Abstract
Background: Type 2 diabetes mellitus, as a noncommunicable disease, is the main public health challenge in the 21st century. The prevalence of diabetes mellitus adjusted for the world population in Iran was 8% until the year 2010.Lipid levels are considered as important parameters to be evaluated, as high serum lipid levels are often reported as a complication in patients with diabetes mellitus.It is claimed that functional foods may improve complications of diabetes mellitus, so this study was designed to evaluate the effects of high performance inulin on glycemic status and lipid profile of women with type 2 diabetes.
Methods: The study was a randomized controlled clinical trial. Forty-nine type 2 diabetic females (fiber intake <30g/d, 25<BMI<35 kg/m2) were divided into two groups. Patients in the intervention group (n=24) received 10g/d inulin and patients in the control group (n=25) received 10g/d maltodextrin for 8 weeks.Glycemic status and lipid profile indices were measured pre and post intervention. Data were analyzed using SPSS software (verision11.5). Paired, unpaired t-test and ANCOVA were used to compare quantitative variables.
Results: Supplementation with inulin caused a significant reduction in FBS (8.50%), HbA1c (10.40%), total cholesterol (12.90%), triglyceride (23.60 %), LDL-c (35.30 %), LDL-c/HDL-c ratio (16.25%) and TC/HDL-c ratio (25.20%) and increased HDL-c (19.90%). The changes for the control group parameters were not significant at the end of study.
Conclusion: Inulin may help to control diabetes and its complications via improving glycemic and lipid parameters.
Keywords: Inulin, Glycemic status, Lipid profile, Type 2 diabetes
Introduction
Diabetes mellitus (DM) is considered as a non-communicable disease and main public health challenge in developing and developed countries1. The prevalence of diabetes was 171 million in the year 2000. The total number of diabetic patients will be 366 million by the year 20302. The prevalence of DM adjusted for the world population in Iran was 8%, and its health expenses was approximately 600 million US dollars in the year 20103. Diabetes mellitus is associated with insulin resistance,hyperinsulinemia, hyperglycemia and biochemical alterations in lipid metabolism4. Cardiovascular disease (CVD) is the primary cause of death in type 2 diabetes5. The relative risk for CVD is 2 to 4 fold higher in DM than in nondiabetic patients6. Dyslipidemia has been known as a risk factor for cardiovascular complications in type 2 DM. The typical lipid abnormalities in type 2 diabetic adults comprise low levels of HDL cholesterol, high levels of triglycerides and a dominance of small, dense LDL particles7.
Recently, functional foods are considered in the management of type 2 diabetes and its complications8. Inulin-type fructans are a group of functional foods, which naturally found in leek, wheat, onion, chicory root garlic, and banana. Inulin-type fructans are group of carbohydrate, which is classified as nonviscous, soluble and fermentable fibers. High performance inulin (HP Inulin) is a prebiotic with long-chain; high-molecular weight mixes of inulin-type fructans. This type of inulin is incorporated as sugar and /or fat substitute into drinks and desserts, baked goods, and milk products. Intake of 5-8 g/d inulin should be sufficient to make a positive effect on the gut microflora. Possible side effect of inulin-type fructansintake is gut discomfort due to gas production, reported at doses >20g/d9.
In animal studies, inulin-type fructans can reduce blood glucose and lipid profile, especially triglycerides10,11. According to our knowledge, all human studies have evaluated the effects of fructooligosaccharides on diabetic patients and there is no study to evaluate the impact of inulin HP in these patients12-14. The results obtained from several studies on the effects of fructooligosaccharides in diabetic patients are different. One study showed positive effects of oligofructose on blood glucose and lipid profile in diabetic patients12. In contrast, some other studies reported no effects for inulin-type fructans supplementation in diabetic patients13,14. It has been suggested to design further randomized controlled trials (RCT) in order to determine the effects of inulin-type fructans, inulin, and oligofructose on blood glucose 15 and lipid profile16 in humans.
