Abstract
Abstract: In this article, we outline the plans, protocols and strategies to set up the first nationwide primary Percutaneous Coronary Intervention (PCI) program for ST-elevation myocardial Infarction (STEMI) in Qatar, as well as the difficulties and the multi-disciplinary solutions that we adopted in preparation. We will also report some of the landmark literature that guided our plans. The guidelines underscore the need for adequate number of procedures to justify establishing a primary-PCI service and maintain competency. The number of both diagnostic and interventional procedures in our centre has increased substantially over the years. The number of diagnostic procedures has increased from 1470 in 2007, to 2200 in 2009 and is projected to exceed 3000 by the end of 2012. The total number of PCIs has also increased from 443 in 2007, to 646 in 2009 and 1176 in 2011 and is expected to exceed 1400 by the end of 2012. These figures qualify our centre to be classified as ‘high volume’, both for the institution and for the individual interventional operators. The initial number of expected primary PCI procedures will be in excess of 600 procedures per year. Guidelines also emphasize the door to balloon time (DBT), which should not exceed 90 minutes. This interval mainly represents in-hospital delay and reflects the efficiency of the hospital system in the rapid recognition and transfer of the STEMI patient to the catheterization laboratory for primary-PCI. Although DBT is clearly important and is in the forefront of planning for the wide primary PCI program, it is not the only important time interval. Myocardial necrosis begins before the patient arrives to the hospital and even before first medical contact, so time is of the essence. Therefore, our primary PCI program includes a nationwide awareness program for both the population and health care professionals to reduce the pre-hospital delay. We have also taken steps to improve the pre-hospital diagnosis of STEMI. In addition to equipping all ambulances to perform 12-lead electrocardiograms (ECGs) we will establish advanced wireless transmission of the ECG to our Heart Centre and to the smart phone of the consultant on-call for the primary-PCI service. This will ensure that the patient is transferred directly to the cath lab without unnecessary delay in the emergency rooms. A single phone-call system will allow the first medic making the diagnosis to activate the primary PCI team. The emergency medical system is acquiring capability to track the exact position of each ambulance using GPS technology to give an accurate estimate of the time needed to arrive to the patient and/or to the hospital. We also plan for medical helicopter evacuation from remote or inaccessible areas. A comprehensive research database is being established to enable specific pioneering research projects and clinical trials, either as a single centre or in collaboration with other regional or international centers. The primary-PCI program is a collaborative effort between the Heart Hospital, Hamada Medical Corporation and the Qatar Cardiovascular Research Centre, a member of Qatar Foundation. Qatar will be first country to have a unified nationwide primary-PCI program. This clinical and research program could be a model that may be adopted in other countries to improve outcomes of patients with STEMI.
Introduction
In addition to unstable angina (UA), the term “acute coronary syndrome (ACS) includes all patients with acute myocardial infarction (AMI), either with ST-segment elevation on the electrocardiogram (STEMI) or without ST-elevation (non-STEMI, or NSTEMI). Both share the essential criterion of raised cardiac biomarkers, especially high sensitivity troponin, as opposed to UA, in which the clinical and ECG features of ACS are not accompanied by a rise in cardiac biomarkers. In this article we will concentrate mainly on the interventional management of STEMI, but will touch on NSTEMI ACS where relevant.
Reperfusion Strategies for STEMI
Advances in the management of STEMI have their roots in fundamental research in basic science. In a landmark publication of Libby [1], there was an illustrative diagram of the pathophysiology of atheromatous disease, from the genesis of a “stable” plaque, to “unstable” plaque leading to plaque rupture (Figure 1). This latter event is thought to be culpable in the pathogenesis of ACS. Plaque rupture is a potent activator of platelet aggregation and/or clotting mechanisms, thus resulting in partial or complete occlusion of the afflicted artery. If the patient survives, the subsequent healing process leads to either permanent occlusion of the vessel, or a variable degree of re-canalization. While an excellent demonstration of the overall picture, one must note that the figure gives an impression of a one-way progressive pathology. This is not always true, for we know that plaque rupture and thrombus formations are not inevitable events. Furthermore, we also know from large trials that rigorous modification of the risk factors often leads to regression, rather than progression, of the process. However, the illustration remains a landmark tool for teaching the patho-physiology of coronary artery disease (CAD) and ACS.
