Abstract
Purpose of review
Primary membranous nephropathy is a common glomerular disease characterized by sub-epithelial immune deposits that has become the prototype of an autoimmune glomerular disease. The purpose of this review is to highlight recent advances regarding the pathogenesis of membranous nephropathy as well as potential new therapies.
Recent findings
The discovery of two major podocytes antigens: neutral endopeptidase (NEP), involved in rare cases of neonatal membranous nephropathy, and the M-type phospholipase–A2 receptor 1 (PLA2R1) the first antigen discovered in adults, have been major “breakthroughs” in our understanding of the pathogenesis of human membranous nephropathy. Anti-PLA2R antibodies appear to predict activity of the disease as well as response to therapy. Pediatric and adult cases of membranous nephropathy occurring in the presence of circulating cationic bovine serum album (BSA) and anti-BSA antibodies have also been described raising the possibility that food antigens may be involved in the development of membranous nephropathy. Moreover, the results of genetic susceptibility have become available. Exciting progress has also been made in the treatment of this disease including therapy with ACTH and Rituximab.
Summary
Understanding disease pathogenesis is crucial in guiding patient evaluation and designing appropriate therapy. Recent discoveries have helped to elucidate the pathophysiology of membranous nephropathy and may facilitate a more patient-specific treatment approach in these patients.
Keywords: ACTH, cationic bovine serum albumin, membranous nephropathy, PLA2R antibodies
Introduction
Membranous nephropathy is a common immune-mediated glomerular disease characterized by the presence of immune deposits on the epithelial side of the glomerular capillary wall. It remains the leading cause of nephrotic syndrome in Caucasian adults.[1] Until recently, most of our understanding of the pathogenic mechanisms came from experimental models in rats, i.e. the Heymann nephritis model.[2,3] In this model, megalin is the podocyte antigen involved but megalin is neither expressed in human podocytes nor detected in the subepithelial deposits in patients with idiopathic/primary membranous nephropathy. Thus, for years the membranous nephropathy target in human podocytes remained elusive. Thanks to modern technology, major advances have occurred in our understanding of the autoimmune processes involved in the development of human membranous nephropathy. A number of podocyte antigens, namely neutral endopeptidases (NEP), M-type phospholipase A2 receptor (PLA2R), aldose reductase (AR), and superoxide dismutase (SOD) 2 have been identified as targets for autoantibodies in patients with membranous nephropathy. Non-podocyte circulating antigens, i.e. cationic bovine serum albumin (BSA) responsible for childhood forms of membranous nephropathy have also been described. The presence of some antibodies appears to correlate with disease activity and response to treatment. Genetic studies are elucidating predisposing factors for development of the disease. Although in most patients the disease progresses relatively slowly, approximately 40% of patients eventually develop ESRD.[4] Because of its frequency, it remains the 2nd or 3rd most common type of primary glomerulonephritis resulting in end stage renal disease.[5] Available immunosuppressive therapies are at least partially successful in reducing proteinuria in membranous nephropathy, but their use is controversial and all are associated with a significant adverse effects and a high relapse rate, thus tempering their use. (reviewed in [6]) This review will highlight the most recent findings in the pathogenesis of the disease as well as potential new therapies for patients with membranous nephropathy.
Anti-neutral endopeptidase antibodies
The initial proof that circulating antibodies against a podocyte protein could cause membranous nephropathy in humans came from Debiec and colleagues who first described the case of a patient with neonatal membranous nephropathy due to the transplacental transfer of circulating anti-neutral endopeptidase antibodies to the fetus.[7] Neutral endopeptidase (NEP) is a membrane bound enzyme that is able to digest biologically active peptides and is expressed on the surface of human’s podocytes, syncytiotrophoblastic cells, lymphoid progenitors, and other many epithelials cells and polymorphonuclear leukocytes. Mothers with truncating mutations of the metallomembrane endopeptidase (MME) gene fail to express NEP on cell membranes. NEP-deficient mothers, who were immunized during pregnancy, were able to transplacentally transfer nephritogenic antibodies against NEP to her children causing membranous nephropathy in the newborn.[8] The fact that rabbits injected with the maternal IgG form from these mothers also developed membranous nephropathy was another proof that the disease was related to circulating anti-NEP antibodies, and demonstration of a human counterpart to Heymann nephritis.[9]
PLA2R autoantibodies
The discovery that antibodies to the M-type phospholipase A2-receptor (PLA2R) are present in 70 to 82% of the patients with primary membranous nephropathy has revolutionized the field of membranous nephropathy.[10] The PLA2R is a transmembrane receptor belonging to the mannose receptor family and a receptor for the secreted phospholipase A2, a lipolytic enzyme that cleaves the fatty acid bond of membrane glycerophospholipids.[11] A common functional feature of this family of receptors is their ability to undergo endocytosis and thus involved in the internalization of extracellular ligands. Sera from patients with primary membranous nephropathy contained IgG4 antibodies that specifically recognized PLA2R, but these antibodies are not present in the serum of healthy controls, in patients with secondary causes of membranous nephropathy, or other glomerular and autoimmune diseases.[10,12] Levels of anti-PLA2R have been found correlate strongly correlated with the disease activity: disappearance of the antibody is associated with remission of proteinuria while reappearance of the antibody heralds a relapse of nephrotic syndrome.[10,13,14] How the presence of these antibodies lead to the development of proteinuria is unknown.
