Abstract
Pancreatic islet amyloid deposits are a characteristic pathologic feature of non-insulin-dependent diabetes mellitus and contain islet amyloid polypeptide (IAPP; amylin). We used transgenic mice that express human IAPP in pancreatic beta cells to explore the potential role of islet amyloid in the pathogenesis of non-insulin-dependent diabetes mellitus. Extensive amyloid deposits were observed in the pancreatic islets of approximately 80% of male transgenic mice > 13 months of age. Islet amyloid deposits were rarely observed in female transgenic mice (11%) and were never seen in nontransgenic animals. Ultrastructural analysis revealed that these deposits were composed of human IAPP-immunoreactive fibrils that accumulated between beta cells and islet capillaries. Strikingly, approximately half of the mice with islet amyloid deposits were hyperglycemic (plasma glucose > 11 mM). In younger (6- to 9-month-old) male transgenic mice, islet amyloid deposits were less commonly observed but were always associated with severe hyperglycemia (plasma glucose > 22 mM). These data indicate that expression of human IAPP in beta cells predisposes male mice to the development of islet amyloid and hyperglycemia. The frequent concordance of islet amyloid with hyperglycemia in these mice suggests an interdependence of these two conditions and supports the hypothesis that islet amyloid may play a role in the development of hyperglycemia.
Full text
PDF

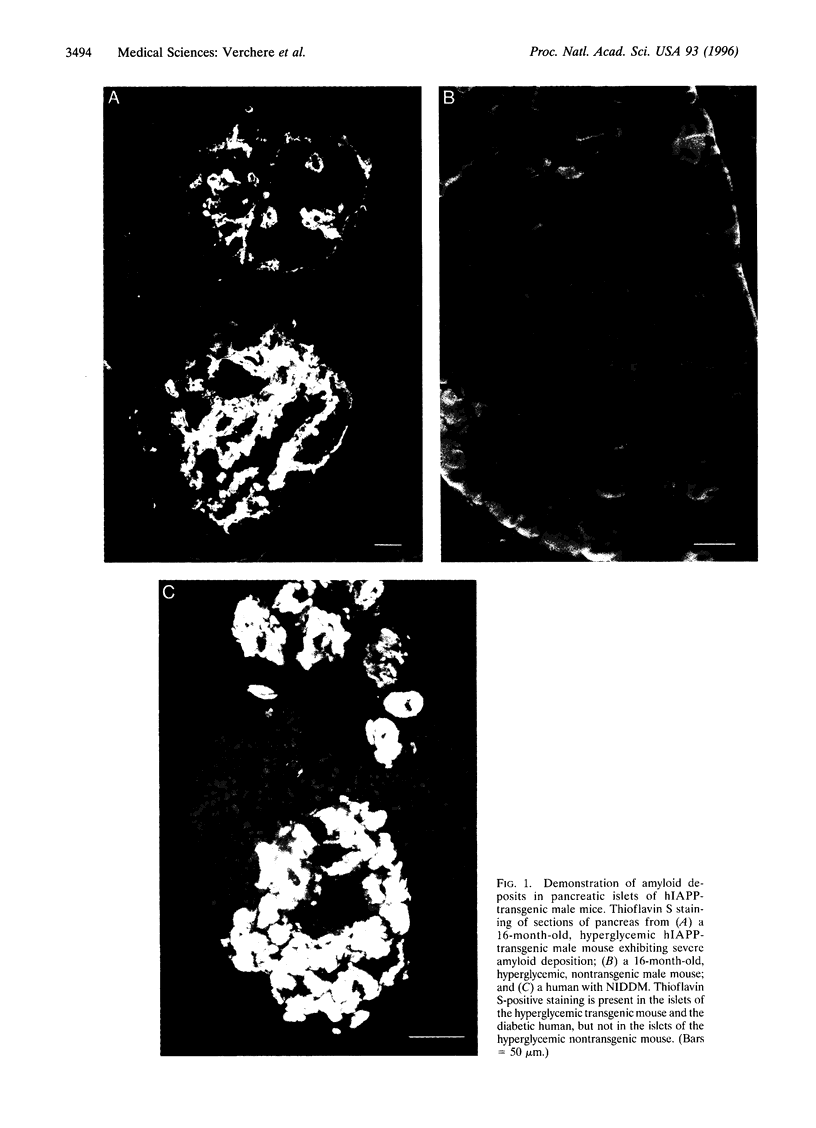


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELL E. T. Hyalinization of the islets of Langerhans in nondiabetic individuals. Am J Pathol. 1959 Jul-Aug;35(4):801–805. [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Christmanson L., Engström U., Rorsman F., Jordan K., O'Brien T. D., Murtaugh M., Johnson K. H., Westermark P. Structure of cat islet amyloid polypeptide and identification of amino acid residues of potential significance for islet amyloid formation. Diabetes. 1990 Jan;39(1):118–122. doi: 10.2337/diacare.39.1.118. [DOI] [PubMed] [Google Scholar]
- Clark A., Cooper G. J., Lewis C. E., Morris J. F., Willis A. C., Reid K. B., Turner R. C. Islet amyloid formed from diabetes-associated peptide may be pathogenic in type-2 diabetes. Lancet. 1987 Aug 1;2(8553):231–234. doi: 10.1016/s0140-6736(87)90825-7. [DOI] [PubMed] [Google Scholar]
- Clark A., Edwards C. A., Ostle L. R., Sutton R., Rothbard J. B., Morris J. F., Turner R. C. Localisation of islet amyloid peptide in lipofuscin bodies and secretory granules of human B-cells and in islets of type-2 diabetic subjects. Cell Tissue Res. 1989 Jul;257(1):179–185. doi: 10.1007/BF00221649. [DOI] [PubMed] [Google Scholar]
