Abstract
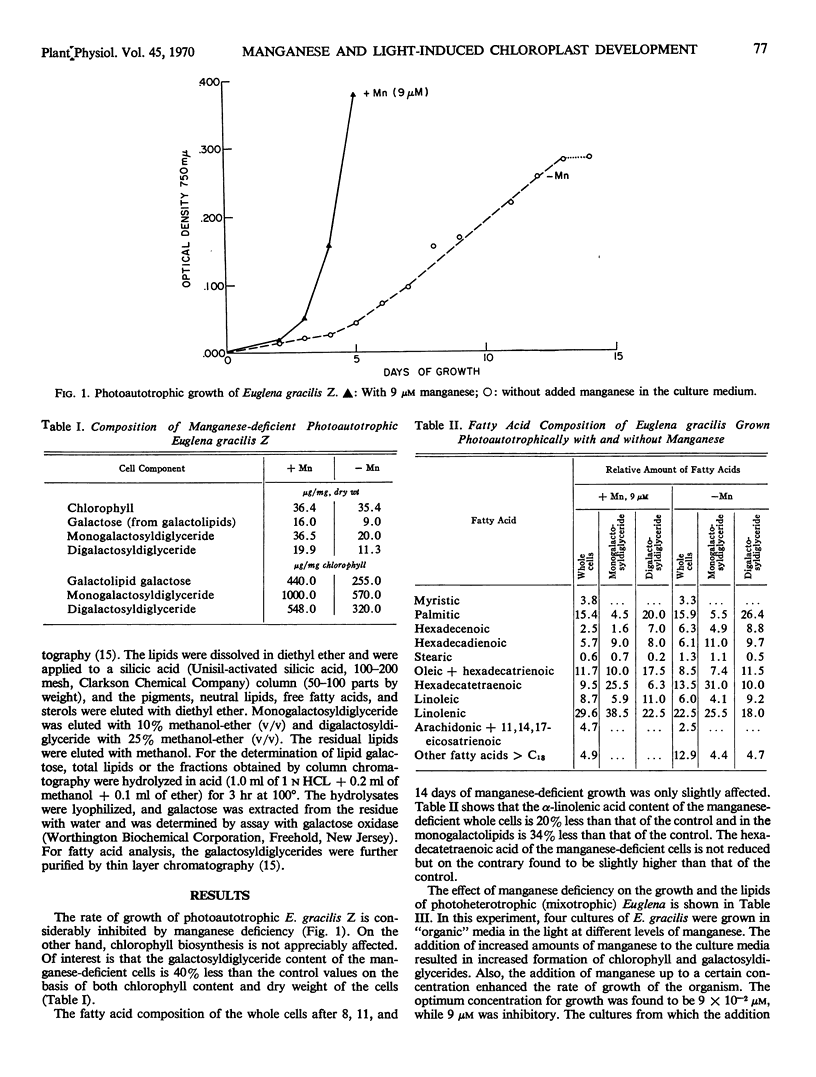
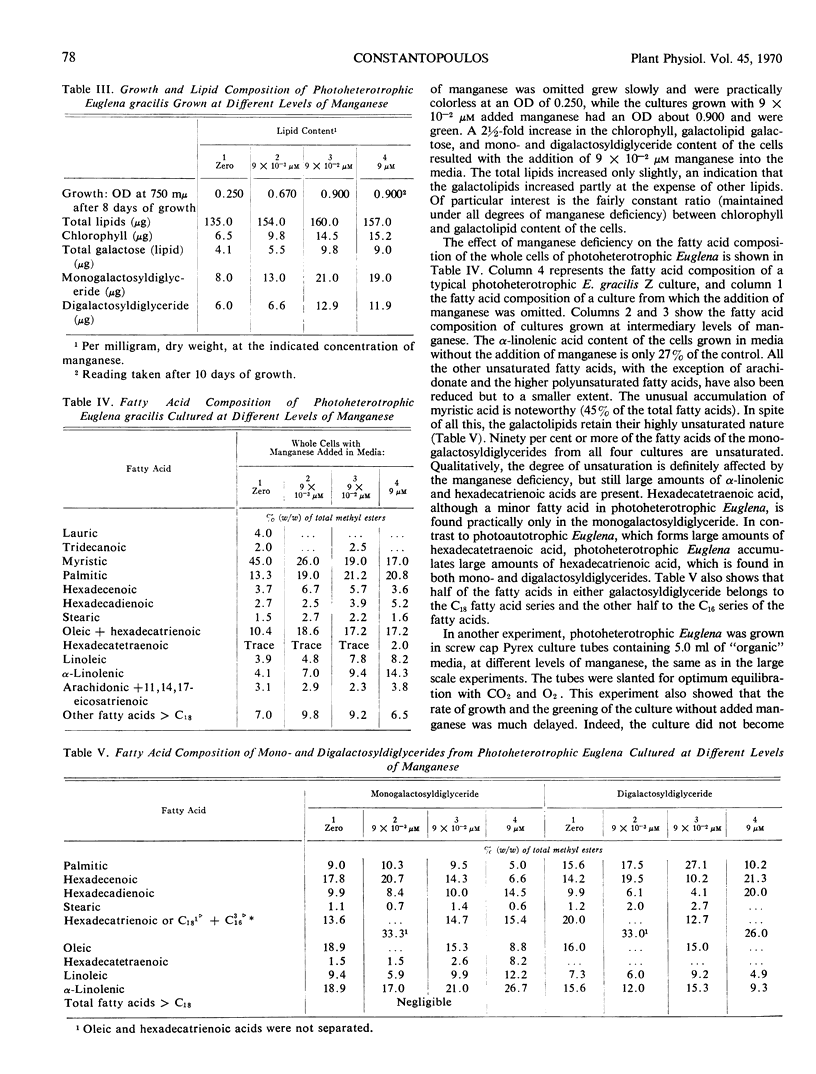
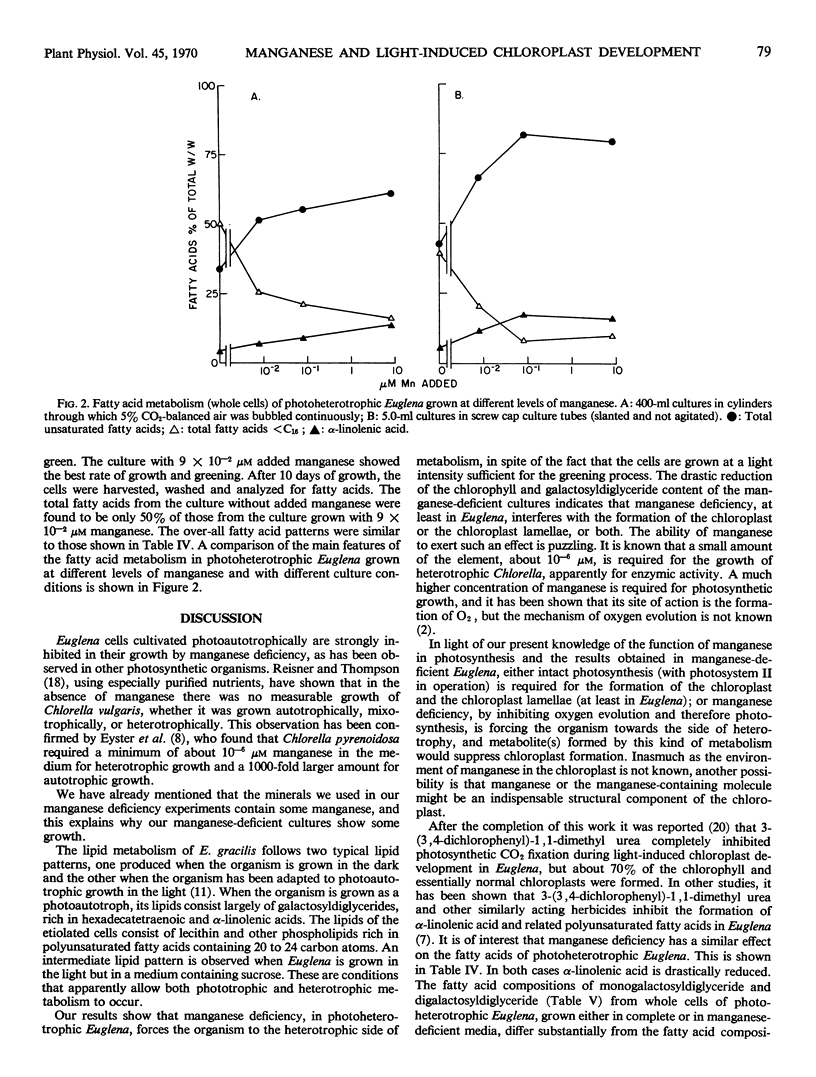
The growth of photoautotrophic Euglena gracilis Z is strongly inhibited by manganese deficiency, whereas chlorophyll formation is not appreciably affected. The galactosyldiglyceride content of the manganese-deficient photo-autotrophic Euglena was about 40% lower on the basis of either chlorophyll content or dry weight. When dark-grown cultures of Euglena were grown photoheterotrophically in light sufficient for the greening of the cells, or photosynthesis, manganese deficiency resulted in a reduction of the cellular content of chlorophyll and galactosyldiglycerides to 40% of control values, indicating interference with chloroplast formation. The fatty acids of the photoheterotrophic manganese-deficient cells were mainly saturated, with an unusual accumulation (about 45%) of the total fatty acids) of myristic acid. In spite of this, the galactosyldiglycerides