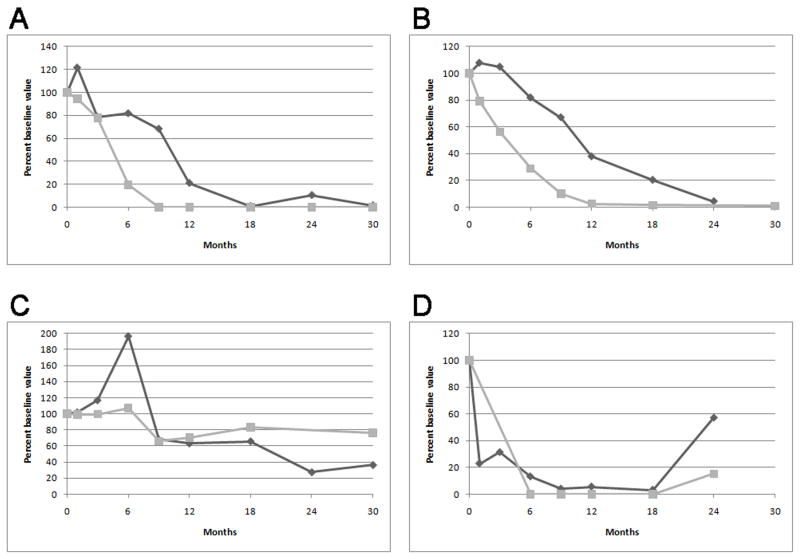
Monitoring anti-PLA2R levels may be also helpful to monitor response to therapy. Hoxha et al., treated 5 patients with membranous nephropathy with rituximab; in 2 of them disappearance of anti-PLA2R from circulation anticipated a complete or partial remission of proteinuria.[12] Patients who failed to clear anti-PLA2R from circulation did not achieve remission of proteinuria. Similarly, we have recently reported on our experience with the use of rituximab in 35 patients with primary membranous nephropathy treated with 2 to 4 doses of rituximab.[13] Pretreatment samples from 25 of 35 (71%) patients contained antibodies against anti-PLA2R, and these autoantibodies declined or disappeared in 17 (68%) of these patients within 12 months after rituximab. Those who demonstrated this immunologic response fared better clinically with 59% and 88% of the patient attaining complete or partial remission of proteinuria at the end of 12 and 24 months, respectively. This compared with 0% and 33% among those patients with persistent anti-PLA2R levels. Changes in antibody levels preceded changes in proteinuria. One subject who relapsed during follow-up had a concomitant return of anti- PLA2R.[13] (Figure 1)
Figure 1.
Representative plots of anti-PLA2R (gray squares) and proteinuria (black diamonds) versus time following initial RTX treatment. Values are plotted as percent of baseline value. Panel (a) and (b) depicts the typical reduction and disappearance of anti-PLA2R followed by resolution of proteinuria exhibited by the majority of patients. Panel (c) is representative of patients in whom anti-PLA2R did not substantially decline following treatment and the associated with persistence of proteinuria. Panel (d) depicts the single patient whose anti-PLA2R level returned with relapse of his disease after having initially disappeared. (From Beck et al. [13] with permission)
Further studies are needed to explain the potential discrepancies between circulating anti- PLA2R antibodies and detectable PLA2R in glomerular deposits in some patients. Debiec and Ronco evaluated the presence of PLA2R autoantibody in the serum and PLA2R in glomerular deposits in 42 consecutive patients with primary membranous nephropathy.[15] In 21 patients, anti-PLA2R antibodies were present in circulation and had PLA2R in glomerular deposits. However, 3 patients with high levels of circulating anti-PLA2R antibodies did not have detectable PLA2R in glomerular deposits. These cases suggest that antibodies were not nephritogenic or that epitopes were poorly accessible at the time of kidney biopsy. On the other hand, 18 patients had no detectable circulating anti-PLA2R antibodies, although 10 of them had PLA2R in glomerular deposits. Debiec and Ronco suggest that these apparently discordant findings might be due to rapid clearance of antibodies from the circulation but deposition in glomeruli or patients with persistent proteinuria due immunologically inactive disease but with irreversible glomerular ultrastructural changes.[15]
The recognition that proteinuria may persist despite the absence of immunological disease activity but as a consequence of an altered/remodeled glomerular basement membrane due to longstanding disease is of great clinical relevance. In this situation, further immunosuppression would be unnecessary and potentially harmful, and management should be conservative. Alternatively, the persistent proteinuria in the presence of circulating anti-PLA2R could be due to ongoing immunological disease and continued, increased, or altered immunosuppressive therapy should be considered.[13] Taken all together, these observations suggest that detection of circulating anti-PLA2R antibodies and PLA2R in biopsy samples and quantification of circulating anti-PLA2R levels may provide a tool for monitoring disease activity and treatment efficacy in patients with membranous nephropathy.