- Clark A., Saad M. F., Nezzer T., Uren C., Knowler W. C., Bennett P. H., Turner R. C. Islet amyloid polypeptide in diabetic and non-diabetic Pima Indians. Diabetologia. 1990 May;33(5):285–289. doi: 10.1007/BF00403322. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Willis A. C., Clark A., Turner R. C., Sim R. B., Reid K. B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio D. A., Verchere C. B., Kahn S. E., Hoagland V., Baskin D. G., Palmiter R. D., Ensinck J. W. Pancreatic expression and secretion of human islet amyloid polypeptide in a transgenic mouse. Diabetes. 1994 Dec;43(12):1457–1461. doi: 10.2337/diab.43.12.1457. [DOI] [PubMed] [Google Scholar]
- Fox N., Schrementi J., Nishi M., Ohagi S., Chan S. J., Heisserman J. A., Westermark G. T., Leckström A., Westermark P., Steiner D. F. Human islet amyloid polypeptide transgenic mice as a model of non-insulin-dependent diabetes mellitus (NIDDM). FEBS Lett. 1993 May 24;323(1-2):40–44. doi: 10.1016/0014-5793(93)81444-5. [DOI] [PubMed] [Google Scholar]
- Games D., Adams D., Alessandrini R., Barbour R., Berthelette P., Blackwell C., Carr T., Clemens J., Donaldson T., Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995 Feb 9;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Eanes E. D., Wiley C. A. Amyloid fibrils formed from a segment of the pancreatic islet amyloid protein. Biochem Biophys Res Commun. 1988 Sep 15;155(2):608–614. doi: 10.1016/s0006-291x(88)80538-2. [DOI] [PubMed] [Google Scholar]
- Howard C. F., Jr Longitudinal studies on the development of diabetes in individual Macaca nigra. Diabetologia. 1986 May;29(5):301–306. doi: 10.1007/BF00452067. [DOI] [PubMed] [Google Scholar]
- Höppener J. W., Verbeek J. S., de Koning E. J., Oosterwijk C., van Hulst K. L., Visser-Vernooy H. J., Hofhuis F. M., van Gaalen S., Berends M. J., Hackeng W. H. Chronic overproduction of islet amyloid polypeptide/amylin in transgenic mice: lysosomal localization of human islet amyloid polypeptide and lack of marked hyperglycaemia or hyperinsulinaemia. Diabetologia. 1993 Dec;36(12):1258–1265. doi: 10.1007/BF00400803. [DOI] [PubMed] [Google Scholar]
- Johnson K. H., O'Brien T. D., Hayden D. W., Jordan K., Ghobrial H. K., Mahoney W. C., Westermark P. Immunolocalization of islet amyloid polypeptide (IAPP) in pancreatic beta cells by means of peroxidase-antiperoxidase (PAP) and protein A-gold techniques. Am J Pathol. 1988 Jan;130(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Kahn S. E., D'Alessio D. A., Schwartz M. W., Fujimoto W. Y., Ensinck J. W., Taborsky G. J., Jr, Porte D., Jr Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990 May;39(5):634–638. doi: 10.2337/diab.39.5.634. [DOI] [PubMed] [Google Scholar]
- Kahn S. E., Prigeon R. L., McCulloch D. K., Boyko E. J., Bergman R. N., Schwartz M. W., Neifing J. L., Ward W. K., Beard J. C., Palmer J. P. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993 Nov;42(11):1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- Kaku K., Fiedorek F. T., Jr, Province M., Permutt M. A. Genetic analysis of glucose tolerance in inbred mouse strains. Evidence for polygenic control. Diabetes. 1988 Jun;37(6):707–713. doi: 10.2337/diab.37.6.707. [DOI] [PubMed] [Google Scholar]
