Abstract
Using manometric techniques, H2 evolution in both darkness and light has been studied in the green alga, Chlamydomonas moewusii.
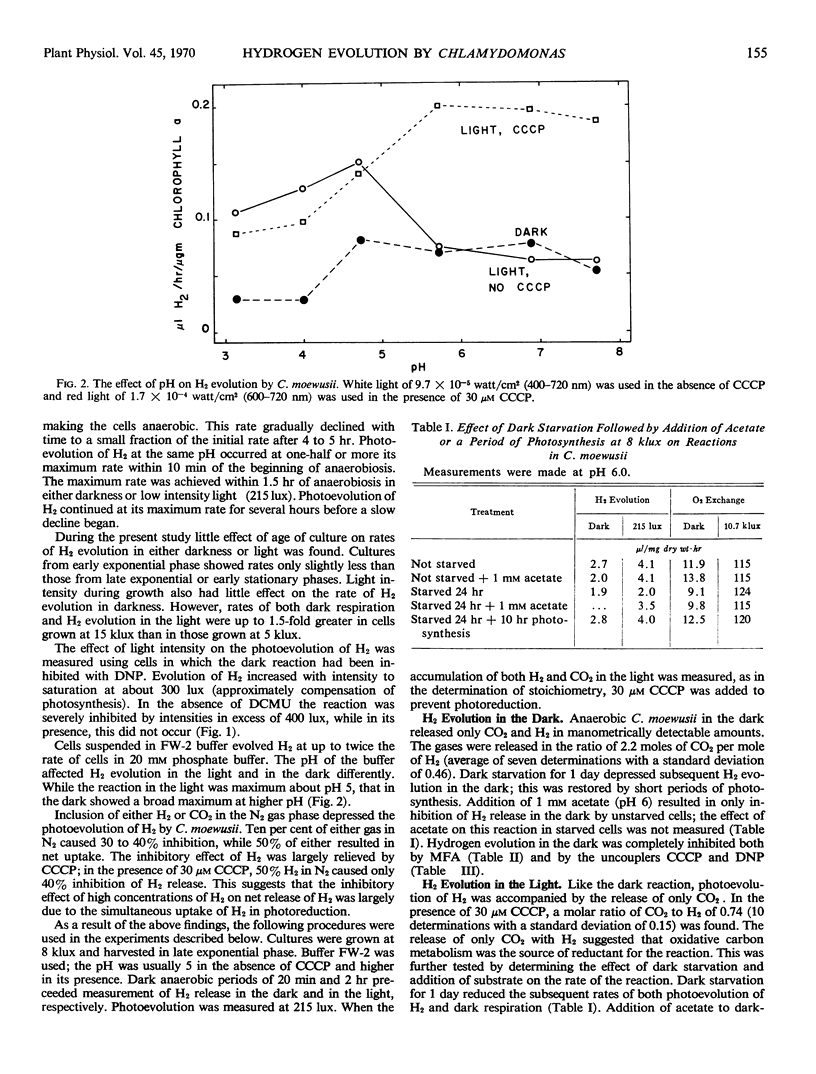
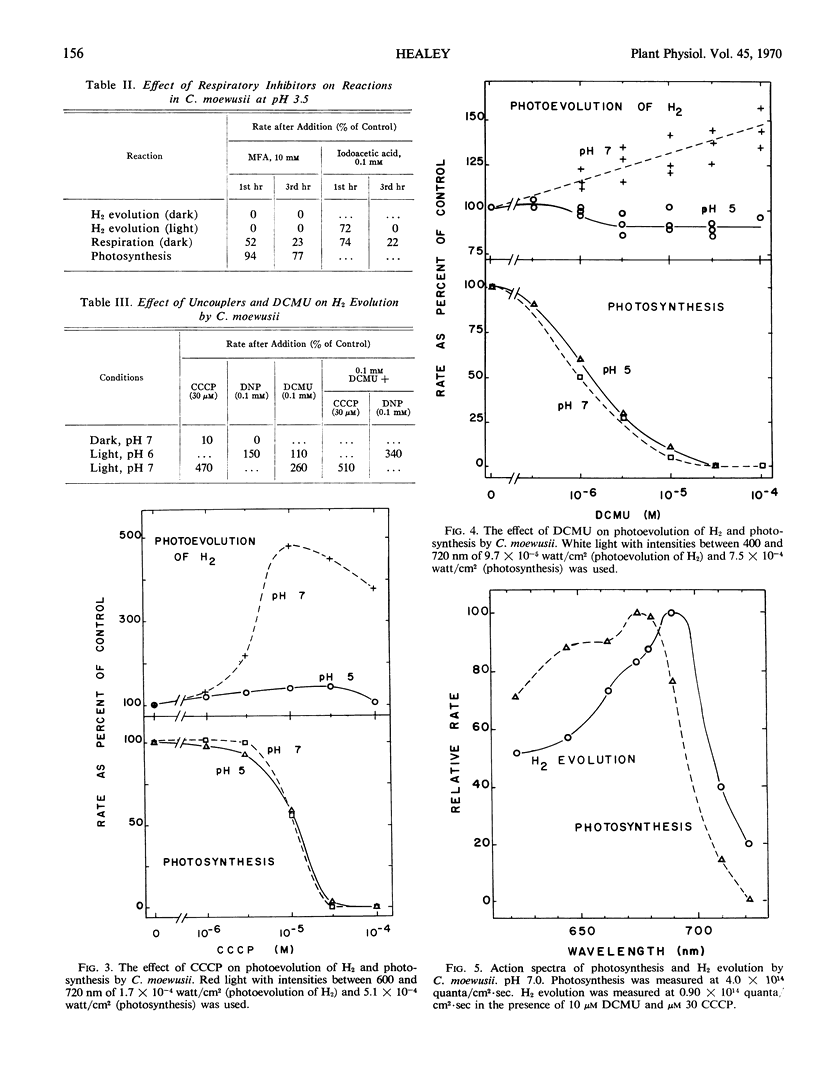
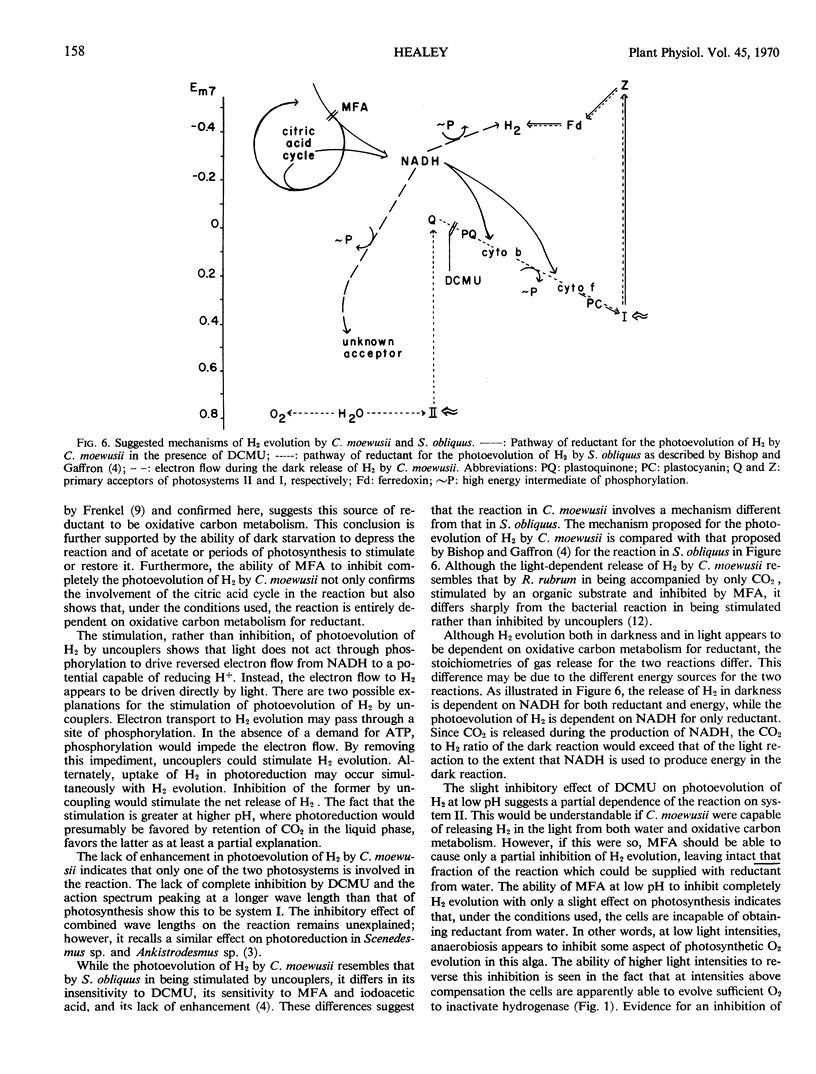
Hydrogen evolution in the dark is accompanied by the release of only CO2 in manometrically detectable amounts. It is depressed by dark starvation and inhibited both by monofluoroacetic acid and by uncouplers of phosphorylation. This evidence suggests that the reaction is dependent on oxidative carbon metabolism for reductant and phosphorylation for energy to raise the reductant to a redox potential capable of reducing H+.