The present trial was conducted to assess the effects of HP inulin on serum glucose indices and lipid profile in women with type 2 diabetes.
Materials and Methods
Subjects
In this trial, sixty- five DM females aged between 20-65 yr were voluntarily recruited from Iran Diabetes Society and Endocrinology as well as Metabolism clinics of Tabriz University of Medical Sciences. Inclusion criteria were: having DM > 6 months, using anti-diabetic drugs, normal diet and Body Mass Index (BMI) >25 kg/m2 during and in the last 3 months. Diabetes mellitus was defined as a fasting glucose ≥126 mg/dl17. Patients were excluded if they had history of gastrointestinal, cardiovascular, renal, thyroid, liver, pancreatic diseases, being pregnant or lactating, consuming pre or probioticsُ products during and 2 weeks prior to the intervention, antibiotics, antacids, alcohol, anti-diarrheal, anti-inflammatory, lipid-lowering, laxatives drugs and individual with a typical fiber intake >30g. At the beginning of trial, demographic data including age, drugs, diabetes duration (years) were collected using a questionnaire. The Ethics Committee of the Tabriz University of Medical Sciences provided ethical approval for the trial, and written informed consent was obtained from each patient. The approval of trial was registered on the Iranian Registry of Clinical Trials website (www.irct.ir/, IRCT201110293253N4).
Experimental design
Participants were randomly divided into 2 groups using a block randomization procedure based on BMI and age. Intervention group received 10 g/d inulin HP supplement (Sensus, Borchwef 3, 4704 RG Roosendaal the Netherlands) and control group received similar amounts of maltodextrin as placebo (Jiujiang Hurirong Trade CO., LTD, China (mainland)) for 8 weeks. Both maltodextrin and inulin have similar taste and appearance18, which were provided to subjects in similar opaque packages. Patients received half of packages at the beginning and the remaining at 4th week of the study. In order to minimize withdrawal and ensure to consumption of supplements, the patients received a phone call every week. Throughout the trial, subjects were asked to have usual physical activity and diet.
Anthropometric and dietary intake assessment
Anthropometric indices including body weight and height were measured at baseline and at the end of the trial. Dietary intakes were evaluated using a 3-day food dairy (two usual days and one weekend day) at baseline and at the end of the trial. Before the intervention, all patients were provided instructions how to use food scale and record their food intake. After recording day, each patient received a phone call for renewed recording food intake by trained person. Dietary intakes were analyzed using the nutritionist 4 software (First Databank Inc., Hearst Corp., San Bruno, CA) containing the database from tables of content and nutritional value of Iranian food products.
Biochemical indices
At baseline and at the end of trial, after an overnight fasting 10 ml venous blood samples were collected and transferred into two vacutainer tube, one containing EDTA for measurement of blood HbA1c and the other containing sodium fluoride for glucose and lipid profile. Serum samples were separated from whole blood by centrifugation at 2500 rpm for 10 min (Beckman Avanti J-25; Beckman Coulter, Brea, CA) at room temperature. All parameters were analyzed on the day of sampling. FBS concentration was measured by the enzymatic methods using an Abbot Model Aclyon 300, USA auto analyzer with kits from Pars-Azmone (Tehran, Iran). Glycosylated hemoglobin (HbA1c) was determined in whole blood using an automated high performance liquid chromatography analyzer with commercially Bio-Rad D-10 Laboratories, Schilti-gheim, France kit.
The levels of serum total cholesterol (TC), high-density lipoprotein (HDL-c) and triglyceride (TG) were measured by enzymatic colorimetric methods with commercial kits (Cholesterol CHOD-PAP and Triglycerides GPO-PAP; Pars-Azmone, IRI) on an automatic analyzer (Abbott, model Alcyon 300-USA)19. Serum Low-density lipoprotein (LDL-c) was calculated according to the Friedewald equation20. Since the TC/HDL-C and LDL-C/HDL-C ratios determine the relative risk of coronary artery disease, they were also calculated in this trail21.
