Abstract
Background and Aims
Insulin-like growth factor (IGF-1) stimulates cell proliferation and inhibits cell apoptosis. Recent studies underline its importance as anabolic hormone and nutritional marker in older individuals. IGF-1 synthesis and bioactivity are modulated by nutritional factors including selenium intake. However, whether circulating IGF-1 levels are positively influenced by plasma selenium, one of the most important human antioxidants, is still unknown.
Methods
Selenium and total IGF-1 were measured in 951 men and women ≥65 years from the InCHIANTI study, Tuscany, Italy.
Results
Means (SD) of plasma selenium and total IGF-1 were 0.95 (0.15) µmol/L and 113.4 (31.2) ng/mL, respectively. After adjustment for age and sex, selenium levels were positively associated with total IGF-1 (ß ± SE: 43.76±11.2, p=0.0001).After further adjustment for total energy and alcohol intake, serum alanine amino transferase (ALT), congestive heart failure, selenium remained significantly associated with IGF-1 (β ± SE: 36.7 ± 12.2, p=0.003). The association was still significant when IL-6 was introduced in the model (β ± SE: 40.1 ± 12.0, p=0.0008).
Conclusions
We found an independent, positive and significant association between selenium and IGF-1 serum levels in community dwelling older adults.
Keywords: aging, total IGF-1, selenium
The activity of the hypothalamic- GH (Growth Hormone) - insulin like growth factor (IGF) axis decreases with aging, leading to relative GH and IGF-I deficiency. Low levels of circulating GH and IGF-I, have been associated with age related adverse conditions such as osteoporosis, sarcopenia, and depression. IGF-1 promotes muscle protein synthesis and cell proliferation and inhibits cell apoptosis. Whether low IGF-1 is a risk factor for sarcopenia, frailty and poor mobility is still under investigation (1–2). Nutritional intake and especially protein intake tend to upregulate the IGF-1 synthesis while energy restriction is associated with lower IGF-1 levels (3–6) suggesting that IGF-1 is a nutritional marker. It has been suggested that selenium is particularly important for IGF bioactivity (7). Selenium modulates somatic growth and is an important antioxidant (8). Selenium deficiency is an independent correlate of growth retardation, influencing triidothyronine and GH axis (8). A recent study conducted in college-aged women found an association between selenium intake and bioactive IGF-1 (7). However, whether selenium intake is important for IGF-1 biological activity has not been studied. Recent data from the InCHIANTI study show that low selenium is an independent predictor of poor skeletal muscle strength (9). In the same population serum IGF-1 levels were also significantly associated with muscle strength and physical performance in men and to a lesser extent in women (2). These findings raise the possibility that beneficial effects of selenium on muscle strength might be mediated by IGF-1. Using data from participants aged 65 and older in the “Aging in the Chianti Area” (InCHIANTI) study, Tuscany, Italy, we tested the hypothesis that plasma selenium concentrations are positively associated with total IGF-1 in older adults.
We tested this hypothesis in men and women. The methods have been described elsewhere. In August 1998, 1270 people 65 years and older were randomly selected from the population registry of Greve in Chianti and Bagno a Ripoli), and of 1256 eligible subjects, 1155 (90.1%) agreed to participate. Of the 1155 participants, 1055 (91.3%) participated in the blood drawing. 951 (90.0%) participants with plasma selenium concentrations and total IGF-1 were available for this analysis. Participants gave a written, informed consent. The study protocol was approved by the Italian National Institute of Research and Care on Aging Ethical Committee (10).
Smoking exposure was reported as pack-year. Average daily intake of energy (kcal) and alcohol were estimated using the European Prospective Investigation into Cancer and Nutrition food frequency questionnaire. Diseases were ascertained according to standard criteria. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Depression was defined by using the Center for Epidemiologic Studies Depression Scale (CES-D).
Blood samples were collected in the morning after a 12-h fast. Aliquots of serum and plasma were stored at −80° C..Plasma selenium was measured by graphite furnace atomic absorption spectrometry using a Perkin Elmer Analyst 600 with Zeeman background correction. Samples were diluted 1:4 with a triton-X (Sigma Chemical, St. Louis, MO) and nitric acid solution (Fisher Scientific, Pittsburgh, PA), and the matrix modifier was a palladium and magnesium nitrate solution. Within-run and between-run coefficients of variation (CV), were 3.1% and 7.1%, respectively. Serum IGF-I was measured by immunoradiometric assay, using commercial reagents (DSL, Webster). Inter-assay and intra-assay CV were all less than 10%. Serum levels of interleukin 6 (IL-6) were measured by ELISA using ultrasensitive commercial kits.
