Abstract
Background: Gastric cancer is the fourth most common malignancy in the world. Honey is a complex mixture of special biological active constituents. Honey possesses antioxidant and antitumor properties. Nutritional studies have indicated that consumption of honey modulates the risk of developing gastric cancer. On the other hand, apoptosis has been reported to play a decisive role in precancerous changes. Our chief study was conducted to assess the relationship between consumption of honey and apoptosis in human gastric mucosa.
Method: This cross-sectional study was conducted on 98 subjects over 18 years old, referred to two hospitals in Tabriz, Iran. Subjects were undergone an upper gastrointestinal endoscopy, 62 subjects were finally enrolled. Honey consumption was assessed by a Food Frequency Questionnaire (FFQ) and apoptosis was detected by TUNEL technique. We tested polynomial curve to find the best fit between honey consumption and apoptosis.
Results: A positive relation between honey consumption and apoptosis was found (P=0.024). Our results indicated that the final and the best fit curve was: apoptosis = 1.714+1.648(honey amount) - 0.533(honey amount)2 +1.833×10-5(honey amount)7.
Conclusion: Honey consumption had positive effects on gastric cancer by inducing apoptosis in gastric mucosa.
Keywords: Gastric cancer, Apoptosis, Honey, Polynomial curve
Introduction
Prevalence of gastric cancer is high around the world. Although, the prevalence of this cancer in the last 70 years was decreased, it is still the fourth most common malignancy in the world and the second most prevalent cause of cancer-induced death [1]. Incidence of gastric cancer is particularly high in East Asia, Eastern Europe and parts of Central and South America [1]. According to a population-based cancer registry, Iran has the highest rate of stomach cancer among the Middle East countries [2]. Sporadic gastric cancer is the result of genotypic changes due to an adverse environment (that is, diet and Helicobacter pylori) [3-4].
Honey is a food product, which is collected from different plants and processed by honeybees. Honey has been used as a traditional medicine for centuries in different cultures, not only for its dietary value but also for its therapeutic properties. Recently, honey has been approved scientifically for its functional and biological properties including anti-oxidant, anti-inflammatory, anti-bacterial, anti-viral, anti-ulcerous, anti-lipid, and anti-cancer properties [5-11]. These activities are mainly attributed to the phenolic compounds such as flavonoids with antioxidant properties and radical scavenging activities seen among all types of honeys in different proportions, depending on the geographical areas, source of honeybee food and climate [12-14]. Moreover, honey has also been used in palliative care of various cancers like radiation-induced mucositis, radiotherapy and chemotherapy induced skin reactions and wounds [15]. It has also been shown to produce antiproliferative effects in bladder cancer [9], colon cancer [16], mammary carcinoma, and fibrosarcoma [10].
However, to the best of our knowledge, there is no report on antiproliferative or proapoptotic effects of honey on prevention of gastric cancer. Since apoptosis is an early indicator of carcinogenesis in gastric mucosa [17], we assessed cross sectionally the relationship between consumption of honey and apoptosis in gastric mucosa of patients undergoing upper gastrointestinal endoscopy.
Materials and Methods
This cross–sectional study was carried out in Shahid Madani and Imam Reza hospitals of Tabriz University of Medical Sciences, Tabriz, Iran; the north west of Iran during October to December 2008 and the study was approved by the Ethics Committee of Tabriz University of Medical Sciences.
Patients over 18 years old, referred to two main hospitals in Tabriz to undergo an upper gastrointestinal endoscopy were asked to participate in this study. For volunteers, a checklist was filled to understand which exclusion criteria were present. Among 109 volunteers, finally 98 subjects had no exclusion criteria. Participants who had at least one of the exclusion criteria were excluded from the study.
The following were the exclusion criteria: Alcohol consumption, smoking, any type of cancer, gastric surgery, certain cancer syndromes (hereditary nonpolyposis colorectal cancer, familial adenomatous polyposis, Peutz Jeghers syndrome), familial gastric cancer (defined according to the criteria proposed by International Gastric Cancer Linkage Consortium), gastric polyp (detected either in previous or present endoscopy), Menetrier’s disease, Pernicious anemia and non-elective endoscopy.
Participants who had no exclusion criteria were informed about the goals and the procedure of the study and a written informed consent was obtained from all the selected subjects. They were advised that the participant could quit at any time and without providing any reasons.