Figure 1. .
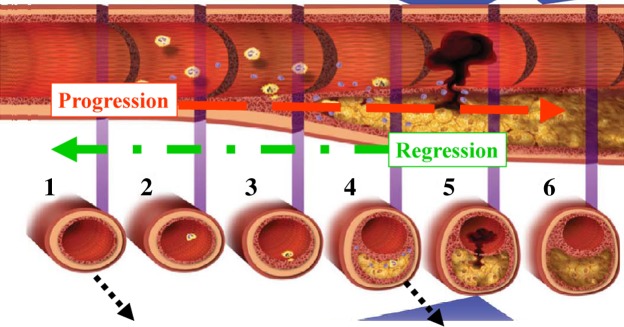
A model for the natural history of coronary artery disease, from stable to unstable disease. Although this process typically progresses, it may also stabilize and even reverse with optimal medical therapy. Modified from Libby [1].
Fibrinolysis to dissolve the culprit thrombus was the first largely accepted reperfusion therapy in STEMI [2]. However, initial experience with fibrinolytic therapy was surrounded by skepticism, mainly because of lack of guidance on the appropriate dose range; too little did not adequately dissolve the thrombus and too much gave hemorrhagic complications. It took sometime before the right dose was eventually found. For many years, fibrinolytic therapy remained the mainstay therapy for STEMI and different agents and different regimens were refined with significant, but somehow limited improvement to the original agents. Furthermore, there remained groups of high-risk patients in whom fibrinolytic therapy was either suboptimal or ineffective, most notably in high-risk patients with STEMI, particularly with cardiogenic shock. Another important element which boosted the advantage of primary-PCI came from studies, like the GUSTO Angiographic Trial [3,4] that included an angiographic limb shortly after the administration of fibrinolytic therapy. Firstly, it was shown that only 50–60% of patients who received fibrinolysis actually achieved complete re-canalization of the infarct related artery (IRA). Secondly, it was also shown that unless complete re-canalization of the IRA is achieved (TIMI-3 flow), the mortality was not greatly affected. In contrast, when primary-PCI was performed timely, TIMI-3 flow was achieved in over 90% of patients [5]. It is also important to state that the effect of TIMI flow on mortality continued to affect mortality for months to years after the initial event [6,7] Thirdly, there was a also relatively higher rate of re-occlusion after fibrinolysis than after primary-PCI [8].
Primary PCI in perspective
The first “primary” PCI for AMI was reported by Meyer in 1982 [9]. However, the first randomized trials were not published until 10 years later by Grines et al. and Zijlstra et al. [6,8]. In experienced hands, primary-PCI is usually straightforward and often leads to spectacular angiographic results (Figure 2). However, as with fibrinolytic therapy earlier experience was somewhat disappointing, with some of the early trials showing a better survival in the fibrinolysis treated groups [10]. It was soon realized, however, that the time between clinical presentation and successful reperfusion was as important in primary-PCI [11] as it was already proven in fibrinolysis [2,12]. There was also a difference in the risk profile of patients allocated in the two groups, with a greater proportion of higher risk patients included in the primary-PCI compared to the fibrinolysis groups.
Figure 2. .
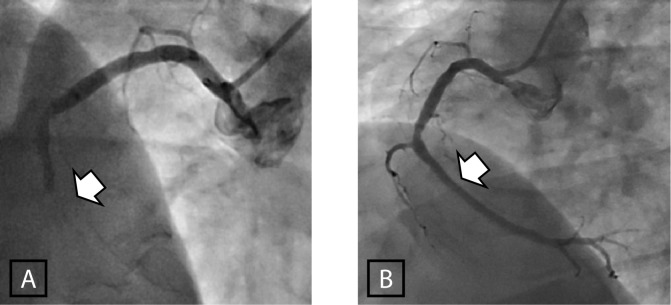
Primary Angioplasty for occluded Right Coronary Artery in ST-Elevation Myocardial Infarction. The arrow indicates the site of occlusion before (A), and after full restoration of blood flow (B).
Several randomized trials have demonstrated the superiority of primary PCI compared to fibrinolysis [13], even though in the largest of these trials (DANAMI-2 [14] and PRAGUE-2 [15]), most of the patients did not initially present to hospitals with PCI facilities. Also bare-metal, rather than drug eluting stents were used.