Statistical Analyses
Data were analyzed using SPSS software version 11.5 (SPSS Inc., Chicago,IL, USA). The results were expressed as mean ± standard deviation. The normality of the distribution of data was evaluated by the one-sample Kolmogorov-Smirnov test. Paired, independent t test and ANCOVA were used to compare numerical data. Drugs used in two groups were compared using the Mann-Whitney U test. Analysis of covariance was used to identify any differences between the two groups post intervention, adjusting for baseline measurements and covariates. For calculating mean changes of markers between groups, first mean changes of markers from baseline were calculated by [(8wk values-baseline values) / baseline values)] * 100. Then, mean changes of markers between groups were calculated. P<0.05 were considered to be statistically significant.
Results
From 65 subjects recruited, 49 subjects completed the trial (n= 24 in the intervention group; n=25 in the control group; (Fig. 1). As shown in Table 1, initial characteristics were similar at baseline in both groups.
Fig. 1.
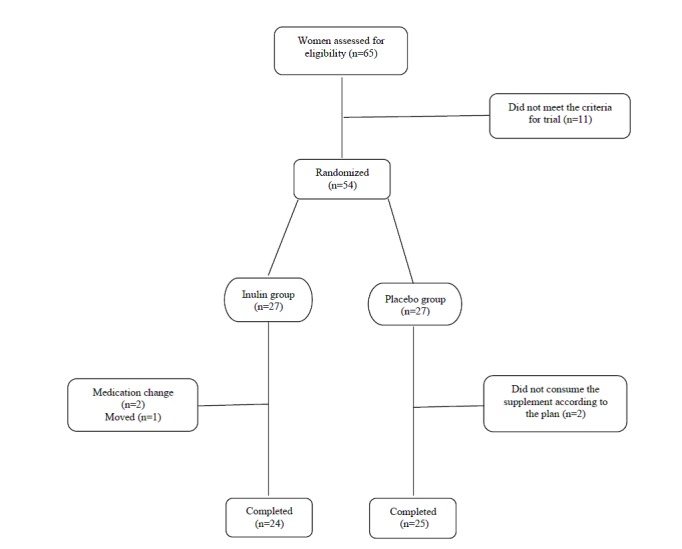
Trial design implemented in this study
Table1. Baseline characteristics of trial patients1 .
| Characteristics |
Control
group (n=25) |
Intervention
group (n=24) |
| Age (yr) | 48.70 ± 9.70 | 47.80 ± 10.10 |
| Weight (kg) | 70.50 ±11.05 | 75.45 ± 11.30 |
| Height (cm) | 153.50 ± 6.50 | 154.40 ± 5.80 |
| BMI (kg/m2) | 29.90 ± 4.20 | 31.60 ± 4.09 |
| Diabetes duration (yr) | 5.30 ± 4.60 | 7.30 ± 5.40 |
| Metformin 500 mg (tablets/d) | 2.70 ± 0.90 | 2.85 ± 1.08 |
| Glibenclamide 5mg (tablets/d) | 1.90 ±1.20 | 2.35 ± 0.99 |
BMI: body mass index. 1Rsults are presented as means ± standard deviation. / For all Characteristics, there were no significant differences between Maltodextrin and Inulin groups (all Non significant, based on independent samples t tests)
Effect of inulin supplementation on anthropometric indices and dietary intakes
As shown in Table 1, there was no significant difference at baseline body weight and BMI between two groups. Two groups did not show significant difference in baseline dietary intakes, except for dietary fiber which was significantly higher in control group than in the intervention group (data not shown, presented in another paper22). Post intervention, body weight and BMI were significantly decreased in inulin group (75.45 ± 11.35 to 72.85 ± 11.20 kg, 31.60 ± 4.09 to 30.50 ± 4.02 kg/m2, respectively; P<0.05) while they remained unchanged in maltodextrin group. These changes were significant in inulin group compared to baseline ( P<0.05). The results for dietary assessment are presented in detail in another paper22.