A skilled gastroenterologist who was completely familiar with the inclusion criteria of the study performed an upper GI endoscopy and when any suspicious lesion or tumor was detected during the endoscopy, immediately the subject was excluded from study. Otherwise, two biopsy samples from the antrum were taken by the physician. One of the two sample was immediately put in formalin 20% and was sent to clinical pathology laboratory for studying by a skilled pathologist and the second sample was kept in buffered formalin 10% and was taken to histology laboratory in Tabriz Faculty of Medicine for detecting apoptosis by TUNEL (Terminal Deoxynucleotidyl Transferas Nick –end labeling) technique.
After endoscopy, the patient was taken to another room to be relaxed and when he/she was ready, a skilled nutritionist completed a demographic and a Food Frequency Questionnaire for patients. Anthropometric measurements were also performed and Body Mass Index (BMI) was calculated as BMI = weight (kg)/stature (m2).
Food frequency questionnaire, developed for Iranian population was applied previously. Frequency of consumption of the total standard portion size included questions about the frequency of honey consumption in 5 categories (never consumed, annually, monthly, weekly, daily) and the amount of consumption was tablespoon and teaspoon.
Pathology and TUNEL technique
Infection with H. pylori affects the rate of apoptosis in gastric mucosa. Approximately 2% of epithelial cells in the normal stomach are apoptotic. In gastritis by H. pylori infection, epithelial proliferation and apoptosis are moderately increased, with approximately 8% apoptotic epithelial cells [18]. On the other hand, infection with H. pylori is common in Iran, and the rate of infection is 69 – 89% in different parts of Iran [19]. We decided to examine apoptosis in patients with H. pylori – induced chronic nonspecific gastritis in antrum, so that all the samples would be in the same condition and the H. pylori infection would be omitted as a confounding factor on apoptosis. Finally, after excluding the subjects who did not have one or more of the above-mentioned requirements, 62 samples entered the final part of the study. Figure 1 shows the flow chart of the participants of the study. Apoptosis was assessed by the terminal deoxynucleotidyl transferase -mediated deoxyuridine triphosphate end labeling (TUNEL) method.
In this phase, sections (4 μm thick) were cut from paraffin-embedded blocks and mounted on microscope slides. The slides were then incubated in 37°C for 36 h before being deparaffinized and rehydrated and after the sections were deparaffinized, they were rehydrated in a graded alcohol series, and incubated with proteinase K (Roche diagnostics, Germany) in 37°C for 30 min. TUNEL solution (Roche diagnostics, Germany) was prepared according to the instruction manual and the samples were incubated with this solution for 1 h in 37°C. After washing, POD (peroxidase) solution (Roche diagnostics, Germany) was added and the slides were incubated for another 30 min in 37°C. Then (di-amino banzidil) DAB solution (Roche diagnostics, Germany) was added and after 15 min in room temperature, the slides were washed and counter stained with methylene blue. The prepared slides were examined under light microscopy (Nikon x 40). The number of apoptotic cells was counted in 10 high power fields (HPFs) for each slide and the mean number of apoptotic cells for each section was calculated (According to kit instruction).
Statistical Analysis
For all continuous variables, normality was tested by Q-Q test. All values are expressed as means ± SE at each time interval. Stepwise regression was exploited to assess the relationship between apoptosis rate and consumption of honey. The level of significance was set at P≤0.05. Data was analyzed by SPSS version 16.00.
Results
Sixty-two subjects were finally enrolled in this study. The mean age was 43.60 ± 1.67 years. The youngest participant was 21 and the oldest was 65 years old with the same ethnic group (Iranian Turk), and 52.3% of the participants were female. Mean BMI was 25.31 ± 0.45 kg/m2. The lowest calculated BMI was 17.86 and the highest was 34.29 kg/m2. The mean honey consumption was 4.2 ± 0.11 g/day and the mean for apoptosis in gastric mucosa was 2.15 ± 0.22 cells.
We used stepwise regression technique and tested polynomial curve (e.g. Y = a + b1x1 + b2x2 + b3x3 +…..+ bmxm for m = 1, 2, 3 …) with different digress to find better fitting curve for showing complete relationship between honey consumption and apoptosis. We increased digress of polynomial curve from one to …, with increased coefficient R in different models. Finally, the polynomial curve with seven digress and large R= 0.29 was accepted and the reminding components in this model were only one, two and seven digress and after removing other components, our final result was apoptosis =1.714+1.648 (honey amount)-0.533(honey amount)2+1.833×10-5(honey amount)7 (Table 1) and apoptotic cells is shown in Fig. 1.