When performed timely, primary-PCI is superior in terms of mortality and morbidity to fibrinolytic therapy. In the guidelines, primary-PCI now holds Class 1, level of Evidence A in the management of STEMI [16]. However this superiority is not uniform in all patients with STEMI. The spectrum extends from maximum superiority in cases of cardiogenic shock, to almost equal benefit from both reperfusion mortalities in low risk patients with small STEMI, early presentation, and good left ventricular (LV) function. In contrast, diabetic patients overall draw greater benefit from primary-PCI than thrombolysis. In a study by Timmer et al. [17], the combined one-month mortality and re-infarction rate in diabetic patients was 6.7% with primary-PCI and 12.5% with fibrinolysis (p = 0.001).
Trials investigating both fibrinolysis and primary-PCI have consistently emphasized the crucial factor of time. The difference is most pronounced in the early hours of the infarction process. It has been amply demonstrated that the outcome from re-perfusion therapy delivered < 2 hours from onset of chest pain was markedly different from those who presented > 4 hours [18]. This difference not only affected the in-hospital and 30-day mortality, but the survival curves continued to diverge over the long term [18,19].
The Importance of time in Primary-PCI
Although it is true that primary-PCI is the perfusion modality of choice in STEMI, this is not an absolute statement, as time to reperfusion is crucial. If primary-PCI is delayed, then much of the edge over fibrinolysis is lost and, as it is often said, “early” fibrinolysis may be better than “late” primary-PCI, at least in low risk patients. The time between the onset of symptoms and the actual balloon dilatation has several important components which can be crudely divided into those prior the arrival to the hospital (pre-hospital delay) and those from the arrival to the hospital to the actual balloon dilatation of the IRA. In the GUSTO-IIb trial, 5 lives per 1000 were saved with every hour of earlier fibrinolysis [11]. Similarly, the TIMI I-2 trial revealed that 10 lives were saved with every hour earlier fibrinolysis for the first four hours [12].
Almost all trials and meta-analyses emphasize the importance of in-hospital delay [15,16,19–21] which is represented by the “Door to Balloon Time” (DBT). It is generally agreed that this should not exceed 90 minutes [16,21]. This interval reflects the efficiency of the hospital systems in the rapid recognition and transfer of the STEMI patient to the catheterization laboratory and prompt performance of the primary-PCI procedure. Although DBT is clearly important and is in the forefront of our planning for a national wide primary-PCI program in Qatar, it is not the only important time interval. One must remember that myocardial necrosis does not start when the patient arrives at the hospital or when he/she makes the first medical contact. It starts at, or maybe even before, the onset of symptoms. Surely if the time between appearance of symptoms and seeking medical advice is prolonged, even a shorter DBT (even though important) may not reduce the muscle damage or mortality. Therefore while our nationwide program will strive to beat the 90-minute threshold for DBT, it is also vital that we sensibly look at the other equally important time intervals.
Each time interval has a different set of factors that cause delay and a unique set of solutions different from the other intervals. For example, unlike DBT, the time from symptoms to first medical contact does not reflect the hospital delay, but it reflects multiple other factors in the pre-hospital phase. These include the patient's own delay in recognizing the major symptoms of a heart attack and thus delay in seeking early medical advice. Data from even the most developed countries still show unacceptable delays in seeking medical help in the very regions where hospitals have excellent records of DBT [22–25]. The most important factor in pre-hospital delay is the lack of awareness of the most typical presentations of heart attacks. Available studies from around the globe provide evidence of staggering delays and often a total lack of knowledge among the public of what to do when presented with heart attack symptoms. In a US study up to 32% of patients who were treated by primary PCI presented late [26]. Surveys in Portugal and France, evaluating the need of awareness programs, discovered a very low level of awareness about the possible symptoms of ACS and about the need to call the emergency medical system (EMS) if and when such symptoms occur. Furthermore, the surveys revealed that less than half the patients called the EMS in France, while over two thirds of patients in Portugal arrived to the hospital by their own transport.
