Abstract
Background: The prevalence of obesity is increasing throughout the world.Obesity assessed by body mass index (BMI) has shown to be associated with gestational complications while the relationship using waist circumference (WC) is not clear yet. The present study was aimed to determine the relationship between WC and adverse pregnancy complications.
Methods: In this prospective cohort study, 1140 nulliparous pregnant women at 1st trimester of pregnancy referred to health care centers in Tabriz, Iran were enrolled in 2009-2010. Anthropometric indexes including (weight, height and WC) were measured using standardized measures and methods. BMI was classified into normal, overweight and obesity based on WHO classification. Abdominal obesity was defined as WC ≥ 88 cm. Pregnancy complication including gestational diabetes, hypertension and preeclamsia. Data were analyzed using SPSS, version 16.
Results: Mean of BMI and WC were 24.32±4.08 kg/m2, 81.84±9.25cm at 1st trimester of pregnancy, respectively. Prevalence of overweight (BMI=25-29.9kg/m2) and obesity (BMI>29.9 kg/m2) was 27.6%, 8.8%, respectively. Abdominal obesity based on WC was 34.8%. Significant correlations were found between BMI and WC (r=0.73, P =0.0001). Women with BMI>29.9 kg/m2 and WC>88 cm were more likely to suffer from gestational pregnancy and hypertension, as well as preeclampsia and preterm delivery.
Conclusion: Early maternal WC similar to BMI is related with pregnancy complications.
Keywords: Body Mass Index, Waist Circumference, Pregnancy complications
Introduction
Obesity is an important public health problem worldwide, and its prevalence is increasing in both developed and developing nations with changes in dietary habits and activity level1,2,3. The increasing prevalence of overweight and obesity among women of childbearing age is a growing public health concern. Obesity is strongly associated with adverse gestational and perinatal outcomes4. Obesity is defined as a condition of excessive body fat, is usually assessed clinically by BMI, obtained by dividing weight by height squared3 and has long been recognized that body mass index (BMI; in kg/m2) is a predictor of the morbidity and mortality that are due to numerous chronic diseases, including type 2 diabetes, cardiovascular disease, and stroke1,4. In addition, it has been established that abdominal obesity, assessed by waist circumference (WC), predicts obesity-related health risk1,4,5-7, and the weighted evidence indicates that WC coupled with BMI predicts health risk better than does BMI alone3,8-15, but there are limited research about WC during pregnancy. People with a large waist are many times more at risk of ill health, including features of metabolic syndrome (such as diabetes, hypertension, and dyslipidemia) as well as shortness of breath and poor quality of life. People with normal BMI but large waist circumference have shown increased risk of obesity complications16-18.
In fact, recent findings indicate that WC is a stronger marker of health risk than is BMI2. As pregnancy progresses, this index are influenced by gestational weight gain in lean tissues, thus limiting its use in pregnancy. Weight gain over pregnancy period affects WC, therefore, it could not be as a useful index in this condition5. An alternative, the use of pre-pregnancy BMI as an indicator of obesity in pregnancy, maybe complicated by the fact that the weight used for this calculation is frequently self-reported, producing inaccuracies. Abdominal adiposity used only, measured by WC, is frequency used as a risk factor for diabetes and cardiovascular disease2 rather than for pregnancy. However, it is seldom used to predict risk in pregnancy, probably because it is believed to be unduly influenced by the increasing volume. Measuring WC at the lowest circumference point is less likely to be influenced by pregnancy progressing and uterus growing.
The purpose of this research was to comparison of total and abdominal obesity prevalence using BMI and WC in relation to pregnancy complications.
Materials and Methods
This cross-sectional study was carried out on 1140 nulliparous pregnant women at 1st trimester of pregnancy women referred to health care centers in Tabriz, Iran and followed up to delivery from July 2009 to March 2010. This study was ethically approved by Ethics Committee of Tabriz University of Medical Sciences.