Table 1. Determination better polynomial curve for finding completed relationship between honey consumption and apoptosis.
| Digress of polynomial | R coefficient | Reminding component | B coefficient | SE | P |
| Constant | 1.714 | 0.318 | 0.000 | ||
| 1 | 1.648 | 0.703 | 0.024 | ||
| 7 | 0.293 | 2 | -0.533 | 0.233 | 0.031 |
| 7 | 1.833×10-5 | 0.000 | 0.046 |
Fig. 1.
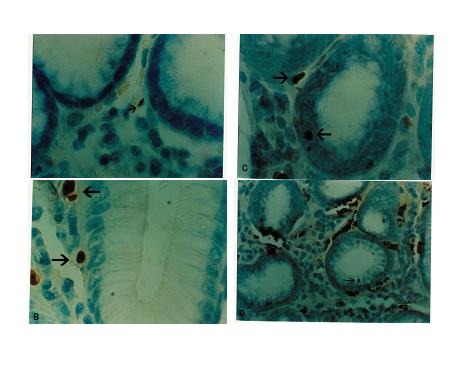
Apoptosis in human gastric mucosa .The arrows show apoptotic cells
Discussion
Epidemiological surveys and experimental studies have provided evidence that environmental factors, including dietary substances, play a major role in the incidence of cancer [20]. Honey, a part of traditional medicine, has recently become the focus of attention for treating certain diseases as well as promoting overall health and well-being. It has been reported that honey contains many phenolic compounds, which act as antioxidants and exhibit anti-carcinogenic, anti inflammatory, antimicrobial, anti-atherogenic, anti-thrombotic, immune modulating and analgesic activities [21-22].
Since, this is the first study to report the proapoptotic activity of honey on gastric mucus, in this study; we investigated the relationship between honey consumption and apoptosis in human gastric mucosa. No study has been found to show antiproliferative or proapoptotic effects of honey on prevention of gastric cancer. Most of the previous studies on honey have focused on its anti-microbial and wound healing properties [23], only a small number of papers have looked into the anticarcinogenic properties of honey. The apoptosis inducing effect of honey observed in the present investigation was similar to the effect of some studies reported previously. Honey had revealed moderate antitumor and pronounced antimetastatic effects [24]. Besides, honey was also shown to potentiate the antitumor activity of 5-fluorouracil and cyclophosphamide. In a study [20], the authors illustrated the apoptosis effect of honey. They showed honey induced apoptosis in human colon cancer cells by arresting the cells at subG1 phase. Furthermore, another study [25] showed that honey had very effective agents for repressing the growth of bladder cancer cell lines (T24, RT4, 253J, and MBT- 2) in vitro. Antitumor properties of honey could be attributed to flavonoids and phenolic acids [26-27], certain enzymes (glucose oxidase, catalase), ascorbic acid [28], carotenoid-like substances [29], organic acids [26], amino acids, and proteins [30] in the honey.
In the present study, we confirmed that there was a positive relationship between honey consumption and apoptosis in gastric mucosa. This result showed that the rate of apoptosis in gastric mucosa could be changed by different consumption of honey. Results of this study may provide some additional clues about the mechanisms through which consumption of honey may alter the process of gastric carcinogenesis. Considering the high prevalence of this cancer in some parts of the world including Iran, these findings will help planning and supporting dietary habits which will result in reducing the prevalence of this malignancy, which is usually detected in its final stages when little can be done for the patient. The advantage of this study was using polynomial curve for finding the complete relationship between apoptosis and amount of honey consumption. The concurrency of analysis in this study minimized the probability of false positive and negative results.
Our study has several limitations, because cell proliferation and cell death are two sides of a coin, both of which influence the process of carcinogenesis assessing cell proliferation in addition to apoptosis rate could have strengthened our study. Furthermore, in spite of the fact that TUNEL is a very well known method for detecting apoptosis, it suffers from some shortcomings. TUNEL will stain necrotic cells as well as apoptotic cells and on the other hand, apoptosis can be induced, although rarely, without extensive deoxynucleotidil transferase (DNA) degradation that is the phenomena detected by TUNEL, therefore apoptosis detection exploiting TUNEL has false positives and false negatives. Moreover, the length of time that tissue is left before fixation can also affect the results of TUNEL. Therefore, if a complementary method were added for detection of apoptosis, the results would have been more reliable. Another limitation is that the actual composition of honey varies, depending on many factors such as the pollen source, climate, environmental conditions, and the processing it undergoes.