contain mainly unsaturated fatty acids. Ninety per cent of the fatty acids of the monogalactosyldiglyceride are unsaturated, including large amounts of α-linolenic acid. The ratio of chlorophyll to galactosyldiglyceride content of the cells was remarkably constant at all manganese deficiency levels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Site of manganese function in photosynthesis. Biochim Biophys Acta. 1968 May 28;153(4):819–837. doi: 10.1016/0005-2728(68)90009-1. [DOI] [PubMed] [Google Scholar]
- ERWIN J., BLOCH K. POLYUNSATURATED FATTY ACIDS IN SOME PHOTOSYNTHETIC MICROORGANISMS. Biochem Z. 1963;338:496–511. [PubMed] [Google Scholar]
- Eyster C., Brown T. E., Tanner H. A., Hood S. L. Manganese Requirement with Respect to Growth, Hill Reaction and Photosynthesis. Plant Physiol. 1958 Jul;33(4):235–241. doi: 10.1104/pp.33.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- HULANICKA D., ERWIN J., BLOCH K. LIPID METABOLISM OF EUGLENA GRACILIS. J Biol Chem. 1964 Sep;239:2778–2787. [PubMed] [Google Scholar]
- Homann P. H. Studies on the manganese of the chloroplast. Plant Physiol. 1967 Jul;42(7):997–1007. doi: 10.1104/pp.42.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLS B. W. SEPARATION OF THE LIPIDS OF PHOTOSYNTHETIC TISSUES: IMPROVEMENTS IN ANALYSIS BY THIN-LAYER CHROMATOGRAPHY. Biochim Biophys Acta. 1963 Aug 27;70:417–422. doi: 10.1016/0006-3002(63)90771-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg A., Gouaux J., Milch P. Monogalactosyl and digalactosyl diglycerides from heterotrophic, hetero-autotrophic, and photobiotic Euglena gracilis. J Lipid Res. 1966 Nov;7(6):733–738. [PubMed] [Google Scholar]
- Schiff J. A., Zeldin M. H., Rubman J. Chlorophyll Formation and Photosynthetic Competence in Euglena During Light-Induced Chloroplast Development in the Presence of 3, (3,4-dichlorophenyl) 1,1-Dimethyl Urea (DCMU). Plant Physiol. 1967 Dec;42(12):1716–1725. doi: 10.1104/pp.42.12.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]