Pregnant women at 1st trimester of pregnancy without history of nulliparous, hyperemesis gravidarom, recurrent spontaneous abortion, uterine surgery, molar pregnancies, any chronic diseases (e.g. cardiovascular, pulmonary, renal, nervous, gastrointestinal, diabetes, drug addiction, mental retardation, limb abnormalities), or special diet were included. Those with disproportionate weight gain in pregnancy according to initial BMI without preeclampsia or gestational diabetes or incomplete delivery file were excluded from the study. Anthropometric indices inc. (weight, height and WC) were measured using standardized measures and methods. Standing height (stature) was measured without shoes and heel against the wall and head in the plan to the nearest centimeter using the height measure stadiometer to the nearest 0.1cm and weight was measured with light clothing by calibrated vertical scale (Seca, Germany) to the nearest 100 g at the central health care office. Waist circumference was measured by placing a tape measure around the bare abdomen just above the hipbone without compressing the skin with 0.1cm precision. All measurements were done three times and the mean was recorded and used for statistical analysis. BMI was calculated as the weight in kg divided by the square of the height in meters.
BMI was classified into overweight and obesity based on WHO classification2. Abdominal obesity- characterized by high WC or WC to hip circumference (HP) ratio (WHR)- was defined as WC 88 cm and more. Including criteria were being of client of Tabriz health care centers, nulliparous and at 1st trimester of pregnancy, without hyperemesis gravidarom, special diet, and any history of recurrent spontaneous abortion, uterine surgery, molar pregnancies, and any disease, willingness to participating. The clients who were not accessible or showed disproportionate weight gain over pregnancy were excluded. Gestational age less than 37 weeks was defined as “preterm delivery”.
Pregnancy complications including gestational hypertension (Blood pressure ≥140/ 90mmHg without proteinuria after 20 weeks of gestation), preeclampsia (Blood pressure ≥140/ 90mmHg with proteinuria >+1 dipstick or > 300 mg protein in urine per 24 h after 20 weeks of pregnancy) and gestational diabetes (Glucose intolerance of variable severity with its onset during pregnancy or first detection in pregnancy) were adapted and recorded in the questionnaire. Pregnant women were followed and monitored over the pregnancy period and the occurrence of any complications were investigated prospectively and recorded efficiently. The subjects were followed up to delivery time and studies pregnancy complications were recorded.
Statistical Analysis
All data were analyzed using the Statistical Package for Social Sciences (SPSS for Windows, release 11.5, 2002, Chicago, IL, USA). The normality of continuous variables was tested using Kolmogorov-Smirnov test. After testing the normality of the distribution of continuous variables, differences in mean between two categories was tested using student t-test. Association between categorical or ordinal variables was tested using χ 2 test. Binary logistic regression models were used to analyze the relationship between certain factors such as pregnancy complications and anthropometric indexes and socio-demographic factors. Multivariate logistic regression was employed to find best predictors after adjusting for the possible confounders and odd ratios (OR) and 95% confidence interval (CI) were estimated. Statistically significant level was defined at P<0.05 for all tests.
Results
Of total 1140 nulliparous women at 1st trimester of pregnancy, 169 and 23 cases were excluded because of uncompleted file and excessive weight gain over pregnancy, respectively. Table 1 shows that majority of the pregnant women were housewife and aged 20-35 years. More than one third of them got university degree. At the 1st trimester of pregnancy, the mean BMI and WC were 24.32 ± 0.12) kg/m2 and 81.84 ± 0.35 cm, respectively (Table1). More than one third of them were overweight (27.6%) or obese (8.8%) according to BMI (Fig. 1).
Table 1. Demographic characteristics and anthropometric measures in pregnant women at 1st trimester of pregnancy (n=1140) .
| Demographic characteristic | ||||
| No. (%) | ||||
| Age (yr) |
<20 20-35 >35 |
244 (21.4) 883 (77.5) 13 (1.1) |
||
| Educational level |
Illiterate Under diploma Diploma University |
27 (2.4) 652 (57.2) 403 (35.3) 58 (5.1) |
||
| Occupation |
Housewife Employed (Medical science) Employed (Non-medical) Private job |
1057 (92.7) 12 (1.1) 22 (1.9) 49 (4.3) |
||
| Mean± SEM * | CI** 95% | |||
| Weight (kg) | 62.27 ± 0.35 | 61.58- 62.95 | ||
| Height (cm) | 159.67±0.15 | 159.46-160.05 | ||
| Body Mass Index (kg/m2) | 24.32±0.12 | 24.19- 24.43 | ||
| WC (cm) | 81.84±0.35 | 81.15-82.52 | ||
*Standard error of mean
** Confidence interval
Fig. 1.