The role of anti-PLA2R antibodies has also been evaluated in recurrent and de novo membranous nephropathy after kidney transplantation.[16] Anti-PLA2R antibodies were present in 5 of 10 patients with recurrent membranous nephropathy, but in none of the 9 patients with de novo membranous nephropathy. Some patients with ESRD due to membranous nephropathy and circulating anti-PLA2R antibodies at the time of kidney transplant did not develop recurrence. We have a similar experience in our own transplant population in which positive anti-PLA2R antibodies at the time of transplant do not predicting recurrence of membranous nephropathy. Because anti-PLA2R antibodies are not always associated with recurrence of the disease, the presence of these antibodies should not preclude kidney transplantation in patients with ESRD secondary membranous nephropathy and positive anti-PLA2R antibodies.
Antibodies to superoxide dismutase 2 (SOD2), aldose reductase (AR) and α-enolase
Using a combined proteomic approach Prunotto et al. identified specific IgG4 antibodies against the cytosolic proteins SOD2, AR, and α-enolase in both serum and glomeruli of patients with primary membranous nephropathy but not from biopsies of patients with other glomerular diseases or normal kidney.[17] It is unclear what the role of these antibodies is in the pathogenesis of primary membranous nephropathy. As opposed to NEP and PLA2R, these are cytosolic proteins and are not present or minimally expressed on normal podocyte membranes but are “neo-expressed” in glomeruli of patients with membranous nephropathy. As in the case of anti- PLA2R antibodies, the predominance of IgG4 in the glomerular immune deposits supports the concept of an isotype-specific mechanism. Mechanisms of translocation of these intracellular molecules have been proposed as way of explaining the development of these autoantibodies. Preliminary in vitro data showed an increase of SOD2 expression on podocyte plasma membrane after treatment with hydrogen peroxide, suggesting that oxidative stress may drive glomerular expression of SOD2, whereas induction of AR seems less specific.[17] It is also possible that antibody spreading occurs, whereby the development of a particular autoantibody (e.g. anti-NEP, anti-PLA2R, etc.) induces the expression of other antigens that, in turn, form targets for the development of additional autoantibodies.[18]
Most recently, the same group also identified glomerular α-enolase as an additional target for autoantibodies in patients with membranous nephropathy.[19] Alpha-enolase is one of the most abundant cytosolic protein, is particularly expressed in tubular kidney cells but it is absent in normal glomeruli. IgG4 antibodies against α-enolase were present in circulation, were eluted from microdissected glomeruli, and co-localized with C5b-9 (membrane attack complex) in sub-epithelial deposits from kidney biopsies of patients with membranous nephropathy. Antibodies against α-enolase have been reported in patients with primary and secondary membranous nephropathy [20] as well as in patients with a number of other autoimmune diseases including lupus erythematosus, ANCA associated vasculitis and inflammatory bowel diseases[21–25] and whether these antibodies are simply a reflection of a nonspecific/molecular epitope spreading immune response in membranous nephropathy awaits further research.
Antibodies to Bovine Serum Albumin
Debiec and colleagues recently evaluated a cohort of 9 children and 41 adults with primary membranous nephropathy and found high levels of circulating anti-bovine serum albumin antibodies, of both IgG1 and IgG4 subclasses, in 4 children and 7 adults with membranous nephropathy.[26] These patients also exhibited high levels of circulating bovine serum albumin but without an increase on immune complex levels in circulation. Bovine serum albumin (BSA) immunopurified from these 4 children’s serum differed from BSA purified from adult patients by migrating in the basic range of pH, whereas BSA from adults migrated in neutral regions and similar as native bovine serum albumin. In children who were negative for anti-PLA2R antibodies but had both high levels of circulating cationic BSA and BSA-specific antibodies, BSA colocalized with IgG in subepithelial immune deposits. IgG1 and IgG4 eluted from kidney-biopsy specimens showed reactivity that was specific for BSA.
The mechanism of BSA-induced membranous nephropathy in these patients is unclear. Human exposure to BSA is common (e.g. cow’s milk) and antibodies to BSA are common in the general population.[27] A variety of modern food processing conditions could induce protein-modifications affecting the proteolysis of BSA as well as facilitating the absorption and passage into the bloodstream. The relative elevated PH in infants (pH 3 or 4 versus pH 2 in adults) and immaturity of the gastrointestinal tract could be make children more susceptible to absorb not digested or only partially digested BSA, especially in the setting of childhood gastroenteritis. In this context, positively charged circulating cationic BSA could become attached to the negatively charged glomerular endothelial glycocalyx and heparan sulphate proteoglycans in the GBM acting as a target for the deposition of anti-BSA IgG and in situ formation of immune-complexes.(Figure 2) In animal models, cationic form of BSA can induce membranous nephropathy (“planted” antigen model).[28–30] The studies by Ronco and Debiec suggest that the “planted” antigen model can also be applied to human disease.[26]
Figure 2.