- Kava R. A., West D. B., Lukasik V. A., Greenwood M. R. Sexual dimorphism of hyperglycemia and glucose tolerance in Wistar fatty rats. Diabetes. 1989 Feb;38(2):159–163. doi: 10.2337/diab.38.2.159. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Chapman H. D., Coleman D. L. The influence of genetic background on the expression of mutations at the diabetes locus in the mouse. V. Interaction between the db gene and hepatic sex steroid sulfotransferases correlates with gender-dependent susceptibility to hyperglycemia. Endocrinology. 1989 Feb;124(2):912–922. doi: 10.1210/endo-124-2-912. [DOI] [PubMed] [Google Scholar]
- Leiter E. H. The genetics of diabetes susceptibility in mice. FASEB J. 1989 Sep;3(11):2231–2241. doi: 10.1096/fasebj.3.11.2673897. [DOI] [PubMed] [Google Scholar]
- Lorenzo A., Razzaboni B., Weir G. C., Yankner B. A. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994 Apr 21;368(6473):756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- Lowell B. B., S-Susulic V., Hamann A., Lawitts J. A., Himms-Hagen J., Boyer B. B., Kozak L. P., Flier J. S. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993 Dec 23;366(6457):740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- Lukinius A., Wilander E., Westermark G. T., Engström U., Westermark P. Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia. 1989 Apr;32(4):240–244. doi: 10.1007/BF00285291. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes. 1991 Feb;40(2):166–180. doi: 10.2337/diab.40.2.166. [DOI] [PubMed] [Google Scholar]
- Sako Y., Grill V. E. A 48-hour lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and B cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology. 1990 Oct;127(4):1580–1589. doi: 10.1210/endo-127-4-1580. [DOI] [PubMed] [Google Scholar]
- Surwit R. S., Kuhn C. M., Cochrane C., McCubbin J. A., Feinglos M. N. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988 Sep;37(9):1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- Verchere C. B., D'Alessio D. A., Palmiter R. D., Kahn S. E. Transgenic mice overproducing islet amyloid polypeptide have increased insulin storage and secretion in vitro. Diabetologia. 1994 Jul;37(7):725–728. doi: 10.1007/BF00417699. [DOI] [PubMed] [Google Scholar]
- Westermark P. Quantitative studies on amyloid in the islets of Langerhans. Ups J Med Sci. 1972;77(2):91–94. doi: 10.1517/03009734000000014. [DOI] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Hayden D. W., O'Brien T. D., Johnson K. H. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986 Nov 14;140(3):827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- de Koning E. J., Morris E. R., Hofhuis F. M., Posthuma G., Höppener J. W., Morris J. F., Capel P. J., Clark A., Verbeek J. S. Intra- and extracellular amyloid fibrils are formed in cultured pancreatic islets of transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8467–8471. doi: 10.1073/pnas.91.18.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]