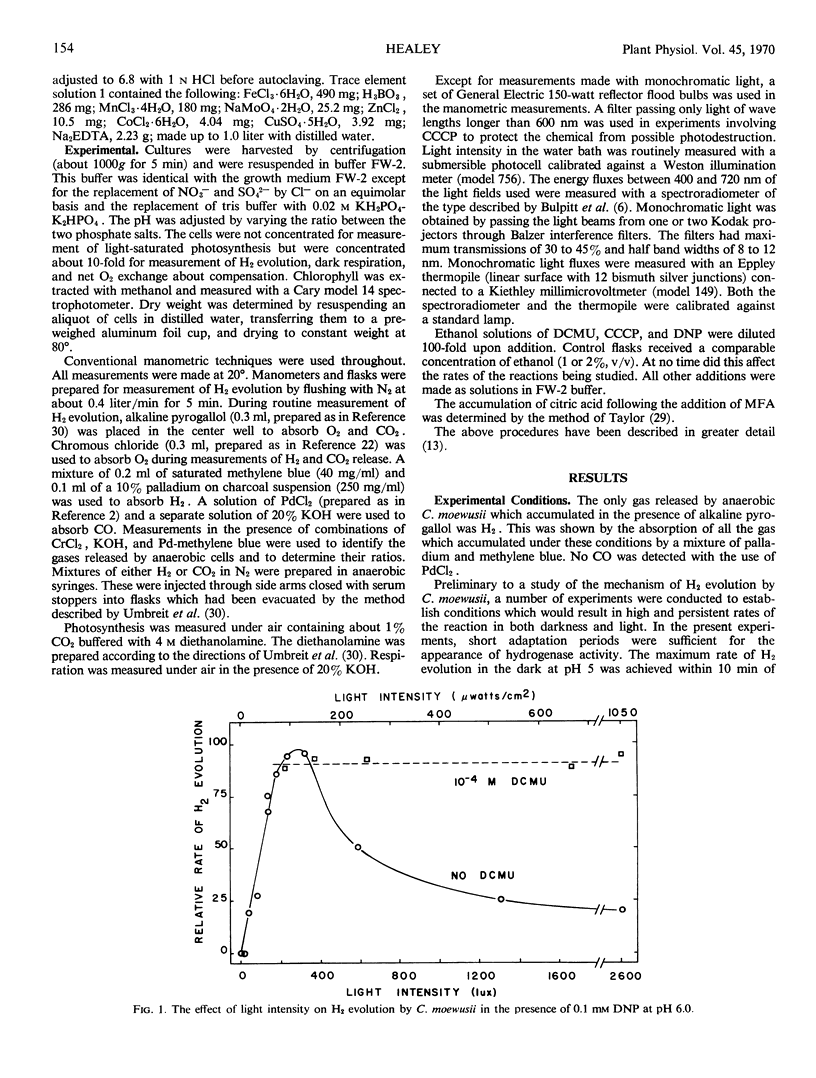
Photoevolution of H2 is also accompanied by the release of only CO2. It is depressed by dark starvation and stimulated by acetate or a period of photosynthesis. Monofluoroacetic acid causes complete inhibition, while 3-(3,4-dichlorophenyl)-1,1-dimethylurea causes no or only slight inhibition. These results indicate that oxidative carbon metabolism is the source of reductant for the reaction. Photoevolution of H2 does not show Emerson enhancement, and it has an action spectrum peaking at a longer wave length than that of photosynthesis. These characteristics, together with the slight effect of 3-(3,4-dichlorophenyl)-1,1-dimethylurea on the reaction, show that only system I of photosynthetic electron transport is involved in the reaction. Photoevolution of H2 is stimulated by uncouplers; this indicates that the role of light is not to provide energy by phosphorylation. Rather, the results support an electron flow driven directly by light through system I from reductant produced in oxidative carbon metabolism to a redox potential capable of reducing H+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN T. H., ROOT W. S. Colorimetric determination of carbon monoxide in air by an improved palladium chloride method. J Biol Chem. 1955 Sep;216(1):309–317. [PubMed] [Google Scholar]
- Abeles F. B. Cell-free Hydrogenase from Chlamydomonas. Plant Physiol. 1964 Mar;39(2):169–176. doi: 10.1104/pp.39.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP N. I., GAFFRON H. Photoreduction at lambda 705 millimicrons in adapted algae. Biochem Biophys Res Commun. 1962 Aug 31;8:471–476. doi: 10.1016/0006-291x(62)90299-1. [DOI] [PubMed] [Google Scholar]
- BOSE S. K., GEST H. Hydrogenase and light-stimulated electron transfer reactions in photosynthetic bacteria. Nature. 1962 Sep 22;195:1168–1171. doi: 10.1038/1951168a0. [DOI] [PubMed] [Google Scholar]
- Cramer W. A., Butler W. L. Light-induced absorbance changes of two cytochrome b components in the electron-transport system of spinach chloroplasts. Biochim Biophys Acta. 1967 Sep 6;143(2):332–339. doi: 10.1016/0005-2728(67)90087-4. [DOI] [PubMed] [Google Scholar]