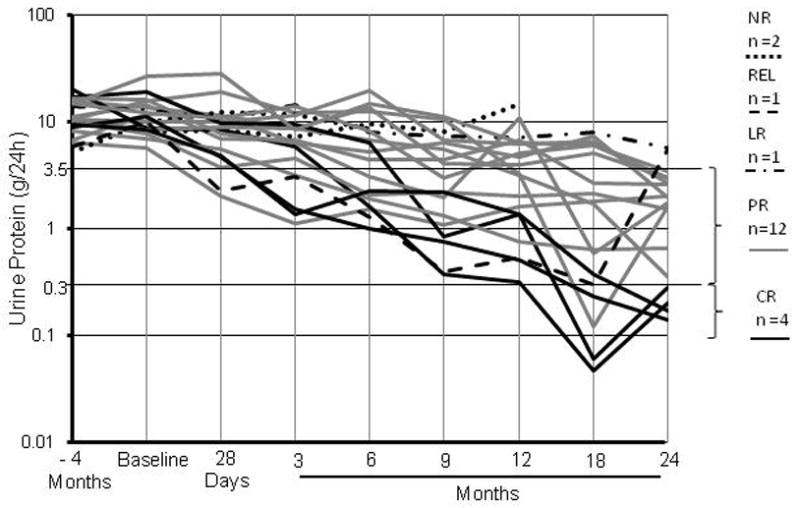
Longitudinal effect of rituximab on proteinuria (log transformed). CR (complete remission) defined as proteinuria (P) less than 0.3 g/24h; PR (partial remission) defined as reduction in P of greater than 50% and final P less than 3.5g but greater than 0.3 g/24h; LR (limited response) defined as reduction in P of greater than 50% and final P greater than 3.5g/24h; NR (no response), a reduction in P of less than 50%; REL, relapse. (From Fervenza et al. [50] with permission)
In patients with BSA-mediated membranous nephropathy, the antibodies predominantly targeted the BSA peptide 147–161 (containing two linear epitopes not present in human albumin and without cross reactivity to podocyte proteins), whereas controls with high anti-BSA antibodies but no membranous nephropathy had a broader spectrum of peptides reactivity. Debiec et al. suggest that the psychochemical properties of the BSA (e.g. charge; BSA modification during food processing/digestion) together with the amount of circulating BSA as well as a predominant T-helper type 2 (Th2) immune response resulting in production of IgG4 are the conditions necessary for the development of membranous nephropathy.[31]
The four children with membranous nephropathy had both high levels of anti-BSA antibodies as well as BSA in circulation, and similar findings were seen in four of the seven adults with membranous nephropathy. BSA could specifically be detected in glomerular immune deposits only in patients who had both circulating cationic BSA and anti-BSA antibodies suggesting that both are required for the development of the disease.[26] Levels of anti-BSA IgG1 and IgG4 antibodies and circulating cationic BSA correlated with disease activity: high in patients with nephrotic range proteinuria and low in patients in remission.
Further studies are needed to explain the origin of circulating cationic BSA. BSA immuno-purified from the serum of children migrate in the basic range of pH, whereas the BSA from adult patients migrated in the neutral region as native BSA. BSA colocalized with IgG immune deposits only in four children with circulating cationic BSA, but in none of the 18 adults patients with membranous nephropathy for whom biopsy specimens were available, implying that only cationic BSA can induce membranous nephropathy. On the other hand, positive PLA2R staining was detected in 14 of the 20 adult biopsy specimens again pointing to a different pathogenic process in adults with membranous nephropathy. Why only antibodies against BSA amino acid residues 147–161 are associated with membranous nephropathy? Is genetic susceptibility the additional hit that triggers membranous nephropathy? Antibodies against other regions of BSA have been reported in patients with rheumatoid arthritis and multiple sclerosis but these patients do not have an associated membranous nephropathy.[32,33] Whether or not dietary proteins could play a role in other cases of membranous nephropathy is unknown, but in children, the diagnosis of membranous nephropathy should raise the possibility of BSA-induced membranous nephropathy.
Genetic susceptibility - the HLA-DQA1 and PLA2R1 risk alleles
Interaction of genetic susceptibility and environmental factors could play a role in the development of glomerular diseases such as IgA nephropathy and primary membranous nephropathy.[34–37] Using genome-wide association studies (GWAS) Stanescu and a group of international collaborators recently linked single-nucleotide polymorphisms (SNPs) in the genes encoding M-type phospholipase A2 receptor 1 (PLA2R) and HLA complex class II HLA-DQ alpha chain 1 (HLA-DQA1) in Caucasian populations with membranous nephropathy.[38] Although the risk for primary membranous nephropathy was higher with the HLA-DQ1 allele than with the PLA2R1 allele, it adds support to the findings of positive anti-PLA2R antibodies in the majority of patients with membranous nephropathy.[10] For person who are homozygous for both risk alleles, the odds ration for developing membranous nephropathy is close to 80, with additive increase in the odds ration, depending on the combination of genotypes.[38]Although these findings do not prove causality, they suggest that genetic background plays a significant role in the predisposition of the primary membranous nephropathy, as previously noted in cases of familial primary membranous nephropathy.[39,40] The HLA region of the reported association spans a 6-Mb interval, extending far beyond the linkage-disequilibrium block of HLA-DQ1 raising the possibility that that several independently associated variants account for this signal.[41] Further work is required to confirm causative variants within the genomic regions, the causative link between the presence of the HLA-DQA1 risk allele and PLA2R1 autoantibodies as well as identify specific gene-environment interactions that “trigger” membranous nephropathy.
