J., Chen X.Y. (2012). Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 24: 2635–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe G.A., Jander G. (2008). Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 59: 41–66 [DOI] [PubMed] [Google Scholar]
- Hu P., Zhou W., Cheng Z., Fan M., Wang L., Xie D. (2013a). JAV1 controls jasmonate-regulated plant defense. Mol. Cell 50: 504–515 [DOI] [PubMed] [Google Scholar]
- Hu Y., Jiang L., Wang F., Yu D. (2013b). Jasmonate regulates the inducer of cbf expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 25: 2907–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J., Meyerowitz E.M. (1998). Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Jeong S.W., Das P.K., Jeoung S.C., Song J.Y., Lee H.K., Kim Y.K., Kim W.J., Park Y.I., Yoo S.D., Choi S.B., Choi G., Park Y.I. (2010). Ethylene suppression of sugar-induced anthocyanin pigmentation in Arabidopsis. Plant Physiol. 154: 1514–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju C., et al. (2012). CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 109: 19486–19491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K., Manners J.M. (2013). MYC2: The master in action. Mol. Plant 6: 686–703 [DOI] [PubMed] [Google Scholar]
- Kieber J.J. (1997). The ethylene response pathway in Arabidopsis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48: 277–296 [DOI] [PubMed] [Google Scholar]
- Kieber J.J., Rothenberg M., Roman G., Feldmann K.A., Ecker J.R. (1993). CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Kliebenstein D.J., Rowe H.C., Denby K.J. (2005). Secondary metabolites influence Arabidopsis/Botrytis interactions: Variation in host production and pathogen sensitivity. Plant J. 44: 25–36 [DOI] [PubMed] [Google Scholar]
- Knight L.I., Rose R.C., Crocker W. (1910). Effects of various gases and vapors upon etiolated seedlings of the sweet pea. Science 31: 635–636 [Google Scholar]
- Lehman A., Black R., Ecker J.R. (1996). HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85: 183–194 [DOI] [PubMed] [Google Scholar]
- Li Z., Peng J., Wen X., Guo H. (2013). Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell 25: 3311–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkies A., Leubner-Metzger G. (2012). Beyond gibberellins and abscisic acid: How ethylene and jasmonates control seed germination. Plant Cell Rep. 31: 253–270 [DOI] [PubMed] [Google Scholar]
- Linkies A., Müller K., Morris K., Turecková V., Wenk M., Cadman C.S., Corbineau F., Strnad M., Lynn J.R., Finch-Savage W.E., Leubner-Metzger G. (2009). Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: A comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell 21: 3803–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O., Chico J.M., Sánchez-Serrano J.J., Solano R. (2004). JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16: 1938–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandaokar A., Thines B., Shin B., Lange B.M., Choi G., Koo Y.J., Yoo Y.J., Choi Y.D., Choi G., Browse J. (2006). Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J. 46: 984–1008 [DOI] [PubMed] [Google Scholar]
- Mason H.S., Mullet J.E. (1990). Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell 2: 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M., Browse J. (1996). The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell 8: 403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M., Creelman R.A., Bell E., Mullet J.E., Browse J. (1997). Jasmonate is essential for insect defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 94: 5473–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M., Underwood W., Koczan J., Nomura K., He S.Y. (2006). Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Mewis I., Appel H.M., Hom A., Raina R., Schultz J.C. (2005). Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol. 138: 1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewis I., Tokuhisa J.G., Schultz J.C., Appel H.M., Ulrichs C., Gershenzon J. (2006). Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways. Phytochemistry 67: 2450–2462 [DOI] [PubMed] [Google Scholar]
- Miersch O., Neumerkel J., Dippe M., Stenzel I., Wasternack C. (2008). Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol. 177: 114–127 [DOI] [PubMed] [Google Scholar]
- Mikkelsen M.D., Petersen B.L., Glawischnig E., Jensen A.B., Andreasson E., Halkier B.A. (2003). Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiol. 131: 298–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi S.A., Chauvin A., Pascaud F., Kellenberger S., Farmer E.E. (2013). GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500: 422–426 [DOI] [PubMed] [Google Scholar]
- Nakata M., Mitsuda N., Herde M., Koo A.J., Moreno J.E., Suzuki K., Howe G.A., Ohme-Takagi M. (2013). A bHLH-type transcription factor, ABA-INDUCIBLE BHLH-TYPE TRANSCRIPTION FACTOR/JA-ASSOCIATED MYC2-LIKE1, acts as a repressor to negatively regulate jasmonate signaling in Arabidopsis. Plant Cell 25: 1641–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y., Figueroa P., Browse J. (2011). Characterization of JAZ-interacting bHLH transcription factors that regulate jasmonate responses in Arabidopsis. J. Exp. Bot. 62: 2143–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara T., Higashi K., Kamada H., Ezura H. (2003). Ethylene advances the transition from vegetative growth to flowering in Arabidopsis thaliana. J. Plant Physiol. 160: 1335–1340 [DOI] [PubMed] [Google Scholar]
