Abstract
Abstract: Cardiothoracic surgeons have utilized the surgical robot to provide a minimally invasive approach to a number of intracardiac operations, including tumor resection, valve repair, and ablation of atrial arrhythmia. We report the case of a 58 year-old woman who was found to have a mobile mass on her aortic valve during evaluation of atrial fibrillation. Both of these conditions were addressed when she underwent a combined robotic biatrial Maze procedure and excision of the mass, which proved to be a papillary fibroelastoma of the aortic valve.
Introduction
Atrial fibrillation is associated with significant morbidity and mortality, and for some patients it may be the cause of particularly bothersome symptoms.
Over the last two and a half decades, surgical treatment of atrial fibrillation with the Cox Maze procedure has been demonstrated to be a safe and effective method of restoring sinus rhythm [1,2]. A number of variations of the operation have been developed to allow for its adaptation to a minimally invasive technique. These have included the use of cryoablation through a minithoracotomy [3], and more recently a robotic approach [4].
With widespread use of echocardiography, asymptomatic cardiac valvular lesions are found with some frequency. Mobile masses associated with left-sided heart valves are particularly worrisome because they present a risk of embolization of tumor or its associated thrombus. Although the majority of these valvular lesions will be found upon resection to be benign fibroelastomas, surgical excision is recommended to avoid the potentially catastrophic complications of cerebrovascular accident, retinal artery occlusion or cardiac event [5,6].
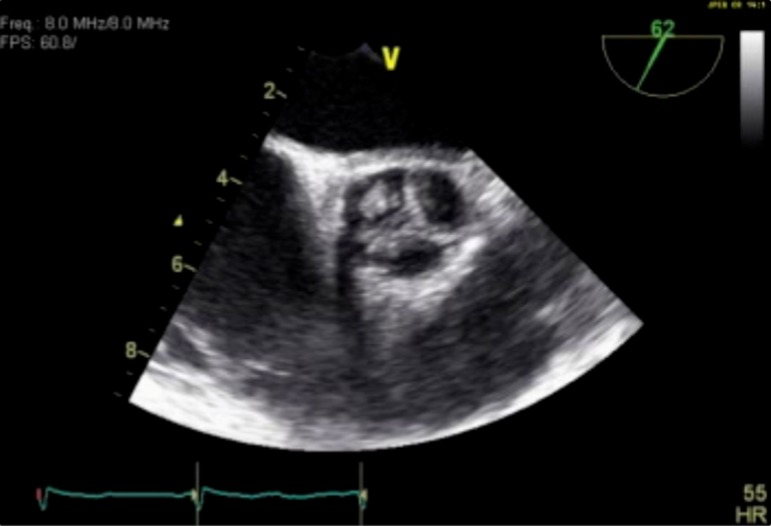
This patient, a 58 year-old woman with a history of hypertension, dyslipidemia, and an uncharacterized bleeding disorder, experienced uncomfortable paroxysms of atrial fibrillation. These occurred two to three times per week, and were accompanied by chest pressure, dizziness, and palpitations. The episodes persisted despite medical therapy first with flecainide and then with propafenone. During evaluation for a possible catheter ablation procedure she was found, by transesophageal echocardiography, to have a mobile mass on the left coronary cusp of the aortic valve measuring 0.6 by 0.7 cm. (Figures 1 and 2) The remainder of her echocardiographic evaluation was remarkable only for mild mitral valve regurgitation. Cardiac CTA demonstrated no significant atherosclerotic disease. Chest and abdominal CTA revealed no anatomic contraindication to a minimally invasive surgical approach with retrograde femoral artery perfusion. Because of the history of easy bleeding and bruising, hematology consultation was obtained, but no identifiable defect of coagulation was identified.
Figure 1. .
Transesophageal echocardiography of aortic valve showing mass on left coronary cusp, transverse view.
Figure 2. .
Transesophageal echocardiography of aortic valve showing mass on left coronary cusp, longitudinal view.