NEW THERAPIES
Adrenocorticotropics hormone
Berg and colleagues were the first to report that adrenocorticotropics hormone (ACTH) lowered the albumin excretion and improved GFR in patients with membranous nephropathy.[42] Subsequent case series strengthened this observation.[43,44] A randomized controlled study in 32 patients with membranous nephropathy found similar remission rates in patients treated with ACTH compared to a 6-month course of alternating corticosteroid and cyclophosphamide.[45] These studies were conducted using a synthetic version of ACTH (Synacthen®; not available in the US), but a retrospective case series of 11 patients with treatment-resistant membranous nephropathy using a natural, highly-purified ACTH gel formulation (H.P. Acthar gel®), reported similar encouraging results.[46]. The exact mechanism by which ACTH mediates its effects in proteinuria is not completely understood, but is likely independent of its induction of cortisol production, as its production remains low and there is concomitant evidence that steroids alone do not affect the outcome of the disease.[47] ACTH is derived from the pro-opiomelanocortin (POMC) precursor. POMC is proteolytically cleaved by endopeptidases to yield various polypeptide fragments with varying physiological activity such as ACTH and α-melanocyte-stimulating hormone (α-MSH). The melano-corticotropic receptor (MCR1) is located on various cells, including B cells, T cells, antigen presenting cells and human podocytes. In a recent study, treatment with ACTH, α-MSH or MS05, a specific MCR1 agonist, showed similar but significant reduction in proteinuria in rats with passive Heymann nephritis.[48]. These results suggest that ACTH mediates its effects via α-MSH interaction on MCR1 on podocytes, and may explain why patients who are resistant to previous immunosuppressive therapies respond to ACTH. A randomized, placebo-controlled, study using Acthar® Gel in treatment-resistant patients with membranous nephropathy and nephrotic syndrome is currently underway. (ClinicalTrials.gov Identifier: NCT01386554)
Rituximab
Previous studies have suggested that selective B-cell depletion with rituximab can induce remission of proteinuria in approximately 70% of the patients with v.[4,49] We recently extended these observations in a study involving 20 patients (11 failures to prior therapy) with membranous nephropathy and proteinuria >5g/24h who received rituximab (375mg/m2 × 4), with retreatment at 6 months regardless of proteinuria response.[50] Baseline proteinuria of 11.9±4.9g/24h decreased to 4.2±3.8g/24h and 2.0±1.7g/24h at 12 and 24 months, respectively (p<0.001) while creatinine clearance increased from 72.4±33 at baseline to 88.4 ±31.5 ml/min/1.73m2 at 24 months (p=0.02) (Figure 3). Of 18 patients who completed 24-months follow up, 4 were in complete remission, 12 were in partial remission (CR + PR = 80%), 1 had a limited response (>50% drop in P but >3.5g/24h) and 1 patient relapsed. Similar observation have been reported in a matched-cohort study compared two-year outcomes of 11 consecutive patients with primary membranous nephropathy who received rituximab as second line therapy for persisting nephrotic syndrome or relapsing disease.[51] A multicenter randomized control study comparing the use of Rituximab versus cyclosporine in the treatment of membranous nephropathy is currently on the way. (ClinicalTrials.gov Identifier: NCT01180036)
Conclusion
As stated by Dr. Richard Glassock in his summation remarks at the 2nd International Conference on Membranous Nephropathy in Bergamo, Italy, in May 2011 “The field of membranous nephropathy is now in the early stages of what Thomas Kuhn called a “paradigm shift”. After years of relative stasis, and as a result of major breakthroughs we now find the field in a state of disequilibrium where the accelerated rate of new information generated greatly exceeds the discard rate of older information and the total information has expanded markedly”. We now know that auto-immunity accounts for 70–80% of the cases of membranous nephropathy and new auto-antibodies are been discovered, including antibodies against common food products. Further research will be needed to elucidate the trigger (s) for the production of these antibodies (molecular mimicry, activation of auto-reactive B/T cells, others) as well as to understand how they disrupt podocyte function. Genetic susceptibility is certainly involved in the development of membranous nephropathy as demonstrated by the linkage of these patients to HLA-DQ gene loci and to specific PLA2R SNPs. Promising new therapies are being evaluated by two important ongoing clinical trials. When commercially available, quantification of antibodies (e.g. anti-PLA2R antibodies) may help monitoring disease activity and response to therapy more efficiently than proteinuria alone. Soon, we should be able to reach the goal of “personalized” therapy in patients with membranous nephropathy.
KEY POINTS.
Membranous nephropathy has become the prototype of an autoimmune glomerular disease.
A number of autoantibodies have been described in these patients, including antibodies to common food products.
Some of these autoantibodies, for example anti-PLA2R, may help in monitoring disease activity as well as response to therapy.
Genetic susceptibility appears to be involved.
Novel approaches to treatment with the use of ACTH and rituximab are being tested currently.