Briefly, there was no significant difference between groups regarding to intakes of energy, carbohydrate and total fat at the end of trial. The intake of energy and macronutrients remained unchanged in control group, while intake of energy and total fat decreased significantly in intervention group22.
Effects of inulin supplementation on fasting blood sugar and lipid profile
At the beginning of trial, we did not observe a significant difference between intervention and control groups in FBS, HbA1c and lipid profile (Table 2). At the end of trial, there was a significant decrease in FBS (8.50%), HbA1c (10.40%), TC (12.90%), TG (23.60 %), LDL-c (35.30 %), LDL-c/HDL-c ratio (16.25%) and TC/HDL-c ratio (25.20%) in the intervention group compared with the control group ( P<0.05). Inulin supplementation caused a 19.90% increase in HDL-c, compared with the control group after adjusting for dietary intakes and baselines values ( P<0.05). In the control group FBS, HbA1c, TC, TG, LDL-c, HDL-c, TC/HDL-c ratio and LDL-c/HDL-c ratio were not significantly changed at the end of trail.
Table 2. The effects of 8 weeks of inulin supplementation on anthropometrics indices, FBS and lipid profile during trial1 .
| Variables | Period |
Control
group (n=25) |
Intervention
group (n=24) |
| FBS (mg/dl) | Initial | 157.80 ± 10.60 | 161.70 ± 15.10 |
| End | 156.10 ± 14.20 | 146.60 ± 19.90a ,b | |
| HbA1c (%) | Initial | 8.20 ± 0.90 | 8.40 ± 0.90 |
| End | 8.30 ± 1.09 | 7.70 ± 0.70 a ,b | |
| TC (mg/dl) | Initial | 197.90 ± 37.80 | 192.50 ± 42.80 |
| End | 203.10 ± 45.60 | 171.00 ± 39.70 a, b | |
| TG (mg/dl) | Initial | 213.10 ± 68.10 | 223.30 ± 84.20 |
| End | 216.80 ± 59.80 | 169.95 ± 65.60 a, b | |
| LDL-c (mg/dl) | Initial | 114.60 ± 35.30 | 110.60 ± 40.90 |
| End | 116.30 ± 42.96 | 89.60 ± 41.40 a, b | |
| HDL-c (mg/dl) | Initial | 40.60 ± 5.65 | 37.20 ± 6.05 |
| End | 43.50 ± 4.20 | 47.40 ± 7.65a, b | |
| TC / HDL-c | Initial | 4.90 ± 0.90 | 5.30 ± 1.40 |
| End | 4.70 ± 0.10 | 3.70 ±1.09a, b | |
| LDL-c / HDL-c | Initial | 2.80 ± 0.80 | 3.00 ± 1.20 |
| End | 2.70 ± 0.95 | 1.95 ± 0.10a, b |
BMI: body mass index; FBS: fasting blood sugar; TC: total cholesterol; TG: triglyceride; HDL-c: high-density lipoprotein; LDL-c: low-density lipoprotein. / 1Values are presented as mean ± standard deviation. aP<0.05, paired t test.b P<0.05 analysis of covariance adjusted for dietary intakes and baseline values.
Discussion
Beneficial effects of high fiber diets on prevention and management of diabetes are claimed23. Therefore, we assayed the effects of inulin on glycemic status and lipid profile in type 2 diabetic patients. Our results showed that the inulin supplementation significantly decreased body weight and BMI, FBS and HbA1c in intervention group compared to control group. In addition, we observed inulin consumption caused a significant decrease in TC, TG, LDL-c, TC/HDL-c ratio and LDL-c/HDL-c ratio in intervention compared to control group. The inulin supplementation significantly increased HDL-c compared to the control group.
Pourmoradian et al. reported that royal jelly supplementation significantly decreased the mean body weight (72.45 ± 4.42 vs. 71.00 ± 6.44 kg)24. The addition of royal jelly improves the growth of L. acidophilus and B. bifidum. Probably, royal jelly supplementation may decrease weight via microfloura change25.