High-sensitivity C Reactive Protein (CRP) was measured in duplicate by enzyme-linked immunosorbent assay using purified protein and polyclonal CRP antibodies. The minimum detectable concentration was 0.03 mg/L, and the interassay CVs 5%.
Plasma concentrations of thyroid-stimulating hormone (TSH), free triiodothyronine (FT3), and free thyroxine (FT4) were measured using a chemiluminescent immunoassay. Interassay CVs were less than 9% for all three hormones.
Alanine aminotransferase (ALT) levels were determined using a commercial kit.Variables are reported as means (standard deviations) for normally distributed parameters or as number and percentages. Means and percentages were compared using t-test and chi square tests. To approximate normal distributions, log-transformed values for IL-6 were used in the analysis and back transformed for data presentation. Plasma selenium was analyzed as a continuous variable. Factors statistically correlated with total IGF-1 were identified using age-adjusted partial correlation coefficients and Spearman partial rank-order correlation coefficients, as appropriate. Parsimonious models obtained by backward selection from initial fully adjusted models were used to identify independent factors of total IGF-1. General linear models were used to test the relationship between selenium and total IGF-1 after adjusting for age and sex (Model 1) and further adjustment for energy and alcohol intake, ALT, and congestive heart failure (Model 2) and for IL-6 (Model 3). All analyses were performed using SAS (v. 8.2, SAS Institute, Inc., Cary, NC).The characteristics of the study population at enrollment are shown in Table 1. Means (SD) of plasma selenium and total IGF-1 were 0.95 (0.15) µmol/L and 113.4 (31.2) ng/mL, respectively. The relationship between IGF-1 and selenium levels is depicted in Figure 1.
Table 1.
Characteristics of the Study Population.
| Characteristic | (n = 951) |
|---|---|
| Selenium (µmol/L)1 | 0.93 (0.16) |
| Total IGF-1 (ng/mL)1 | 113.4 (31.2) |
| Age (years)1 | 75.8 (7.5) |
| Sex (% female) | 57.0 |
| Body mass index (kg/m2)1 | 27.4 (4.0) |
| Energy Intake (kcal/d)1 | 1894.3 (556.9) |
| Alcohol (g/d)2 | 10.0 [5.7–20.0] |
| Smoking (pack-year)2 | 0 [0–20] |
| IL-6 (pg/mL)2 | 1.47 [0.86–2.36] |
| GPT. (U/L)1 | 19.4 (12.2) |
| Congestive Heart Failure(%) | 50 (5.3) |
| COPD (%) | 76(8.0) |
| Cancer (%) | 63 (6.6) |
| CRP (mg/L)1 | 2.03 [1.38–5.62] |