While the DBT has received great attention, the importance of the pre-hospital delay has not received the same attention in the literature. Admittedly, this latter time has not been shown to affect the outcome as consistently as the DBT. Several explanations have been postulated for the lack of a strict relationship between pre-hospital delay and mortality, when a strong relationship is well demonstrated with DBT. However, none is totally convincing and investigations to solve this puzzle will continue. This presumably reflects the difficulty in the precise recognition of when symptoms begin and when first medical contact is made. This is unlike the DBT, which can be determined more precisely. Nonetheless, until this issue is settled by more studies, it will be illogical to develop systems for improved STEMI care that ignore the pre-hospital phase and concentrate only on the hospital DBT.
It may sound odd, but large registries such as the National Registry of Myocardial Infarction (NRMI) and GUSTO–IIb have suggested that the adjusted in-hospital mortality does not increase significantly with increasing pre-hospital delay, while a similar increase in the DBT did increase the odds for in-hospital mortality from around 40% to around 60% [11,27]. The GUSTO-IIb study showed that the in-hospital mortality for patients with DBT of less than one hour was only 1% as compared to 6.4% in those with DBT of more than 90 minutes (P = 0.001) [11]. The in-hospital mortality was approximately 1% when DBT was < 60 minutes, but it jumped to 6.4% when DBT was >90 minutes. In between these extreme ranges of DBT, there is a clear relationship between in-hospital mortality and each increment of DBT. This raises a logical question: why did a 30 minute decrease in DBT result in a significant survival benefit, while a similar decrease in pre-hospital delay did not? However, the two time intervals are not entirely unrelated. There is an interesting relationship indicating that the pre-hospital delay and the in-hospital delay times are related when it comes to the outcome of primary-PCI patients. In a study by Brodie et al. [28] using data from the CADILLAC and HORIZONS –AMI trials, the DBT made a significant impact on survival among patients who presented early after the appearance of symptoms (short pre-hospital delay). Thus, mortality in those who presented soon after the onset of symptoms was 1.9%, if DBT time was < 90 minutes, but was 3.8% if DBT was >90 minutes [28]. In contrast, in patients who presented late, the DBT did not seem to have the same impact on mortality. In another analysis [29], while the 7-year mortality after primary-PCI showed a clear difference when DBT was less than 120 minutes (mortality 15%)–as opposed to patients with DBT greater than 120 minutes (mortality 21%)–the difference was only noted in those patients who presented < 3 hours after the onset of chest pain. In those who presented greater than 3 hours, the DBT had less impact on survival. This emphasizes that in primary PCI, it is not only the DBT that determines outcome, but the pre-hospital delay may also play an important role [29].
It is also relevant to mention that in trials using fibrinolysis for STEMI, the interval from appearance of symptoms and fibrinolysis was indeed a very strong predictor of survival. Therefore, it should be logical to expect the same for primary-PCI, not only for DBT, but the entire delay from symptom onset to reperfusion. The relationship between time delays and mortality is clearer with fibrinolysis than with primary-PCI. This may reflect the relative simplicity to recruit large numbers of patients in fibrinolytic trials and the fact fibrinolysis has been with us for a much longer time period. On the contrary, it is more difficult to gather large groups for controlled trials of primary PCI, and the logistics for primary-PCI protocols are much more complicated than those of fibrinolysis.
In a study by Zwolle group [30], there was a significant and linear correlation between the one-year mortality and the delay from the onset of symptoms to balloon dilatation (which includes both the pre-hospital delay and DBT). Furthermore, a delay of more than four hours was an independent predictor or mortality. In a separate publication, these investigators calculated that every 30 minutes delay is associated with 1.075 increases in the Relative Risk of one-year mortality [31].
Bearing in mind the importance of the pre-hospital on outcome, and the crucial role of both the public and health care professionals, our primary-PCI program has plans for a nationwide awareness program to go hand in hand with efforts to reduce the pre-hospital delay, as well as the DBT. It is true that TV programs about the symptoms and signs of a heart attack are very likely to increase the flow of “false positive” patients to the emergency rooms and increase the demand on the already stretched ambulance service, therefore, the message should be balanced and realistic. This awareness program should be well thought of and preceded by well-studied plans in both the EMS and the hospital emergency room (ER) service to cope with the extra load. Furthermore, in announcing the primary-PCI program, the public should be made aware that primary-PCI has specific indications and is not for all types of chest pain, or even all heart attacks. Otherwise patient after patient will demand this “magic” treatment whether indicated or not.