- Ortega-Martínez O., Pernas M., Carol R.J., Dolan L. (2007). Ethylene modulates stem cell division in the Arabidopsis thaliana root. Science 317: 507–510 [DOI] [PubMed] [Google Scholar]
- Overmyer K., Tuominen H., Kettunen R., Betz C., Langebartels C., Sandermann H., Jr, Kangasjärvi J. (2000). Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 12: 1849–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L., et al. (2010). NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464: 788–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx I.A., Eggermont K., Terras F.R., Thomma B.P., De Samblanx G.W., Buchala A., Métraux J.P., Manners J.M., Broekaert W.F. (1996). Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 8: 2309–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx I.A., Thomma B.P., Buchala A., Métraux J.P., Broekaert W.F. (1998). Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10: 2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potuschak T., Lechner E., Parmentier Y., Yanagisawa S., Grava S., Koncz C., Genschik P. (2003). EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115: 679–689 [DOI] [PubMed] [Google Scholar]
- Pré M., Atallah M., Champion A., De Vos M., Pieterse C.M., Memelink J. (2008). The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol. 147: 1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T., Song S., Ren Q., Wu D., Huang H., Chen Y., Fan M., Peng W., Ren C., Xie D. (2011). The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 23: 1795–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H., Shen Z., Huang S.S., Schmitz R.J., Urich M.A., Briggs S.P., Ecker J.R. (2012). Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 338: 390–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M.V., Lee H., Creelman R.A., Mullet J.E., Davis K.R. (2000). Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12: 1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson F., Okamoto H., Patrick E., Harris S.R., Wasternack C., Brearley C., Turner J.G. (2010). Jasmonate and phytochrome A signaling in Arabidopsis wound and shade responses are integrated through JAZ1 stability. Plant Cell 22: 1143–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo E., León J., Sánchez-Serrano J.J. (1999). Cross-talk between wound signalling pathways determines local versus systemic gene expression in Arabidopsis thaliana. Plant J. 20: 135–142 [DOI] [PubMed] [Google Scholar]
- Rowe H.C., Walley J.W., Corwin J., Chan E.K., Dehesh K., Kliebenstein D.J. (2010). Deficiencies in jasmonate-mediated plant defense reveal quantitative variation in Botrytis cinerea pathogenesis. PLoS Pathog. 6: e1000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüzicka K., Ljung K., Vanneste S., Podhorská R., Beeckman T., Friml J., Benková E. (2007). Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19: 2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer F., Fernández-Calvo P., Zander M., Diez-Diaz M., Fonseca S., Glauser G., Lewsey M.G., Ecker J.R., Solano R., Reymond P. (2013). Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25: 3117–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J.S., Joo J., Kim M.J., Kim Y.K., Nahm B.H., Song S.I., Cheong J.J., Lee J.S., Kim J.K., Choi Y.D. (2011). OsbHLH148, a basic helix-loop-helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant J. 65: 907–921 [DOI] [PubMed] [Google Scholar]
- Shan X., Wang J., Chua L., Jiang D., Peng W., Xie D. (2011). The role of Arabidopsis Rubisco activase in jasmonate-induced leaf senescence. Plant Physiol. 155: 751–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X., Yan J., Xie D. (2012). Comparison of phytohormone signaling mechanisms. Curr. Opin. Plant Biol. 15: 84–91 [DOI] [PubMed] [Google Scholar]
- Sheard L.B., et al. (2010). Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Tian S., Hou L., Huang X., Zhang X., Guo H., Yang S. (2012). Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 24: 2578–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R., Stepanova A., Chao Q., Ecker J.R. (1998). Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Qi T., Fan M., Zhang X., Gao H., Huang H., Wu D., Guo H., Xie D. (2013b). The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genet. 9: e1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Qi T., Huang H., Ren Q., Wu D., Chang C., Peng W., Liu Y., Peng J., Xie D. (2011). The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect jasmonate-regulated stamen development in Arabidopsis. Plant Cell 23: 1000–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Qi T., Huang H., Xie D. (2013a). Regulation of stamen development by coordinated actions of jasmonate, auxin, and gibberellin in Arabidopsis. Mol. Plant 6: 1065–1073 [DOI] [PubMed] [Google Scholar]