Mean (Standard Deviation) for continuous variables or percentages as noted,
Median [Interquartile Range]
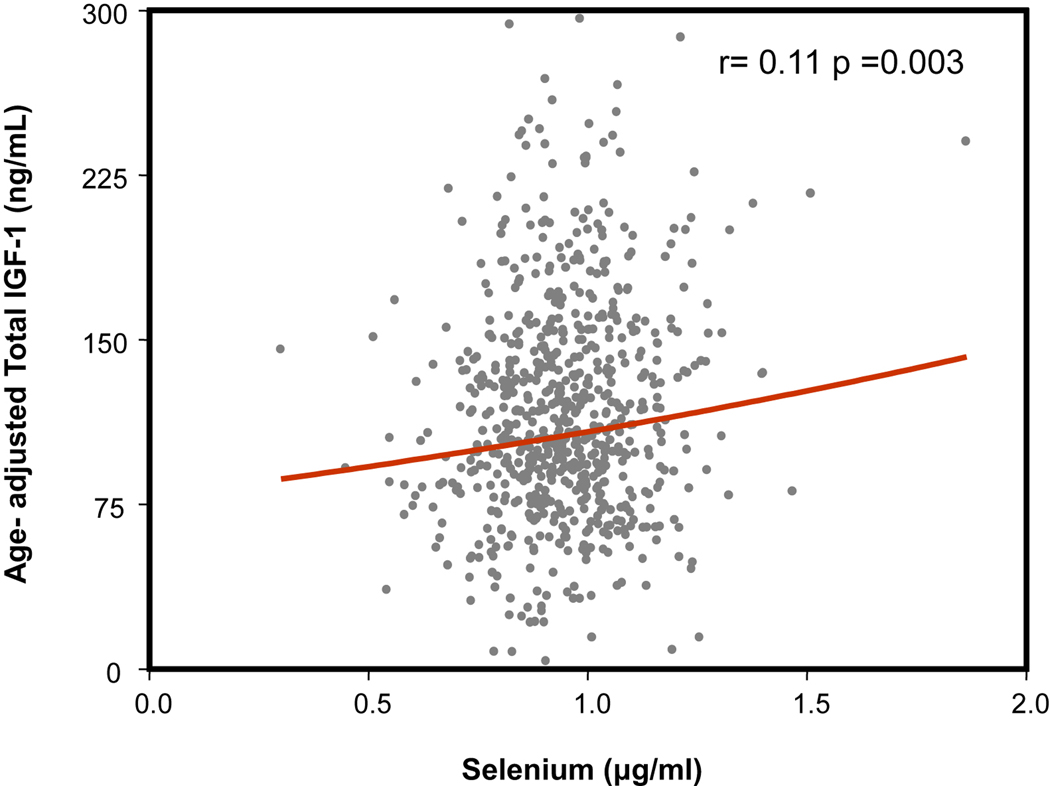
Figure 1.
Relationship between selenium levels expressed in µg /ml (horizontal axis) and age adjusted total IGF-1 levels (ng/ml) (vertical axis). As shown, the relationship is statistically significant (r=0.11, p=0.003).
Age adjusted partial correlation analysis was used to examine the relationship between different demographic, nutritional, and disease variables and total IGF-1 as shown in Table 2. Selenium and energy intake were positively associated while alcohol intake and ALT were negatively associated with IGF-1. Multivariate logistic regression models were used to examine the relationship between IGF-1 and selenium (Table 3). After adjusting for age and sex (model 1) selenium and IGF-1 were positively associated. In models (model 2) adjusted for age, sex, total energy intake, alcohol intake, ALT, congestive heart failure, selenium levels remained significantly associated with total IGF-1 (β ± SE: 36.7 ± 12.2, p=0.003). The significant association between selenium and IGF-1 was not affected by introducing IL-6 in the model (β ± SE: 40.1 ± 12.0, p=0.0008) (model 3). Consistently, when in the Model 3 was introduced CRP, instead of IL-6, the strength and the significance of the association between selenium and total IGF-1 were unaffected (β ± SE: 38.1 ± 12.0, p=0.0001).
Table 2.
Age- Adjusted Spearman Partial Rank-Order Correlation Coefficients of Total IGF-1 and Selenium and Other Factors at Enrollment.
| Characteristic (n = 951) | Total IGF-1 (ng/mL) r (p value) |
|---|---|
| Selenium (µmol/L) | 0.11 (0.003)* |
| Body mass index (kg/m2) | 0.15 (0.68) |
| Energy Intake (kcal/d) | 0.08 (0.03)* |
| Alcohol (g/d) | −0.10 (0.009)* |
| Smoking (pack-year) | −0.02 (0.54) |
| Log (IL-6) (pg/mL) | 0.009 (0.80) |
| ALT (U/L) | −0.07 (0.07)* |
| Congestive Heart Failure(%) | 0.06 (0.08) |
| COPD (%) | −0.003 (0.94) |
| Cancer (%) | 0.04 (0.28) |
| CRP (mg/L) | −0.03 (0.29) |
p<0.1
Table 3.
Relationship Between Selenium and IGF-1^.
| Characteristic | Total IGF-1 (ng/mL) (At Enrolment, n=951) |
||
|---|---|---|---|
| Beta | SE | P | |
| Model 1 | |||
| Selenium (µmol/L) | 43.76 | 11.25 | 0.0001 |
| Model 2 | |||
| Selenium (µmol/L) | 36.69 | 12.243 | 0.003 |
| Model 3 | |||
| Selenium (µmol/L) | 40.11 | 11.970 | 0.0008 |
Model 1: Adjusted for Age and Sex.