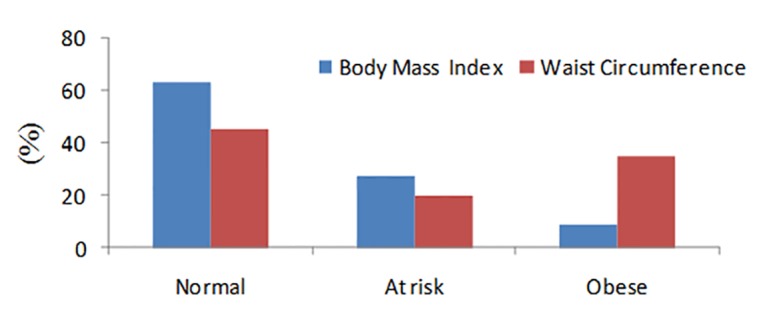
Body mass index and waist circumference status at 1st trimester of pregnancy
Table 2. Association between obesity indexes and pregnancy-delivery complication and outcomes after adjusting for the confounders .
| Body Mass Index (kg/m2) | Waist circumference (cm) | |||||
| <25 | 25-29.9 | >29.9 | <80 | 80-88 | >88 | |
|
Gestational hypertension |
1.00 | 1.98 (0.95, 4.13)* | 13.66 (6.98, 26.75) | 1.00 | 2.02 (0.74, 5.48) | 6.32 (2.91, 13.71) |
| Preeclampsia | 1.00 | 1.68 (0.78, 3.62) | 6.78 (3.18, 14.26) | 1.00 | 2.29(0.87, 6.03) | 3.93 (1.75, 8.80) |
|
Gestational diabetes |
1.00 | 1.91(0.63, 5.74) | 8.78 (3.18, 24.17) | 1.00 | 0.39 (0.04, 3.38) | 3.77 (2.91, 10.41) |
|
Preterm delivery |
1.00 | 1.25 (0.47, 3.20) | 3.72 (1.40, 9,86) | 1.00 | 1.24 (0.47, 3.25) | 3.14 (1.16, 8.50) |
* Odds ratio (CI 95%) after adjusting for preeclampsia, gestational hypertension, gestational diabetes and pregnancy delivery using multivariate logistic regression
More than one third (34.8%) of the nulliparous women at the 1st trimester of pregnancy had WC greater than 88cm (as clinical hazard as cut offs), indicating abdominal obesity (fig. 1). BMI at the 1st trimester of pregnancy was significantly correlated with WC (r=0.73, P=0.001). The percentage of gestational hypertension and diabetes, preeclampsia were 5.9%, 4.3% and 4.5%, respectively. There were significant associations between early pregnancy BMI and WC with pregnancy complications using univariate logistic regression analysis (Early pregnancy BMI and WC were significantly associated with gestational hypertension ( P=0.0001), gestational diabetes ( P =0.0001), preeclampsia ( P=0.001), preterm delivery ( P=0.001), respectively. After adjusting for significant confound-ders in this study including preeclampsia, gestational hypertension, gestational diabetes and pregnancy delivery, women with BMI>29.9 kg/m2 at the 1st trimester of pregnancy 13.66, 6.78, 8.78 and 3.72 times were more likely to suffer from gestational hypertension, preeclampsia, gestational diabetes and preterm delivery compared with those with healthy BMI (BMI<25 kg/m2), respectively. Early maternal WC was also found as a significant predictor for gestational hypertension (OR=6.32, 95%CI 2.91-13.71), gestational diabetes (OR=3.77, 95%CI 2.91-10.41), preeclampsia (OR=3.93, 95%CI 1.75-8.80) and preterm delivery (OR=3.14, 95%CI 1.16-8.50).
Discussion
Results of the present study indicated significant association between maternal high BMI and WC of early pregnancy and pregnancy-delivery complications including gestational hypertension and diabetes, preeclampsia and preterm delivery) i.e. high maternal BMI and WC could be significant predictors of pregnancy complications also maternal low BMI was found as significant risk factor of preterm delivery.