- FRENKEL A. W. Hydrogen evolution by the flagellate green alga, Chlamydomonas moewusii. Arch Biochem Biophys. 1952 Jul;38:219–230. doi: 10.1016/0003-9861(52)90026-x. [DOI] [PubMed] [Google Scholar]
- GEST H., ORMEROD J. G., ORMEROD K. S. Photometabolism of Rhodospirillum rubrum: light-dependent dissimilation of organic compounds to carbon dioxide and molecular hydrogen by an anaerobic citric acid cycle. Arch Biochem Biophys. 1962 Apr;97:21–33. doi: 10.1016/0003-9861(62)90039-5. [DOI] [PubMed] [Google Scholar]
- Hiyama T., Nishimura M., Chance B. Energy and Electron Transfer Systems of Chlamydomonas reinhardi. I. Photosynthetic and Respiratory Cytochrome Systems of the Pale Green Mutant. Plant Physiol. 1969 Apr;44(4):527–534. doi: 10.1104/pp.44.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES L. W., MYERS J. A COMMON LINK BETWEEN PHOTOSYNTHESIS AND RESPIRATION IN A BLUE-GREEN ALGA. Nature. 1963 Aug 17;199:670–672. doi: 10.1038/199670a0. [DOI] [PubMed] [Google Scholar]
- KESSLER E., MAIFARTH H. [The presence and activity of hydrogenase in various green algae]. Arch Mikrobiol. 1960;37:215–225. [PubMed] [Google Scholar]
- STIFFLER H. J., GEST H. Effects of light intensity and nitrogen growth source on hydrogen metabolism in Rhodospirillum rubrum. Science. 1954 Dec 17;120(3129):1024–1026. doi: 10.1126/science.120.3129.1024. [DOI] [PubMed] [Google Scholar]
- Stiller M. Hydrogenase mediated nitrite reduction in chlorella. Plant Physiol. 1966 Feb;41(2):348–352. doi: 10.1104/pp.41.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR T. G. A modified procedure for the microdetermination of citric acid. Biochem J. 1953 Apr;54(1):48–49. doi: 10.1042/bj0540048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon L. P. Photochemical and electron transport reactions of bacterial photosynthesis. Bacteriol Rev. 1968 Sep;32(3):243–261. doi: 10.1128/br.32.3.243-261.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