Acknowledgments
F.C.F has received unrestricted research grants from Genentech Inc., South San Francisco, CA., Biogen Idec, San Diego CA, and Questcor Pharmaceutical, Hayward, CA, the Fulk Family Foundation, the Mayo Clinic Foundation, and support from the UL1-RR24150 grant to the Center for Translational Science Activities. SS is the recipient of a Career Development Award from the Fulk Family Foundation.
References
- 1.Medawar W, Green A, Campbell E, Carmody M, Donohoe J, Doyle G, Walshe JJ. Clinical and histopathologic findings in adults with the nephrotic syndrome. Irish journal of medical science. 1990;159:137–140. doi: 10.1007/BF02937405. [DOI] [PubMed] [Google Scholar]
- 2.Kerjaschki D, Farquhar MG. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982;79:5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerjaschki D, Farquhar MG. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. The Journal of experimental medicine. 1983;157:667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggenenti P, Chiurchiu C, Brusegan V, Abbate M, Perna A, Filippi C, Remuzzi G. Rituximab in idiopathic membranous nephropathy: a one-year prospective study. J Am Soc Nephrol. 2003;14:1851–1857. doi: 10.1097/01.asn.0000071511.35221.b3. [DOI] [PubMed] [Google Scholar]
- 5.Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, Wolfe RA, Jones E, Disney AP, Briggs D, et al. Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: results from an international comparative study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2000;35:157–165. doi: 10.1016/S0272-6386(00)70316-7. [DOI] [PubMed] [Google Scholar]
- 6.Fervenza FC, Sethi S, Specks U. Idiopathic membranous nephropathy: diagnosis and treatment. Clin J Am Soc Nephrol. 2008;3:905–919. doi: 10.2215/CJN.04321007. [DOI] [PubMed] [Google Scholar]
- 7.Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschenes G, Ronco PM. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. The New England journal of medicine. 2002;346:2053–2060. doi: 10.1056/NEJMoa012895. [DOI] [PubMed] [Google Scholar]
- 8.Debiec H, Nauta J, Coulet F, van der Burg M, Guigonis V, Schurmans T, de Heer E, Soubrier F, Janssen F, Ronco P. Role of truncating mutations in MME gene in fetomaternal alloimmunisation and antenatal glomerulopathies. Lancet. 2004;364:1252–1259. doi: 10.1016/S0140-6736(04)17142-0. [DOI] [PubMed] [Google Scholar]
- 9.Ronco P, Debiec H. Antigen identification in membranous nephropathy moves toward targeted monitoring and new therapy. J Am Soc Nephrol. 2010;21:564–569. doi: 10.1681/ASN.2009121220. [DOI] [PubMed] [Google Scholar]
- 10.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llorca O. Extended and bent conformations of the mannose receptor family. Cellular and molecular life sciences: CMLS. 2008;65:1302–1310. doi: 10.1007/s00018-007-7497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoxha E, Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, Helmchen U, Stahl RA. An immunofluorescence test for phospholipase-A2-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant. 2011;26:2526–2532. doi: 10.1093/ndt/gfr247. [DOI] [PubMed] [Google Scholar]
- 13**.Beck LH, Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, Cosio FG, Cattran DC, Salant DJ. Rituximab-Induced Depletion of Anti-PLA2R Autoantibodies Predicts Response in Membranous Nephropathy. J Am Soc Nephrol. 2011;22:1543–1550. doi: 10.1681/ASN.2010111125. Largest study to date to evaluate levels of anti-PLA2R antibodies and response to therapy. It also introduces the concept that proteinuria may persist despite disapearance of the antibody due to irreversible glomerular damage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Hofstra JM, Beck LH, Jr, Beck DM, Wetzels JF, Salant DJ. Anti-phospholipase A receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6:1286–1291. doi: 10.2215/CJN.07210810. The study shows a correlation between presence of anti-PLA2R antibodies and disease activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. The New England journal of medicine. 2011;364:689–690. doi: 10.1056/NEJMc1011678. [DOI] [PubMed] [Google Scholar]
- 16**.Debiec H, Martin L, Jouanneau C, Dautin G, Mesnard L, Rondeau E, Mousson C, Ronco P. Autoantibodies Specific for the Phospholipase A(2) Receptor in Recurrent and De Novo Membranous Nephropathy. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:2144–2152. doi: 10.1111/j.1600-6143.2011.03643.x. Study reports that positive anti-PLA2R antibodies prior to kidney transplantation do not necessarely correlate with disease recurrence. [DOI] [PubMed] [Google Scholar]
- 17*.Prunotto M, Carnevali ML, Candiano G, Murtas C, Bruschi M, Corradini E, Trivelli A, Magnasco A, Petretto A, Santucci L, et al. Autoimmunity in membranous nephropathy targets aldose reductase and SOD2. J Am Soc Nephrol. 2010;21:507–519. doi: 10.1681/ASN.2008121259. This study reports on a number of newly discovered antibodies in patients with membranous nephropathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murtas C, Ravani P, Ghiggeri GM. New insights into membranous glomerulonephritis: from bench to bedside. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26:2428–2430. doi: 10.1093/ndt/gfr336. [DOI] [PubMed] [Google Scholar]