Parnell et al. reported oligofructose at a dose of 21 g/day for 12 weeks decreased body weight in healthy adults18. We have showed that supplementation with inulin significantly decreased energy intake of the intervention group (1693.60 ± 250.60 to 1417.90 ± 236.70 kcal/day, P<0.05)22. The mechanism(s) of weight reduction by inulin is not fully understood. Probably, some gut hormones such as GLP-1, PYY, and ghrelin are involved in weight reduction caused by inulin18.
Only three studies investigated the effects of fructans on glucose, insulin and lipid profile in type 2 DM12-14. Yamashita et al showed that oligofructose (OFS) supplementation ( 8 g/day for 2 weeks) decreased fasting blood glucose, TC and LDL-c in type 2 DM patients12. Chen et al. demonstrated that glucomannan, as a prebiotic, at a dose of 3.6 g/day for 4 weeks, reduced fasting blood glucose, TC and LDL-c in type 2 DM patients26. Jackson et al. showed that prebiotic supplementation (10 g/d inulin for 8 weeks in individuals with mild hyperlipidaemia) decreased fasting insulin and TG27. Introducing of inulin-enriched pasta to healthy young volunteers showed a significant decrease in glycemic status and improved lipid profile28. Luo et al. and Alles et al. did not find significant changes with OFS supplementation (20g/d for 4 weeks, 15g/d for 3 weeks, respectively), on FBS and lipid profile in type 2 DM patients13,14. Additionally, the finding of increased HDL-c concentrations is in agreement with data previously obtained in vitro29. In our trial, the TC: HDL-c ratio and LDL-c/HDL-c ratio, as atherogenic indices were significantly reduced in the intervention group compared with the control group. It is indicated that these ratios CVD were better than either serum TC or LDL-c30.
The difference in results obtained in several studies may be due to pathologic state and basal levels of fasting blood sugar and lipid profile in type 2 DM patients and type and dose of supplementation.
Hypoglycemic effect of fibers can be explained by several mechanisms. Inulin can control the level of serum glucose by decreasing the post-meal rise of serum glucose and delaying entry of glucose into blood and retarding gastric emptying31. Furthermore, modification of the gut hormones such as glucagon-like peptide1 (GLP-1)32, short- chain fatty acids (SCFA) which are produced from colonic fermentation of prebiotics33,34 and reduction in the body weight and BMI35 can affect glucose metabolism in the body. SCFA can delay gastric emptying33. Oligofructose can improve glucose metabolism by rising of plasma insulin, β cell mass, pancreatic insulin, GLP-136 and GLP-237.
Based on what is already known about the properties of inulin- fructans, it seems that beneficial effects of inulin on lipid profile mainly mediated by short chain fatty acids (SCFA). Butyrate inhibits liver cholesterol synthesis and provides a source of energy for human colon epithelial cells38. Acetate may act as precursor for cholesterol synthesis, while propionate could inhibit hepatic cholesterol synthesis by decreasing the use of acetate as a precursor of cholesterol39. Rossi et al. found that butyrate was the major fermentation product from inulin40. Reduction of plasma TG may be resulted from decreased hepatic lipogenic capacity41, a reduction in the hepatic lipogenic enzyme gene expression such as acetyl -COA carboxylase, malic enzyme, ATP citrate lyase, glucose6- phosphate, 1-dehydrogenase and fatty acid synthase, increase triacylglycerol-rich lipoprotein catabolism10,42. Glucose and insulin are main stimulator in the control of lipogenesis, reduction in fasting blood sugar and insulin levels can result in reduction lipogenesis43. Moreover, prebiotics may contribute to cholesterol reduction by increasing fecal bile acid excretion38, reducing in intestinal cholesterol absorption by increasing the thickness of the unstirred layer in the small intestine44, increase in the expression of 3-hydroxy-3-methylglutaryl-COA reductase (HMG-COA reductase) and increase in the sterol regulatory element-binding proteins42.