Model 2: Adjusted for Age, Sex, Energy Intake, Alcohol, GPT, Congestive Heart Failure.
Model 3: Adjusted for Age, Sex, Energy Intake, Alcohol, GPT, Congestive Heart Failure, log (IL-6).
In a further analysis, adjusted for thyroid function (TSH, FT4), the association between selenium and IGF-1 remained substantially unchanged (β ± SE: 37.7 ± 12.6, p=0.003).
This study shows that selenium is an independent correlate of IGF-1 in older adults. This is the first study to show an association between selenium and IGF-1 in the elderly. Our findings confirm previous results of smaller female younger population showing a positive association between selenium intake and IGF-1 levels (7). We previously reported that more than 30% of older adults in the InCHIANTI study had plasma selenium <0.88 µmol/L (70 µg/L), the level below which low selenium may be limiting the synthesis of selenoproteins (9). Thus, it is possible that selenium deficiency may lead to insufficient antioxidant buffering by selenoproteins and increased and unopposed oxidative stress with damage to DNA, proteins, and lipid in muscle tissue and other tissues. Data from Uppsala Longitudinal Study of Adult Men show that high serum selenium levels were predictive of lower urinary F2 isoprostane concentrations, a biomarker of lipid peroxidation and oxidative stress (11).
Oxidative stress could contribute to the decline in IGF-1 levels observed in older population. An additional hypothesis considers inflammatory cytokines as the mediators of the relationship between selenium and IGF-1. Plasma levels of inflammatory cytokines increase with age leading to a subclinical inflammatory state. If we hypothesize that oxidative stress is the main trigger to inflammation, decreased circulating levels of antioxidants, such as selenium, may be responsible for the increase in inflammatory markers and ultimately to low IGF-1 levels. This hypothesis is consistent with data from the Women’s Health and Aging Study I showing that participants with low selenium levels have higher serum IL-6 levels (12) and that high IL-6 was associated with low IGF-1 (13). Of note, in our study the relationship between selenium and IGF-1 was independent of IL-6 (Table 3) and CRP. Selenium could also modulate the deiodinase activity and therefore triiodothyronine production but in our results the relationship between selenium and IGF-1 was independent of thyroid hormone levels.
The impact of dietary intake on the relationship between selenium and IGF-1 cannot be neglected. Primary sources of dietary selenium are proteins, namely meat, fish and poultry, with dietary caloric and protein intake important positive modulators of serum IGF-1 concentration. We found a positive and significant relationship between caloric intake and IGF-1 but the inclusion of caloric intake in the multivariate models did not affect the relationship between selenium and IGF-1, probably because of the short period of FFQ investigation.
Because of the cross-sectional design this study cannot address the possible causality of the association between selenium and IGF-1 and neither excludes residual confounding. Older adults with low IGF-1 have a lower dietary intake of selenium because of factors relating to their low strength, i.e., being less able to shop and prepare meals higher in selenium such as fish. Selenium intake was not estimated in this study. However the dietary intake of selenium is difficult to measure using conventional assessment tools, since selenium in food depends upon the selenium content of the soil where plants/animals are grown, and importation of foods from outside the area can add to imprecision of such an assessment. Even, the lack of markers of oxidative stress does not clarify the mechanism by which selenium interacts with IGF-1.
Selenium concentrations in the present study are consistent with previous reports from Italy where plasma selenium concentrations were 0.82 µmol/L among adults of Veneto Region, 1.12 and 0.86 among adults of 65–89 years and ≥90 years, from Bologna (14). Mean serum selenium concentrations in Italian adults varied between 1.09 and 1.17 µmol/L with decreasing values observed in adults over age 60 (15). The identification of selenium deficiency in single older individuals or in groups may allow starting selenium supplementation with potential effect on anabolic status assessed by IGF-1. This hypothesis should be tested in appropriately designed intervention studies.
Acknowledgements
This work was supported by National Institute on Aging Contracts N01-AG-916413, N01-AG-821336, N01-AG-5-0002, and NIA Grant R01 AG027012. This research was supported in part by the Intramural Research Program, National Institute on Aging, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
All the authors declare that they do not have any conflict of interest.