- 19.Bruschi M, Carnevali ML, Murtas C, Candiano G, Petretto A, Prunotto M, Gatti R, Argentiero L, Magistroni R, Garibotto G, et al. Direct characterization of target podocyte antigens and auto-antibodies in human membranous glomerulonephritis: Alfa-enolase and borderline antigens. J Proteomics. 2011 doi: 10.1016/j.jprot.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Wakui H, Imai H, Komatsuda A, Miura AB. Circulating antibodies against alpha-enolase in patients with primary membranous nephropathy (MN) Clinical and experimental immunology. 1999;118:445–450. doi: 10.1046/j.1365-2249.1999.01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratesi F, Moscato S, Sabbatini A, Chimenti D, Bombardieri S, Migliorini P. Autoantibodies specific for alpha-enolase in systemic autoimmune disorders. The Journal of rheumatology. 2000;27:109–115. [PubMed] [Google Scholar]
- 22.Hill GS. Tubulointerstitial nephritis and vasculitis. Current opinion in nephrology and hypertension. 1994;3:356–363. doi: 10.1097/00041552-199405000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Moodie FD, Leaker B, Cambridge G, Totty NF, Segal AW. Alpha-enolase: a novel cytosolic autoantigen in ANCA positive vasculitis. Kidney international. 1993;43:675–681. doi: 10.1038/ki.1993.97. [DOI] [PubMed] [Google Scholar]
- 24.Roozendaal C, de Jong MA, van den Berg AP, van Wijk RT, Limburg PC, Kallenberg CG. Clinical significance of anti-neutrophil cytoplasmic antibodies (ANCA) in autoimmune liver diseases. Journal of hepatology. 2000;32:734–741. doi: 10.1016/s0168-8278(00)80241-x. [DOI] [PubMed] [Google Scholar]
- 25.Vermeulen N, Vermeire S, Arijs I, Michiels G, Ballet V, Derua R, Waelkens E, Van Lommel L, Schuit F, Rutgeerts P, et al. Seroreactivity against glycolytic enzymes in inflammatory bowel disease. Inflammatory bowel diseases. 2011;17:557–564. doi: 10.1002/ibd.21388. [DOI] [PubMed] [Google Scholar]
- 26**.Debiec H, Lefeu F, Kemper MJ, Niaudet P, Deschenes G, Remuzzi G, Ulinski T, Ronco P. Early-childhood membranous nephropathy due to cationic bovine serum albumin. The New England journal of medicine. 2011;364:2101–2110. doi: 10.1056/NEJMoa1013792. The study demonstrates that modified bovine serum albumin is implicated in the pathogenesis of children with membranous nephropathy, it has clinical implications in the evaluation and treatment of children with this disease as well as raises the possibility that other dietary proteins could play a role in auto-immunity. [DOI] [PubMed] [Google Scholar]
- 27.Mogues T, Li J, Coburn J, Kuter DJ. IgG antibodies against bovine serum albumin in humans--their prevalence and response to exposure to bovine serum albumin. Journal of immunological methods. 2005;300:1–11. doi: 10.1016/j.jim.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Border WA, Ward HJ, Kamil ES, Cohen AH. Induction of membranous nephropathy in rabbits by administration of an exogenous cationic antigen. The Journal of clinical investigation. 1982;69:451–461. doi: 10.1172/JCI110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JS, Chen A, Chang LC, Chang WS, Lee HS, Lin SH, Lin YF. Mouse model of membranous nephropathy induced by cationic bovine serum albumin: antigen dose-response relations and strain differences. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2004;19:2721–2728. doi: 10.1093/ndt/gfh419. [DOI] [PubMed] [Google Scholar]
- 30.Adler SG, Wang H, Ward HJ, Cohen AH, Border WA. Electrical charge. Its role in the pathogenesis and prevention of experimental membranous nephropathy in the rabbit. The Journal of clinical investigation. 1983;71:487–499. doi: 10.1172/JCI110793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronco P, Debiec H. Advances in membranous nephropathy: success stories of a long journey. Clinical and experimental pharmacology & physiology. 2011;38:410–416. doi: 10.1111/j.1440-1681.2011.05506.x. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Maceda B, Lopez-Bote JP, Langa C, Bernabeu C. Antibodies to dietary antigens in rheumatoid arthritis--possible molecular mimicry mechanism. Clinica chimica acta; international journal of clinical chemistry. 1991;203:153–165. doi: 10.1016/0009-8981(91)90287-m. [DOI] [PubMed] [Google Scholar]
- 33.Winer S, Astsaturov I, Cheung RK, Schrade K, Gunaratnam L, Wood DD, Moscarello MA, O’Connor P, McKerlie C, Becker DJ, et al. T cells of multiple sclerosis patients target a common environmental peptide that causes encephalitis in mice. Journal of immunology. 2001;166:4751–4756. doi: 10.4049/jimmunol.166.7.4751. [DOI] [PubMed] [Google Scholar]