Our trial had some limitations, including duration of the intervention, which seems to be short, lack of measurement of serum SCFA, serum free fatty acids and plasma apolipoprotein.
Conclusion
Inulin supplementation may control levels of glycemic status and improve lipid profile in type 2 diabetic patients. These findings support the use of inulin as a safe treatment for managing diabetes. Our findings must be scrutinized in further clinical trials.
Acknowledgments
The authors would like to thank all of the patients, Mr. Firuz Purrahim and Mr. Amir M. Vatankhah. This research was financially supported by Health and Nutrition Faculty, Nutrition Research Center and Vice Chancel-lor for Research of Tabriz University of Medi-cal Sciences, Iran. This article was written based on the data for PhD thesis on nutrition, registered in Tabriz University of Medical Sciences.
Competing interests
The authors declare that there is no conflict of
interests.
Citation: Dehghan P, Pourghassem Gargari B, Asgharijafarabadi M. Effects of High Performance Inulin Supplementation on Glycemic Status and Lipid Profile in Women with Type 2 Diabetes: A Randomized, Placebo- Controlled Clinical Trial. Health Promot Perspect 2013; 3(1): 55-63
References
- 1.Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J. et al. The burden of mortality attributable to diabetes: Realistic estimates for the year 2000. Diabetes Care. 2005;28:2130–2135. doi: 10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Golozar A, Khademi H, Kamangar F, Poutschi H, Islami F, Abnet CC. et al. Diabetes mellitus and its correlates in an Iranian adult population. PLoS One. 2011;6:e26725. doi: 10.1371/journal.pone.0026725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tripathi BK, Srivastava AK. Diabetes mellitus: complications and therapeutics. Med Sci Monit. 2006;12:RA130–47. [PubMed] [Google Scholar]
- 5.Ali MK, Narayan KMV, Tandon N. Diabetes & coronary heart disease: Current perspectives. Indian J Med Res. 2010;132:584–597. [PMC free article] [PubMed] [Google Scholar]
- 6.Ray A, Huisman MV, Tamsma JT. Research and Writing-group; van Asten J, Bingen BO, Broeders EA. et al. The role of inflammation on atherosclerosis, intermediate and clinical cardiovascular endpoints in type 2 diabetes mellitus. Eur J Intern Med. 2009;20:253–260. doi: 10.1016/j.ejim.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 7. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–3421. [PubMed]
- 8.Ballali S, Lanciai F. Functional food and diabetes: a natural way in diabetes prevention? Int J Food Sci Nutr. 2012;63 Suppl1:51–61. doi: 10.3109/09637486.2011.637487. [DOI] [PubMed] [Google Scholar]
- 9.Kolida S, Gibson GR. Prebiotic capacity of inulin-type fructans. J Nutr. 2007;137(11 Suppl):2503S–2506S. doi: 10.1093/jn/137.11.2503S. [DOI] [PubMed] [Google Scholar]
- 10.Agheli N, Kabir M, Berni-Canani S, Petitjean E, Boussairi A, Luo J. et al. Plasma lipids and fatty acid synthase activity are regulated by short-chain fructo-oligosaccharides in sucrose-fed insulin-resistant rats. J Nutr. 1998;128:1283–1288. doi: 10.1093/jn/128.8.1283. [DOI] [PubMed] [Google Scholar]
- 11.Busserolles J, Gueux E, Rock E, Demigne C, Mazur A, Rayssiguier Y. Oligofructose protects against the hypertriglyceridemic and prooxidative effects of a high fructose diet in rats. J Nutr. 2003;133:1903–1908. doi: 10.1093/jn/133.6.1903. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita K, Kawai K, Itakura M. Effects of fructooligosaccharides on blood glucose and serum lipids in diabetic subjects. Nutr Res. 1984;4:961–966. [Google Scholar]