Waist circumference is as good as BMI to assess obesity of women at the 1st trimester of pregnancy, which were in normal range (81.84 cm and 24.32 kg/m2, respectively). However, more than one third of the subjects were overweight or obese according to BMI and WC status. Similar results found in a study in Glasgow indicating that median of BMI and WC were 24 (kg/m2) and 79 cm, respectively, between 6 and 16 weeks, of gestation13. 21.9% and 8.3% of pregnant women in the early pregnancy were overweight and obese according to BMI, respectively14. Similar results have also been reported by Siber et al.15 which are in agreement with our study and indicated the positive and significant correlation between BMI and WC at early pregnancy. Mean of BMI and WC were 26.5 (kg/m2) and 89.6cm among Iranian women, respectively and the prevalence of overweight and/or obesity and abdominal obesity were 56.9% and 53.5% in women, respectively16. Lower prevalence of obesity among pregnant women at early pregnancy in our study compared with the latter could be due to non-pregnant women from all age group participated in that study. As most of the studies investigated the association between early pregnancy BMI rather than WC and pregnancy outcomes, there are limited numbers of studies to compare the results regarding WC. Results of the present study showed significant and positive association between BMI and WC with gestational hypertension (OR=13.62 vs. OR=6.32) and diabetes (OR=8.78 vs. OR=3.77) and also preeclampsia (OR=6.78 vs. OR=3.93) (P<0.001 for all) while maternal low BMI (<18.5 kg/m2) at 1st trimester of pregnancy was significantly associated with preterm delivery (P <0.01), even after adjusting for the confounders such as LBW ( P<0.0001). Interestingly, pregnant women with obesity and abdominal obesity were 3.72 and 3.14 times more likely to have preterm infant compared with normal weight pregnant women, respectively. Gestational hypertension and preeclampsia were significantly more common among over weight and obese pregnant women vs. women with healthy BMI (P<0.05)14,15,19. Mean BMI and WC have been reported significantly higher in pregnant women with hypertension, preeclampsia and diabetes over pregnancy ( P=0.002)17,18. Odds for gestational hypertension and diabetes among obese women were higher than normal weight women (OR= 7.14 95%CI: 6.49-7.85) and (OR= 8.60CI 95%:7.15-10.50), respectively17. It has also been reported that the risk of gestational hypertension significantly increased by increasing BMI greater than ≥30kg/m2 ( P<0.0001)20 and obese women had a higher risk of preeclampsia ( P=0.02)21. Similar findings were found elsewhere14,22-24. This study had some limitations such as lack of anthropometric records before gestation, gaining weight, limited available anthropometric measurements in a routine prenatal care system. In order to minimize these types of errors, we measured height, weight and WC in early pregnancy, before any real impact of gestational weight gain. Furthermore, gathering data by a trained assessor and the prospective design of the study on relatively large sample size are the strengths on this study. However, strengths of the present study include the type of study, i.e. obesity indexes at the 1st trimester of a large sample size of pregnant women from different health centers of Tabriz were assessed. Although studies investigating the predictive role of BMI for pregnancy complications without controlling contributing factors are few, such studies for WC is much rare.
Conclusion
Early pregnancy WC as an index for abdominal obesity in agreement with BMI could predict pregnancy complications including gestational hypertension and diabetes, preeclampsia and preterm delivery. Therefore, identifying overweight women at the 1st trimester of pregnancy, particularly those with accumulation of excessive visceral fat, is essential. BMI and WC are well validated and available for all health professionals in weight gain monitoring and directing future intervention.
Acknowledgments
We kindly acknowledge Research Deputy of Tabriz University of Medical Sciences for their financial support.
Competing interests
The authors declare that there is no conflict of interests.