- 34.Feehally J, Farrall M, Boland A, Gale DP, Gut I, Heath S, Kumar A, Peden JF, Maxwell PH, Morris DL, et al. HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol. 2010;21:1791–1797. doi: 10.1681/ASN.2010010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, Sanna-Cherchi S, Men CJ, Julian BA, Wyatt RJ, et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011;43:321–327. doi: 10.1038/ng.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S, Chin HJ, Na KY, Oh J, Chung W, Noh JW, Lee YK, Cho JT, Lee EK, Chae DW. Single nucleotide polymorphisms in the phospholipase A2 receptor gene are associated with genetic susceptibility to idiopathic membranous nephropathy. Nephron Clin Pract. 2011;117:c253–258. doi: 10.1159/000320194. [DOI] [PubMed] [Google Scholar]
- 37.Liu YH, Chen CH, Chen SY, Lin YJ, Liao WL, Tsai CH, Wan L, Tsai FJ. Association of phospholipase A2 receptor 1 polymorphisms with idiopathic membranous nephropathy in Chinese patients in Taiwan. J Biomed Sci. 2010;17:81. doi: 10.1186/1423-0127-17-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38**.Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, et al. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. The New England journal of medicine. 2011;364:616–626. doi: 10.1056/NEJMoa1009742. Report on the significant association of variants in genes encoding M-type phospholipase A2 receptor 1 and HLA-DQ alpha chain 1 in European patients with membranous nephropathy. [DOI] [PubMed] [Google Scholar]
- 39.Bockenhauer D, Debiec H, Sebire N, Barratt M, Warwicker P, Ronco P, Kleta R. Familial membranous nephropathy: an X-linked genetic susceptibility? Nephron Clinical practice. 2008;108:c10–15. doi: 10.1159/000112466. [DOI] [PubMed] [Google Scholar]
- 40.Short CD, Feehally J, Gokal R, Mallick NP. Familial membranous nephropathy. British medical journal. 1984;289:1500. doi: 10.1136/bmj.289.6457.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiryluk K. Risk alleles in idiopathic membranous nephropathy. The New England journal of medicine. 2011;364:2072–2073. doi: 10.1056/NEJMc1103117. author reply 2073–2074. [DOI] [PubMed] [Google Scholar]
- 42.Berg AL, Nilsson-Ehle P, Arnadottir M. Beneficial effects of ACTH on the serum lipoprotein profile and glomerular function in patients with membranous nephropathy. Kidney Int. 1999;56:1534–1543. doi: 10.1046/j.1523-1755.1999.00675.x. [DOI] [PubMed] [Google Scholar]
- 43.Rauen T, Michaelis A, Floege J, Mertens PR. Case series of idiopathic membranous nephropathy with long-term beneficial effects of ACTH peptide 1–24. Clin Nephrol. 2009;71:637–642. doi: 10.5414/cnp71637. [DOI] [PubMed] [Google Scholar]
- 44.Berg AL, Arnadottir M. ACTH-induced improvement in the nephrotic syndrome in patients with a variety of diagnoses. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2004;19:1305–1307. doi: 10.1093/ndt/gfh110. [DOI] [PubMed] [Google Scholar]
- 45.Ponticelli C, Passerini P, Salvadori M, Manno C, Viola BF, Pasquali S, Mandolfo S, Messa P. A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis. 2006;47:233–240. doi: 10.1053/j.ajkd.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 46*.Bomback AS, Tumlin JA, Baranski J, Bourdeau JE, Besarab A, Appel AS, Radhakrishnan J, Appel GB. Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Des Devel Ther. 2011;5:147–153. doi: 10.2147/DDDT.S17521. Preliminary data on the anti-proteinuric effect of natural ACTH on patients with treatment-resistant membranous nephropathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cattran DC, Delmore T, Roscoe J, Cole E, Cardella C, Charron R, Ritchie S. A randomized controlled trial of prednisone in patients with idiopathic membranous nephropathy. N Engl J Med. 1989;320:210–215. doi: 10.1056/NEJM198901263200403. [DOI] [PubMed] [Google Scholar]
- 48.Lindskog A, Ebefors K, Johansson ME, Stefansson B, Granqvist A, Arnadottir M, Berg AL, Nystrom J, Haraldsson B. Melanocortin 1 receptor agonists reduce proteinuria. J Am Soc Nephrol. 2010;21:1290–1298. doi: 10.1681/ASN.2009101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, Leung N, Cohen IM, Wochos DN, Bergstralh E, et al. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73:117–125. doi: 10.1038/sj.ki.5002628. [DOI] [PubMed] [Google Scholar]
- 50**.Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG, et al. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5:2188–2198. doi: 10.2215/CJN.05080610. Largest prospective study to date to evaluate the use of Rituximab in the treatment of membranous nephropathy and with the longest follow-up; also provides the most detailed pharmacokinetic as well as immunological studies in B and T cell subpopulation in these patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cravedi P, Sghirlanzoni MC, Marasa M, Salerno A, Remuzzi G, Ruggenenti P. Efficacy and safety of rituximab second-line therapy for membranous nephropathy: a prospective, matched-cohort study. Am J Nephrol. 2011;33:461–468. doi: 10.1159/000327611. [DOI] [PubMed] [Google Scholar]