- 13.Luo J, Van Yperselle M, Rizkalla SW, Rossi F, Bornet FR, Slama G. Chronic consumption of short-chain fructooligosaccharides does not affect basal hepatic glucose production or insulin resistance in type 2 diabetics. J Nutr. 2000;130:1572–1577. doi: 10.1093/jn/130.6.1572. [DOI] [PubMed] [Google Scholar]
- 14.Alles MS, de Roos NM, Bakx JC, van de Lisdonk E, Zock PL, Hautvast GA. Consumption of fructooligosaccharides does not favorably affect blood glucose and serum lipid concentrations in patients with type 2 diabetes. Am J Clin Nutr. 1999;69:64–69. doi: 10.1093/ajcn/69.1.64. [DOI] [PubMed] [Google Scholar]
- 15.Bonsu NKA, Johnson CS, Mcleod KM. Can dietary fructans lower serum glucose? J Diabetes. 2011;3:58–66. doi: 10.1111/j.1753-0407.2010.00099.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu T, Yang Y, Zhang L, Han J. Systematic review of the effects of inulin-type fructans on blood lipid profiles: a meta-analysis. Wei Sheng Yan Jiu. 2010;39:172–176. [PubMed] [Google Scholar]
- 17.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27 Suppl 1:S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 18.Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 2009;89:1751–1759. doi: 10.3945/ajcn.2009.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moghadasian MH, Frohlich JJ, Scudamore CH. Specificity of the commonly used enzymatic assay for plasma cholesterol determination. J Clin Path. 2002;55:859–861. doi: 10.1136/jcp.55.11.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RRI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without the use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FERHDL) Clin Biochem. 2001;34:583–588. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 22.Pourghassem Gargari B, Dehghan P, Aliasgharzadeh A, Asghari Jafar-Abadi M. Effects of high performance inulin supplementation on glycemic control and antioxidant status in women with type 2 diabetes. Diabetes Metab J. 2013;37:140–148. doi: 10.4093/dmj.2013.37.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson JW, Baird P, Davis Jr RH, Ferreri S, Knudtson M, Koraym A. et al. Health benefits of dietary fiber. Nutr Rev. 2009;67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 24.Pourmoradian S, Mahdavi R, Mobasseri M, Faramarzi E, Mobasseri M. Effects of royal jelly supplementation on body weight and dietary intake in type 2 diabetic females. Health Promot Perspect. 2012;2:231–235. doi: 10.5681/hpp.2012.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haddadin MSY, Haddadin J, Benguiar R. The Effect of Royal Jelly on Growth and Short-Chain Fatty Acid Production of Probiotic Bacteria and Activity of Bacterial Procarcinogenic Enzymes in Rat Faeces. Pol J Food Nutr Sci. 2012;62:251–258. [Google Scholar]
- 26.Chen HL, Sheu WH, Tai TS, Liaw YP, Chen YC. Konjac supplement alleviated hypercholesterolemia and hyperglycemia in type 2 diabetic subjects-a randomized double-blind trial. J Am Coll Nutr. 2003;22:36–42. doi: 10.1080/07315724.2003.10719273. [DOI] [PubMed] [Google Scholar]
- 27.Jackson KG, Taylor GR, Clohessy AM, Williams CM. The effect of the daily intake of inulin on fasting lipid, insulin and glucose concentrations in middle-aged men and women. Br J Nutr. 1999;82:23–30. doi: 10.1017/s0007114599001087. [DOI] [PubMed] [Google Scholar]
- 28.Russo F, Riezzo G, Chiloiro M, De Michele G, Chimienti G, Marconi E. et al. Metabolic effects of a diet with inulin-enriched pasta in healthy young volunteers. Curr Pharm Des. 2010;16:825–831. doi: 10.2174/138161210790883570. [DOI] [PubMed] [Google Scholar]