Conclusions
Our study confirmed that the consumption of honey could induce apoptosis in human gastric mucosa. As apoptosis is one of the effective components in the process of gastric cancer, it is worth designing more elaborate prospective and interventional studies to examine these findings more specifically. Studies performed under more controlled conditions such as cell culture studies, interventional studies with different dosage of honey to study the exact molecular mechanism behind the antitumor activity of honey, measuring rate of gastric cell proliferation and finding the best method for detecting apoptosis will also be very helpful in determining the exact effect of honey on apoptosis and gastric cancer.
Acknowledgments
This study was supported by Tabriz University of Medical Sciences, Nutrition Research Center, Liver, and Gastrointestinal Disease Research Center. We thank the participants of this study for their enthusiastic support. The authors would also like to thank Mohammad Hossein Somi and Leila Roshangar for helping in this study. The authors declare that there is no conflict of interests.
References
- [1].Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Bio. 2009;472:467–77. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- [2].Mohagheghi MA, Mosavi-Jarrahi A, Malekzadeh R, Parkin M. Cancer incidence in Tehran Metropolos: the first report from the Tehran population-based cancer registry,1998-2001. Arch Iranian Med. 2009;12(1):15–23. [PubMed] [Google Scholar]
- [3].Nardone G, Compare D. Epigenetic alterations due to diet and Helicobacter pylori infection in gastric carcinogenesis. Expert Rev Gastroenterol Hepatol. 2008;2:243–8. doi: 10.1586/17474124.2.2.243. [DOI] [PubMed] [Google Scholar]
- [4].Liu H, Merrell DS, Semino-Mora C, Goldman M, Rahman A. Diet synergistically affects helicobacter pylori induced gastric carcinogenesis in nonhuman primates. Gastroenterology. 2009;137:1367–79. doi: 10.1053/j.gastro.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Irish J, Carter DA, Blair SE, Heard TA. Antibacterial activity of honey from the Australian stingless bee Trigona carbonaria. Int J Antimicrob Agents. 2008;32(1):89–90. doi: 10.1016/j.ijantimicag.2008.02.012. [DOI] [PubMed] [Google Scholar]
- [6].Temaru E, Shimura S, Amano K, Karasawa T. Antibacterial activity of honey from stingless honeybees (Hymenoptera; Apidae; Meliponinae) Pol J Microbiol. 2007;56(4):281–285. [PubMed] [Google Scholar]
- [7].Estevinho L, Pereira AP, Moreira L, Dias LG, Pereira E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem Toxicol. 2008;46(12):3774–3779. doi: 10.1016/j.fct.2008.09.062. [DOI] [PubMed] [Google Scholar]
- [8].Wang XH, Andrae L, Engeseth NJ. Antimutagenic effect of various honeys and sugars against Trp-p-1. J Agric Food Chem. 2002;50(23):6923–6928. doi: 10.1021/jf025641n. [DOI] [PubMed] [Google Scholar]
- [9].Swellam T, Miyanaga N, Onozawa M, Hattori K, Kawai K. Antineoplastic activity of honey in an experimental bladder cancer implantation model: in vivo and in vitro studies. Int J Urol. 2003;10(4):213–219. doi: 10.1046/j.0919-8172.2003.00602.x. [DOI] [PubMed] [Google Scholar]
- [10].Orsolic N, Terzic SSL, Basic I. Honey-bee products in prevention and/or therapy of murine transplantable tumours. J Sci Food Agric. 2005;85:363–370. [Google Scholar]
- [11].Boukraa L, Amara K. Synergistic effect of starch on the antibacterial activity of honey. J Med Food. 2008;11(1):195–198. doi: 10.1089/jmf.2007.502. [DOI] [PubMed] [Google Scholar]
- [12].Beretta G, Orioli M, Facino RM. Antioxidant and radical scavenging activity of honey in endothelial cell cultures (EAhy926) Planta Med. 2007;73(11):1182–1189. doi: 10.1055/s-2007-981598. [DOI] [PubMed] [Google Scholar]