Statement of authorship
Marcello Maggio, carried out the studies and data analyses and drafted the manuscript. Marcello Maggio and Fulvio Lauretani carried out the samples analyses. Marcello Maggio, Fulvio Lauretani, Stefania Bandinelli, Luigi Ferrucci participated in the design of the study and Marcello Maggio, Luigi Ferrucci, Fulvio Lauretani, performed the statistical analysis. Gianpaolo Ceda, Elisabetta Dall'Aglio, Jack M. Guralnik, Giuseppe Paolisso, Richard D. Semba, Antonio Nouvenne, Loris Borghi, Graziano Ceresini, Fabrizio Ablondi , Mario Benatti, Luigi Ferrucci, conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.Hoffman AR, Lieberman SA, Butterfield G, Thompson J, Hintz RL, Ceda GP, Marcus R. Functional consequences of the somatopause and its treatment. Endocrine. 1997;7:73–76. doi: 10.1007/BF02778067. [DOI] [PubMed] [Google Scholar]
- 2.Ceda GP, Dall'Aglio E, Maggio M, Lauretani F, Bandinelli S, Falzoi C, Grimaldi W, Ceresini G, Corradi F, Ferrucci L, Valenti G, Hoffman AR. Clinical implications of the reduced activity of the GH-IGF-I axis in older men. J Endocrinol Invest. 2005;28(11 Suppl Proceedings):96–100. Review. [PubMed] [Google Scholar]
- 3.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15(1):80–101. doi: 10.1210/edrv-15-1-80. Review. [DOI] [PubMed] [Google Scholar]
- 4.Holmes MD, Pollak MN, Hankinson SE. Lifestyle correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11:862–867. [PubMed] [Google Scholar]
- 5.Hoppe C, Udam TR, Lauritzen L, Mølgaard C, Juul A, Michaelsen KF. Animal protein intake, serum insulin-like growth factor I, growth in healthy 2.5-y-old Danish children. Am J Clin Nutr. 2004;80(2):447–452. doi: 10.1093/ajcn/80.2.447. [DOI] [PubMed] [Google Scholar]
- 6.Martín MA, Serradas P, Ramos S, Fernández E, Goya L, Gangnerau MN, Lacorne M, Pascual-Leone AM, Escrivá F, Portha B, Alvarez C. Protein-caloric food restriction affects insulin-like growth factor system in fetal Wistar rat. Endocrinology. 2005;146(3):1364–1371. doi: 10.1210/en.2004-0665. [DOI] [PubMed] [Google Scholar]
- 7.Philip Karl J, Alemany JA, Koenig C, Kraemer WJ, Frystyk J, Flyvbjerg A, Young AJ, Nindl BC. Diet, body composition, and physical fitness influences on IGF-I bioactivity in women. Growt Horm IGF Res. 2009 doi: 10.1016/j.ghir.2009.04.001. in press. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Reyes R, Egrise D, Nève J, Pasteels JL, Schoutens A. Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J Bone Miner Res. 2001;16(8):1556–1563. doi: 10.1359/jbmr.2001.16.8.1556. [DOI] [PubMed] [Google Scholar]
- 9.Lauretani F, Semba RD, Bandinelli S, Ray AL, Guralnik JM, Ferrucci L. Association of low plasma selenium concentrations with poor muscle strength in older community-dwelling adults: the InCHIANTI Study. Am J Clin Nutr. 2007;86(2):347–352. doi: 10.1093/ajcn/86.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48(12):1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 11.Helmersson J, Árnlöv J, Vessby B, Larsson A, Alfthan G, Basu S. Serum selenium predicts levels of F2-isoprostanes and prostaglandin F2α in a 27 year follow-up study of Swedish men. Free Radic Res. 2005;39:763–770. doi: 10.1080/10715760500108513. [DOI] [PubMed] [Google Scholar]
- 12.Walston J, Xue Q, Semba RD, Ferrucci L, Cappola AR, Ricks M, Guralnik J, Fried LP. Serum antioxidants, inflammation, and mortality in older women. Am J Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 13.Barbieri M, Ferrucci L, Ragno E, Corsi A, Bandinelli S, Bonafe M, Olivieri F, Giovagnetti S, Franceschi C, Guralnik JM, Paolisso G. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284:E481–E487. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- 14.Morisi G, Patriarca M, Marano G, Giampaoli S, Taggi F. Age and sex specific reference serum selenium levels estimated for the Italian population. Ann Ist Super Sanita. 1989;25:393–403. [PubMed] [Google Scholar]