After informed consent was obtained the patient was brought to surgery, general anesthesia induced, and lung separation accomplished with an endobronchial balloon catheter. Venous drainage for cardiopulmonary bypass was established using a 17 Fr catheter (Medtronic Inc, Minneapolis MN) placed percutaneously in the right internal jugular vein and a 24 Fr catheter (Avalon Laboratories, Rancho Dominguez CA) directed through the right common femoral vein to the right atrium. Arterial return was accomplished with a 21 Fr catheter directed proximally through the right common femoral artery, and a 10 Fr pediatric aortic cannula (Medtronic Inc, Minneapolis MN) directed distally in the right superficial femoral artery to provide lower extremity perfusion. Right chest incisions for the robotic ports were placed anterior to the anterior axillary line (AAL) at the 3rd intercostal space (ICS), posterior to the AAL at the 6th ICS, and at the 5th ICS along the midclavicular line. A working port incision of 5 cm length was placed along the 4th ICS crossing the AAL. A Chitwood aortic clamp was brought through the 2nd ICS at the midaxillary line, and the aorta was cross-clamped. Antegrade cardioplegia was administered via the aortic root and intermittent retrograde cardiplegia was given via a coronary sinus catheter (Edwards Lifesciences, Irvine CA) placed percutaneously via the right internal jugular vein. The daVinci surgical robot (Intuitive Surgical, Sunnyvale, CA) was docked, a 30 degree up-oriented scope utilized, and the aorta opened transversely above the right coronary artery. The mobile mass on the left coronary cusp of the aortic valve was visualized (Figure 3).
Figure 3. .
Intraoperative view/video of left coronary cusp mass.
This grossly appeared to have numerous fronds and a “sea anemone” appearance. The mass was excised by shaving it off the aortic valve, and the aorta was closed with a 4-0 Cardionyl suture (Peters, Paris France) in running to-and-fro fashion. On pathologic evaluation, the diagnosis of papillary fibroelastoma confirmed (Figure 4).
Figure 4. .
Photomicrograph of papillary fibroelastoma.
The biatrial Maze procedure was performed as previously described by Rodriguez and colleagues [4]. The left atriotomy incision was placed anterior to the right pulmonary veins in longitudinal fashion. Two minute applications of the CryoMaze probe (ATS Medical, Plymouth MN) were utilized to create the left atrial lesions at − 140 to − 160 degrees C. A box lesion was begun from the inferior edge of the atriotomy along the floor of the left atrium, then anterior to the left pulmonary veins (Figure 5).
Figure 5. .
Maze lesion forming inferior portion of pulmonary vein isolation box.
The box was completed by creating a lesion from the superior edge of the atriotomy along the roof of the left atrium, the anterior to the pulmonary veins to connect with the first lesion. (Figure 6) The mitral lesion was made from the inferior edge of the box lesion to the P3 portion of the mitral annulus. The mirror image of this lesion was made on the epicardial surface and over the coronary sinus by placing the probe in the oblique pericardial sinus (Figure 7).
Figure 6. .
Maze lesion forming superior portion of pulmonary vein isolation box.
Figure 7. .
Epicardial lesion over the coronary sinus placed with the probe in the oblique coronary sinus.
The atriotomy was closed with CV-2 Goretex (W.L. Gore & Assoc, Flagstaff AZ) in running fashion. Snares at the cavoatrial junctions were tightened and a transverse right atriotomy fashioned. One minute applications of the cryoprobe were utilized. A linear lesion from the lateral aspect of the superior vena cava to the inferior vena cava was fashioned (Figure 8).
Figure 8. .
Intercaval lesion from lateral aspect of SVC to IVC.
A lesion connecting this line to the right atriotomy incision was placed, completing the “T”-shaped set on the right atrial wall (Figure 9).
Figure 9. .
Lesion connecting right atriotomy to intercaval lesion.
Endocardial lesions were then made from the medial edge of the atriotomy to the 2 o'clock position on the tricuspid annulus (Figure 10) and from the 10 o'clock position to the right atrial appendage (Figure 11). The final lesion extended from appendage to approximately 2 cm from the atriotomy.
Figure 10. .
Lesion from right atriotomy to Tricuspid Valve annulus.
Figure 11. .
Lesion from Tricuspid Valve annulus to Right Atrial Appendage.
Warm blood was administered as the atriotomy was closed, and once the heart began to beat, the crossclamp was removed. A bipolar right ventricular temporary pacing wire was placed, the robot undocked, and the patient was weaned from bypass in normal sinus rhythm with normal right and left ventricular contractility by transesophageal echo. The aortic valve showed no insufficiency nor residual mass. Protamine was administered and routine decannulation and closure were accomplished.
Initial post-operative course was uneventful, but on postoperative day six the patient fell, with subsequent development of a significant periorbital hematoma which required operative evacuation.
Despite this, she was able to be discharged on post-operative day seven, and promptly returned to her usual activities. Her symptoms of atrial fibrillation resolved completely, and she remains in sinus rhythm two years post surgery.