- 29.Nazih H, Nazih-Sanderson F, Krempf M, Michel Huvelin J, Mercier S, Marie Bard J. Butyrate stimulates ApoA-IV-containing lipoprotein secretion in differentiated Caco-2 cells. Role in cholesterol efflux. J Cell Biochem. 2001;83:230–238. doi: 10.1002/jcb.1221. [DOI] [PubMed] [Google Scholar]
- 30.Kinosian B, Glick H, Garland G. Cholesterol and coronary heart disease: predicting risks by levels and ratios. Annals of Internal Medicine. 1994;121:641–647. doi: 10.7326/0003-4819-121-9-199411010-00002. [DOI] [PubMed] [Google Scholar]
- 31. Engel JV. The benefits of eating fiber. Retrieved November 18, 2007, from http://www.diabetes.ca/section_about/fiber.asp
- 32.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 33.Cherbut C. Motor effects of short-chain fatty acids and lactate in the gastrointestinal tract. Proc Nutr Soc. 2003;62:95–99. doi: 10.1079/PNS2002213. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Qiao Q, Tuomilehto J, Hammar N, Alberti KG. et al. Blood lipid levels in relation to glucose status in European men and women without a prior history of diabetes: the DECODE Study. Diabetes Res Clin Pract. 2008;82:364–377. doi: 10.1016/j.diabres.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Sohaily S, Yadegary E, Parsian H, Baneifar A. The effect of intermittent aerobic exercise on serum leptin and insulin resistance index in overweight female students. Ann Biol Res. 2012;3:2636–2641. [Google Scholar]
- 36.Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes. 2006;55:1484–1490. doi: 10.2337/db05-1360. [DOI] [PubMed] [Google Scholar]
- 37.Cani PD, Daubioul CA, Reusens B, Remacle C, Catillon G, Delzenne NM. Involvement of endogenous glucagon-like peptide-1(7-36) amide on glycaemia-lowering effect of oligofructose in streptozotocin-treated rats. J Endocrinol. 2005;185:457–465. doi: 10.1677/joe.1.06100. [DOI] [PubMed] [Google Scholar]
- 38.Trautwein EA, Rieckhoff D, Erbersdobler HF. Dietary inulin lowers plasma cholesterol and triacylglycerol and alters biliary bile acid profile in hamsters. J Nutr. 1988;128:1937–1943. doi: 10.1093/jn/128.11.1937. [DOI] [PubMed] [Google Scholar]
- 39.Demigné C, Morand C, Levrat MA, Besson C, Moundras C, Rémésy C. Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br J Nutr. 1995;74:209–219. doi: 10.1079/bjn19950124. [DOI] [PubMed] [Google Scholar]
- 40.Rossi M, Corradini C, Amaretti A, Nicolini M, Pompei A, Zanoni S. et al. Fermentation of Fructooligosaccharides and Inulin by Bifidobacteria: A Comparative Study of Pure and Fecal Cultures. Appl Environ Microbiol. 2005;71:6150–6158. doi: 10.1128/AEM.71.10.6150-6158.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kok N, Roberfroid M, Delzenne N. Dietary oligofructose modifies the impact of fructose on hepatic triacylglycerol metabolism. Metabolism. 1996;45:1547–1550. doi: 10.1016/s0026-0495(96)90186-9. [DOI] [PubMed] [Google Scholar]
- 42.Delzenne NM, Kok NN. Biochemical basis of oligofructose-induced hypolipidemia in animal models. J Nutr. 1999;129(7 Suppl):1467S–1470S. doi: 10.1093/jn/129.7.1467S. [DOI] [PubMed] [Google Scholar]
- 43. Beylot M. Effects of inulin-type fructans on lipid metabolism in man and in animal models. Br J Nutr 2005;93 Suppl 1;S163-S168. [DOI] [PubMed]
- 44.Dikeman CL, Murphy MR, Fahey GC. Dietary fibers affecte viscosity of solutions and simulated human gastric and small intestinal digesta. J Nutr. 2006;136:913–919. doi: 10.1093/jn/136.4.913. [DOI] [PubMed] [Google Scholar]