Citation: Ebrahimi-Mameghani M, Mehrabi E, Kamalifard M, Yavarikia P. Correlation between Body Mass Index and Central Adiposity with Pregnancy Complications in Pregnant Women. Health Promot Perspect 2013; 3(1): 73-79
References
- 1.World health organization. National task force on the prevention and treatment of obesity Overweight, obesity, and health risk. Arch Intern Med. 2000;160:898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- 2.Seidell JC, Flegal KM. Assessing obesity: classification and epidemiology. Br Med Bull. 1997;53:238–252. doi: 10.1093/oxfordjournals.bmb.a011611. [DOI] [PubMed] [Google Scholar]
- 3.World health organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 4.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with under-weight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 5.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 6.Rennie KL, Jebb SA. Prevalence of obesity in Great Britain. Obes Rev. 2005;6:11–12. doi: 10.1111/j.1467-789X.2005.00164.x. [DOI] [PubMed] [Google Scholar]
- 7.Bell AC, Ge K, Popkin BM. Weight gain and its predictors in Chinese adults. Int J Obes Relat Metab Disord. 2001;25:1079–1086. doi: 10.1038/sj.ijo.0801651. [DOI] [PubMed] [Google Scholar]
- 8.Popkin BM, Doak CM. The obesity epidemic is a worldwide phenomenon. Nutr Rev. 1998;56:106–14. doi: 10.1111/j.1753-4887.1998.tb01722.x. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 10.Hodge AM, Dowse GK, Bareeboo H, Tuomileho J, Alberti KG, Zimmet PZ. Incidence, increasing prevalence, and predictors of change in obesity and fat distribution over 5 years in the rapidly developing population of Mauritius. Int J Obes Relat Metab Disord. 1996;20:137–146. [PubMed] [Google Scholar]
- 11.Azizi F, Azadbakht L, Mirmiran P. Trends in overweight, obesity and central fat accumulation among Tehranian adults between 1998–1999 and 2001–2002: Tehran lipid and glucose study. Ann Nutr Metab. 2005;49:3–8. doi: 10.1159/000084171. [DOI] [PubMed] [Google Scholar]
- 12.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current national institutes of health guidelines. Arch Intern Med. 2002;162:2074–2079. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 13. Cunningham G, Leveno KJ, Hauth J, Glistrap L, Wenstrom K. William’s Obstetrics.22nd ed. medical publishing division 2000.
- 14.Bhattacharya SO, Campbell D, Liston W, Bhttacharya SI. Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Pub Health J. 2007;7:168. doi: 10.1186/1471-2458-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW. et al. Maternal obesity and pregnancy outcome: a study of 287 213 pregnancies in London. Int J Obesity. 2001;25:1175–1182. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 16.Janghorbani M, Amini M, Willet W, Gouya M, Delavari A, Alikhani S. et al. First nationwide survey of prevalence of overweight, underweight, and abdominal obesity in Tehran adults. Obesity J. 2007;15:2797–2808. doi: 10.1038/oby.2007.332. [DOI] [PubMed] [Google Scholar]
- 17.Denison FC, Price J, Graham C, Wild S, Liston WA. Maternal obesity, length of gestation, risk of postdates pregnancy and spontaneous onset of labor at term. Int J Obstet Gynecol. 2008;115:720–725. doi: 10.1111/j.1471-0528.2008.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leddy ML, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol J. 2008;1:170–178. [PMC free article] [PubMed] [Google Scholar]
- 19.Margaret E, Samuels K, Edmund F, Funai M, Catalin B, Errol N. et al. Pregnancy body mass index, hypertensive disorders of pregnancy, and long –term maternal mortality. Am J Obstet Gynecol. 2007;197:490. doi: 10.1016/j.ajog.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nohr EA, Timpson NJ, Andersen CS, Smith GD, Olsen J, Sørensen TIA. Severe obesity in young women and reproductive health: The Danish national birth cohort. 2009 available from www.plosone.org [DOI] [PMC free article] [PubMed]
- 21.Heinrich CD, Hansen M, McCulloch A, Archer L. The association of prepregnancy body mass index and adverse maternal and perinatal outcomes. Colorado department of Public health& environment. Health watch. 2009;69:1–6. [Google Scholar]
- 22.Omanwa K, Zimmer M, Tlolka J, Wytrychowska E, Maciejewska J, Drys A. Is low pre-pregnancy body mass index a risk factor for preterm birth and low neonatal birth weight? Ginecol Pol J. 2006;77:618. [PubMed] [Google Scholar]
- 23.Spinillo A, Capuzzo E, Piazzi G, Ferrari A, Morales V, Di Mario M. Risk for spontaneous preterm delivery by combined body mass index and gestational weight gain patterns. Acta J Obstet Gynecol. 1998;77:32–36. [PubMed] [Google Scholar]
- 24. Kalkwarf HJ. Maternal weight gain and risk of preterm delivery: Effect on neonatal mortality and public health impact. Unpublished Ph.D. thesis of Cornel (New York) University 1991.


