Abstract
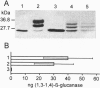
The codon usage of a hybrid bacterial gene encoding a thermostable (1,3-1,4)-beta-glucanase was modified to match that of the barley (1,3-1,4)-beta-glucanase isoenzyme EII gene. Both the modified and unmodified bacterial genes were fused to a DNA segment encoding the barley high-pI alpha-amylase signal peptide downstream of the barley (1,3-1,4)-beta-glucanase isoenzyme EII gene promoter. When introduced into barley aleurone protoplasts, the bacterial gene with adapted codon usage directed synthesis of heat stable (1,3-1,4)-beta-glucanase, whereas activity of the heterologous enzyme was not detectable when protoplasts were transfected with the unmodified gene. In a different expression plasmid, the codon modified bacterial gene was cloned downstream of the barley high-pI alpha-amylase gene promoter and signal peptide coding region. This expression cassette was introduced into immature barley embryos together with plasmids carrying the bar and the uidA genes. Green, fertile plants were regenerated and approximately 75% of grains harvested from primary transformants synthesized thermostable (1,3-1,4)-beta-glucanase during germination. All three trans genes were detected in 17 progenies from a homozygous T1 plant.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. A., Stone B. A. A new substrate for investigating the specificity of beta-glucan hydrolases. FEBS Lett. 1975 Apr 1;52(2):202–207. doi: 10.1016/0014-5793(75)80806-4. [DOI] [PubMed] [Google Scholar]
- Borriss R., Buettner K., Maentsaelae P. Structure of the beta-1,3-1,4-glucanase gene of Bacillus macerans: homologies to other beta-glucanases. Mol Gen Genet. 1990 Jul;222(2-3):278–283. doi: 10.1007/BF00633829. [DOI] [PubMed] [Google Scholar]
- Borriss R., Olsen O., Thomsen K. K., von Wettstein D. Hybrid bacillus endo-(1-3,1-4)-beta-glucanases: construction of recombinant genes and molecular properties of the gene products. Carlsberg Res Commun. 1989;54(2):41–54. doi: 10.1007/BF02907584. [DOI] [PubMed] [Google Scholar]
- Borriss R., Zemek J. beta-1,3-1,4-Glucanase in sporenbildenden Mikroorganismen. IV. Eigenschaften einiger Bacillus-beta-Glucan-HER. Zentralbl Bakteriol Naturwiss. 1981;136(1):63–69. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Edwards K., Johnstone C., Thompson C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 1991 Mar 25;19(6):1349–1349. doi: 10.1093/nar/19.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher G. B., Lock P. A., Morgan M. M., Lingelbach K., Wettenhall R. E., Mercer J. F., Brandt A., Thomsen K. K. Primary structure of the (1-->3,1-->4)-beta-D-glucan 4-glucohydrolase from barley aleurone. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2081–2085. doi: 10.1073/pnas.83.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham H., Fadel J. G., Newman C. W., Newman R. K. Effect of pelleting and beta-glucanase supplementation on the ileal and fecal digestibility of a barley-based diet in the pig. J Anim Sci. 1989 May;67(5):1293–1298. doi: 10.2527/jas1989.6751293x. [DOI] [PubMed] [Google Scholar]
- Hahn M., Olsen O., Politz O., Borriss R., Heinemann U. Crystal structure and site-directed mutagenesis of Bacillus macerans endo-1,3-1,4-beta-glucanase. J Biol Chem. 1995 Feb 17;270(7):3081–3088. doi: 10.1074/jbc.270.7.3081. [DOI] [PubMed] [Google Scholar]
- Hofemeister J., Kurtz A., Borriss R., Knowles J. The beta-glucanase gene from Bacillus amyloliquefaciens shows extensive homology with that of Bacillus subtilis. Gene. 1986;49(2):177–187. doi: 10.1016/0378-1119(86)90278-7. [DOI] [PubMed] [Google Scholar]