- [13].Viuda-Martos M, Ruiz-Navajas Y, Fernandez-Lopez J, Perez-Alvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008;73(9):R117–124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- [14].Hegazi AG, Abd El-Hady FK. Influence of Honey on the Suppression of Human Low Density Lipoprotein (LDL) Peroxidation (In vitro)Evid Based Complement Alternat. Med. 2009;6(1):113–121. doi: 10.1093/ecam/nem071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bardy J, Slevin NJ, Mais KL, Molassiotis A. A systematic review of honey uses and its potential value within oncology care. J Clin Nurs. 2008;17(19):2604–2623. doi: 10.1111/j.1365-2702.2008.02304.x. [DOI] [PubMed] [Google Scholar]
- [16].Rao CV, Desai D, Rivenson A, Simi B, Amin S, Reddy BS. Chemoprevention of colon carcinogenesis by phenylethyl-3-methylcaffeate. Cancer Res. 1995;55(11):2310–2315. [PubMed] [Google Scholar]
- [17].Ikeda M, Shomori K, Endo K, Makino T, Matsuura T, Ito H. Frequent occurrence of apoptosis is an early event in the oncogenesis of human gastric carcinoma. Virchows Arch. 1998;432:43–47. doi: 10.1007/s004280050132. [DOI] [PubMed] [Google Scholar]
- [18].Herbay A, Rudi J. Role of apoptosis in gastric epithelial turnover. Microsc Res Tech. 2000;48:303–311. doi: 10.1002/(SICI)1097-0029(20000301)48:5<303::AID-JEMT7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- [19].Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric Cancer in Iran: Epidemiology and Risk Factors. Arch Iran Med. 2009;12:576–583. [PubMed] [Google Scholar]
- [20].Jaganathan SK, Mandal M. Honey constituents and its apoptotic effect in colon cancer cells. J Api Product Api Medical Sci. 2009;1:29–36. [Google Scholar]
- [21].Gomez-Caravaca AM, Gomez-Romero M, Arraez-Roman D, Segura-Carretero A, Fernandez-Gutierrez A. Advances in the analysis of phenolic compounds in products derived from bees. J Pharm Biomed Anal. 2006;41(4):1220–1234. doi: 10.1016/j.jpba.2006.03.002. [DOI] [PubMed] [Google Scholar]
- [22].Simon A, Traynor K, Santos K, Blaser G, Bode U. Medica honeys for wound care–still the ‘latest resort’? Evid Based Complement Alternat. Med. 2009;6(2):165–173. doi: 10.1093/ecam/nem175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Owen A Moore, Lesley A Smith, Fiona Campbell, Kate Seers, Henry J McQuay, R Andrew Moore. Systematic review of the use of honey as a wound dressing. BMC Complementary and Alternative Medicine 2001; 1:2. Abdulmlik A Ghashm, Nor H Othman, Mohammed N Khattak, Noorliza M Ismail, Rajan Saini. Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complementary and Alternative Medicine 2010; 10:49. [DOI] [PMC free article] [PubMed]
- [24].Tarek S, Naoto M, Mizuki O. Antineoplastic activity of honey in an experimental bladder cancer implantation model: in vivo and in vitro studies. Int J Urol. 2003;10:213–219. doi: 10.1046/j.0919-8172.2003.00602.x. [DOI] [PubMed] [Google Scholar]
- [25].Cherchi L, Spanedda C, Tuberoso Cabras P. Solidphase extraction and HPLC determination of organic acid in honey. J Chromatogr. 1994;669:59–64. [Google Scholar]
- [26].Davies MC, Harris RG. Free amino acid analysis of honeys from England and of the geographical origin of honeys. J Apicultural Res. 1982;21:168–173. [Google Scholar]
- [27].Saravana Kumar Jaganathan, Mahitosh Mandal. Antiproliferative Effects of Honey and of Its Polyphenols: A Review. J of Biomed and Biotech. (2009);10: 1155. doi: 10.1155/2009/830616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tan ST, Wilkins AL, Holland PT, McGhie TK. Geographical discrimination of honeys though the employment of sugar patterns and common chemical quality parameters. J Agric Food Chem. 1989;37:1217–1221. [Google Scholar]
- [29].White JW. The protein content of honey. J Apicultural Res. 1978;17:234–238. [Google Scholar]