Comment
Over the last decade and one half, refinements in robotic cardiac surgery have enabled its use to extend beyond a few pioneering centers [7,8] to more widespread application. The surgical robot is now used routinely to repair mitral valves, close atrial septal defects, create ablative lesions for atrial fibrillation, and remove intracardiac masses and tumors. Robotic resection of an aortic valve papillary fibroelastoma has been described [9], but we believe this is the first description of such a resection concomitant with a biatrial Maze procedure.
Papillary fibroelastomas represent the minority of primary tumors of the heart, but the vast majority of tumors of the cardiac valves [5]. They may be found on any of the heart valves, do not exhibit the potential for malignant transformation, and are usually not symptomatic. Most commonly they are found incidentally when patients undergo echo-cardiography for a different indication, as was the case in our patient.
Despite the benign nature of papillary fibroelastomas, when present in the left heart these lesions have been shown to present a risk of embolism with central nervous system accident, cardiac injury, or end-organ malperfusion, and prompt surgical removal is recommended [5,6].
The presence of paroxysmal atrial fibrillation in this patient presented the opportunity to perform one surgical procedure that would address both conditions. Feasibility of the concomitant performance of robotic mitral repair and a Maze procedure using cryoablation has previously been demonstrated [10].
The successful outcome of the case described here suggests that a robotic approach can similarly be utilized for the infrequent patient who presents with atrial fibrillation and a lesion of the aortic valve.
References
- [1].Cox JL. The first Maze procedure. J Thor Cardiovasc Surg. 2011;141:1093–1097. doi: 10.1016/j.jtcvs.2010.12.012. [DOI] [PubMed] [Google Scholar]
- [2].Gaynor SL, Schuessler RB, Bailey MS, Ishii Y, Boineau JP, Gleva MJ, Cox JL, Damiano RJ. Surgical treatment of atrial fibrillation: Predictors of late recurrence. J Thorac Cardiovasc Surg. 2005;129:104–111. doi: 10.1016/j.jtcvs.2004.08.042. [DOI] [PubMed] [Google Scholar]
- [3].Ad N, Cox JL. The Maze procedure for the treatment of atrial fibrillation: A minimally invasive approach. Jour Card Surg. 2004;19:196–200. doi: 10.1111/j.0886-0440.2004.4036_1.x. [DOI] [PubMed] [Google Scholar]
- [4].Rodriguez E, Cook RL, Chu MW, Chitwood WR. Minimally invasive bi-atrial cryomaze operation for atrial fibrillation. Oper Techniques Thor Cardiovasc Surg. 2009;14:208–223. [Google Scholar]
- [5].Shahian DM. Papillary fibroelastomas. Semin Thorac Cardiovasc Surg. 2000;12:101–110. doi: 10.1053/ct.2000.5082. [DOI] [PubMed] [Google Scholar]
- [6].Bossert T, Gummert JF, Mohr F. Papillary Fibroelastomas and other cardiac tumors should be resected on an urgent basis. Ann Thor Surg. 2005;79:756. doi: 10.1016/j.athoracsur.2004.02.145. [DOI] [PubMed] [Google Scholar]
- [7].Falk V, Walther T, Autschbach R, Diegeler A, Battelini R, Mohr FW. Robot-assisted minimally invasive solo mitral valve operation. J Thor Cardiovasc Surg. 1998;115:470–471. doi: 10.1016/S0022-5223(98)70295-8. [DOI] [PubMed] [Google Scholar]
- [8].Chitwood WR, Nifong LW, Elbeery JE, Chapman WH, Albrecht R, Kim V, Young JA. Robotic mitral valve repair: trapezoidal resection and Prosthetic annuloplasty with the daVinci Surgical system. J Thor Cardiovasc Surg. 2000;120:1171–1172. doi: 10.1067/mtc.2000.110177. [DOI] [PubMed] [Google Scholar]
- [9].Woo YJ, Grand TJ, Weiss SJ. Robotic resection of an aortic valve papillary fibroelastoma. Ann Thorac Surg. 2005;80:1100–1102. doi: 10.1016/j.athoracsur.2004.02.108. [DOI] [PubMed] [Google Scholar]
- [10].Rodriguez E, Nifong LW, Chu MW, Wood W, Vos PW, Chitwood WR. Robotic mitral valve repair for anterior leaflet and bileaflet prolapse. Ann Thorac Surg. 2008;85:438–444. doi: 10.1016/j.athoracsur.2007.04.122. [DOI] [PubMed] [Google Scholar]