- Høj P. B., Fincher G. B. Molecular evolution of plant beta-glucan endohydrolases. Plant J. 1995 Mar;7(3):367–379. doi: 10.1046/j.1365-313x.1995.7030367.x. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel T., Simon O., Borriss R., Heinemann U. Molecular and active-site structure of a Bacillus 1,3-1,4-beta-glucanase. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5287–5291. doi: 10.1073/pnas.90.11.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khursheed B., Rogers J. C. Barley alpha-amylase genes. Quantitative comparison of steady-state mRNA levels from individual members of the two different families expressed in aleurone cells. J Biol Chem. 1988 Dec 15;263(35):18953–18960. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luchsinger W. W., Chen S. C., Richards A. W. Mechanism of action of malt beta-glucanases. 9. The structure of barley beta-D-glucan and the specificity of A11-endo-beta-glucanase. Arch Biochem Biophys. 1965 Dec;112(3):531–536. doi: 10.1016/0003-9861(65)90091-3. [DOI] [PubMed] [Google Scholar]
- Meldgaard M., Harthill J., Petersen B., Olsen O. Glycan modification of a thermostable recombinant (1-3, 1-4)-beta-glucanase secreted from Saccharomyces cerevisiae is determined by strain and culture conditions. Glycoconj J. 1995 Jun;12(3):380–390. doi: 10.1007/BF00731341. [DOI] [PubMed] [Google Scholar]
- Meldgaard M., Svendsen I. Different effects of N-glycosylation on the thermostability of highly homologous bacterial (1,3-1,4)-beta-glucanases secreted from yeast. Microbiology. 1994 Jan;140(Pt 1):159–166. doi: 10.1099/13500872-140-1-159. [DOI] [PubMed] [Google Scholar]
- Olsen O., Borriss R., Simon O., Thomsen K. K. Hybrid Bacillus (1-3,1-4)-beta-glucanases: engineering thermostable enzymes by construction of hybrid genes. Mol Gen Genet. 1991 Feb;225(2):177–185. doi: 10.1007/BF00269845. [DOI] [PubMed] [Google Scholar]
- Perlak F. J., Fuchs R. L., Dean D. A., McPherson S. L., Fischhoff D. A. Modification of the coding sequence enhances plant expression of insect control protein genes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3324–3328. doi: 10.1073/pnas.88.8.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlak F. J., Stone T. B., Muskopf Y. M., Petersen L. J., Parker G. B., McPherson S. A., Wyman J., Love S., Reed G., Biever D. Genetically improved potatoes: protection from damage by Colorado potato beetles. Plant Mol Biol. 1993 May;22(2):313–321. doi: 10.1007/BF00014938. [DOI] [PubMed] [Google Scholar]
- Politz O., Simon O., Olsen O., Borriss R. Determinants for the enhanced thermostability of hybrid (1-3,1-4)-beta-glucanases. Eur J Biochem. 1993 Sep 15;216(3):829–834. doi: 10.1111/j.1432-1033.1993.tb18204.x. [DOI] [PubMed] [Google Scholar]
- Skriver K., Olsen F. L., Rogers J. C., Mundy J. cis-acting DNA elements responsive to gibberellin and its antagonist abscisic acid. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7266–7270. doi: 10.1073/pnas.88.16.7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Lemaux P. G. Generation of Large Numbers of Independently Transformed Fertile Barley Plants. Plant Physiol. 1994 Jan;104(1):37–48. doi: 10.1104/pp.104.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Chang S. Y., Bibb M. J., Bibb M. J. A cassette containing the bar gene of Streptomyces hygroscopicus: a selectable marker for plant transformation. Nucleic Acids Res. 1990 Feb 25;18(4):1062–1062. doi: 10.1093/nar/18.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N. Complete Nucleotide Sequence of a Hordeum vulgare Gene Encoding (1-->3, 1-->4)-beta-Glucanase Isoenzyme II. Plant Physiol. 1991 Aug;96(4):1382–1384. doi: 10.1104/pp.96.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N. Structure of the genes encoding Hordeum vulgare (1----3,1----4)-beta-glucanase isoenzymes I and II and functional analysis of their promoters in barley aleurone protoplasts. Mol Gen Genet. 1992 Jul;234(1):33–42. doi: 10.1007/BF00272342. [DOI] [PubMed] [Google Scholar]