- Stotz H.U., Pittendrigh B.R., Kroymann J., Weniger K., Fritsche J., Bauke A., Mitchell-Olds T. (2000). Induced plant defense responses against chewing insects. Ethylene signaling reduces resistance of Arabidopsis against Egyptian cotton worm but not diamondback moth. Plant Physiol. 124: 1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Zarembinski T.I., Oeller P.W., Liang X., Abel S. (1992). Modification of fruit ripening by suppressing gene expression. Plant Physiol. 100: 549–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B., Katsir L., Melotto M., Niu Y., Mandaokar A., Liu G., Nomura K., He S.Y., Howe G.A., Browse J. (2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Thomma B.P., Eggermont K., Penninckx I.A., Mauch-Mani B., Vogelsang R., Cammue B.P., Broekaert W.F. (1998). Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 95: 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma B.P., Eggermont K., Tierens K.F., Broekaert W.F. (1999). Requirement of functional Ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 121: 1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S.B., Wang X.J., Hagen G., Guilfoyle T.J. (2001). AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13: 2809–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H., Overmyer K., Keinänen M., Kollist H., Kangasjärvi J. (2004). Mutual antagonism of ethylene and jasmonic acid regulates ozone-induced spreading cell death in Arabidopsis. Plant J. 39: 59–69 [DOI] [PubMed] [Google Scholar]
- Turner J.G., Ellis C., Devoto A. (2002). The jasmonate signal pathway. Plant Cell 14 (Suppl): S153–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda J., Kato J. (1980). Isolation and identification of a senescence-promoting substance from wormwood (Artemisia absinthium L.). Plant Physiol. 66: 246–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan P., Shockey J., Lévesque C.A., Cook R.J., Browse J. (1998). A role for jasmonate in pathogen defense of Arabidopsis. Proc. Natl. Acad. Sci. USA 95: 7209–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C., Hause B. (2013). Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. (Lond.) 111: 1021–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X., Zhang C., Ji Y., Zhao Q., He W., An F., Jiang L., Guo H. (2012). Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res. 22: 1613–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D.X., Feys B.F., James S., Nieto-Rostro M., Turner J.G. (1998). COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094 [DOI] [PubMed] [Google Scholar]
- Xu L.H., Liu F.Q., Lechner E., Genschik P., Crosby W.L., Ma H., Peng W., Huang D.F., Xie D.X. (2002). The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 14: 1919–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Li H., Li S., Yao R., Deng H., Xie Q., Xie D. (2013). The Arabidopsis F-box protein CORONATINE INSENSITIVE1 is stabilized by SCFCOI1 and degraded via the 26S proteasome pathway. Plant Cell 25: 486–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Zhang C., Gu M., Bai Z., Zhang W., Qi T., Cheng Z., Peng W., Luo H., Nan F., Wang Z., Xie D. (2009). The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21: 2220–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.X., Stolz S., Chételat A., Reymond P., Pagni M., Dubugnon L., Farmer E.E. (2007). A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19: 2470–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.L., et al. (2012). Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 109: E1192–E1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.D., Cho Y.H., Sheen J. (2007). Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zarei A., Körbes A.P., Younessi P., Montiel G., Champion A., Memelink J. (2011). Two GCC boxes and AP2/ERF-domain transcription factor ORA59 in jasmonate/ethylene-mediated activation of the PDF1.2 promoter in Arabidopsis. Plant Mol. Biol. 75: 321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Q., Yan L., Tan D., Chen R., Sun J., Gao L., Dong M.Q., Wang Y., Li C. (2013). Phosphorylation-coupled proteolysis of the transcription factor MYC2 is important for jasmonate-signaled plant immunity. PLoS Genet. 9: e1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Turner J.G. (2008). Wound-induced endogenous jasmonates stunt plant growth by inhibiting mitosis. PLoS ONE 3: e3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Hull A.K., Gupta N.R., Goss K.A., Alonso J., Ecker J.R., Normanly J., Chory J., Celenza J.L. (2002). Trp-dependent auxin biosynthesis in Arabidopsis: Involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 16: 3100–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X.Y., Spivey N.W., Zeng W., Liu P.P., Fu Z.Q., Klessig D.F., He S.Y., Dong X. (2012). Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 11: 587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S., Shi H., Xue C., Wang L., Xi Y., Li J., Quail P.H., Deng X.W., Guo H. (2012). A molecular framework of light-controlled phytohormone action in Arabidopsis. Curr. Biol. 22: 1530–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Gan L., Shen Z., Xia K. (2006). Interactions between jasmonates and ethylene in the regulation of root hair development in Arabidopsis. J. Exp. Bot. 57: 1299–1308 [DOI] [PubMed] [Google Scholar]
- Zhu J., Jeong J.C., Zhu Y., Sokolchik I., Miyazaki S., Zhu J.K., Hasegawa P.M., Bohnert H.J., Shi H., Yun D.J., Bressan R.A. (2008). Involvement of Arabidopsis HOS15 in histone deacetylation and cold tolerance. Proc. Natl. Acad. Sci. USA 105: 4945–4950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., et al. (2011). Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 12539–12544 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.