Another important component of the pre-hospital delay is the lack of awareness amongst some physicians, in primary health centers and private practice, of the importance of reducing unnecessary investigations in STEMI in favor of fast transfer. Many practitioners still feel that full assessment is necessary before referral of a STEMI patient to the cardiac centre. Furthermore, some may not even be aware of the availability of the primary-PCI program or how to access the system and contact the right site. This is our responsibility and should be part of the nationwide awareness program directed to all health care professionals in the country. Without such awareness, patients will continue to be sent to the general ERs, rather than transfered to the catheterization laboratory, which will inevitably increase the delay. Most of these practitioners are capable of making the diagnosis of STEMI on the 12 lead ECG. However, they are used to sending all patients to the ER, which will inevitably cause delay. Practitioners therefore should be instructed to indicate clearly in their referral call that this is a patient with STEMI and that the patient needs transfer directly to the catheterization laboratory in the Heart Hospital, not to the general ERs. It will also be our duty to work out a mechanism whereby practitioners can also contact the primary-PCI team directly on a national hotline dedicated to this purpose.
Reducing Pre-hospital and In-hospital Delay in Qatar
The DBT can be reduced in many ways. It is obvious if the diagnosis of STEMI is made before the patient arrives to the hospital, and if such information is passed to the primary-PCI team, it will significantly reduce the DBT, thus allowing the primary-PCI team to assemble in advance of the arrival of the patient. We have taken several steps to achieve this pre-hospital diagnosis in Qatar. For example, while all ambulances have ECG monitoring and resuscitation capability, including defibrillation, they are all now being equipped with 12-lead ECG capability. We have designed protocols whereby ambulances with such facilities are preferentially dispatched to calls for chest pain. This takes us to the next step, which is reading the ECG and recognizing STEMI. Most of the paramedics in the ambulance service are competent of recognizing ST-elevation, and focused ECG courses are being planned to update and certify all EMS staff to recognize ST-elevation in 12-lead ECGs.
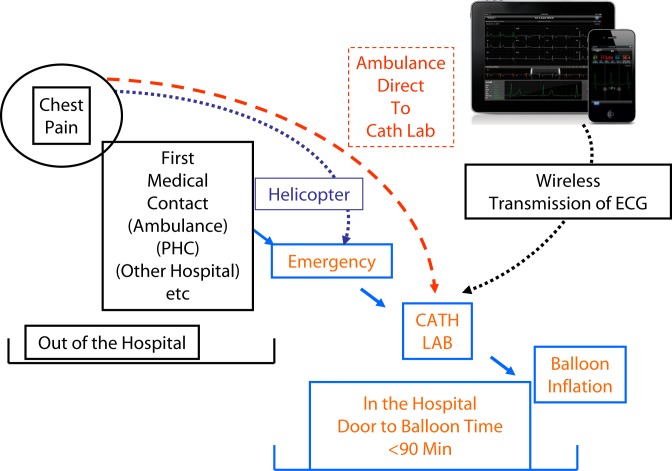
The plans are not only to make all ambulances capable of recording the 12-lead ECG, but also to transmit the tracing wirelessly to our Heart Centre. In collaboration with the EMS and ER, we have commissioned a system which will allow the acquisition and wireless transmission of 12-lead ECG's from the ambulance to our hospital site and to the smart phone of the consultant who is on-call for the primary-PCI service. Thereby, the diagnosis of STEMI is confirmed and the cardiac catheterization laboratory is activated before the arrival of the patient to the hospital.
This procedure will also ensure that the patient is transferred directly to the catheterization laboratory without an unnecessary delay in the ERs of another hospital. It is of note that the protocols also ensure that while the ambulance is dispatched to the patient with chest pain, another, faster, EMS vehicle with an experienced paramedic is dispatched at the same time to get to the exact location of the patient, help identify the address and guide the ambulance to the quickest route, which is particularly valuable in crowded and remote areas. The EMS is also acquiring a system that will track the exact position of each ambulance using the satellite Global Positioning System (GPS). This will speed and optimize the pre-hospital pathway, as the ambulance nearest to the patient location can easily be recognized and dispatched. It will also give an accurate estimate of the time needed to arrive at the patient and to transfer the patient to the hospital.
More recently, we have obtained approval to add patients with STEMI to the list of emergencies qualifying for evacuation by the medical helicopter service, especially from far or inaccessible areas, such as oil rigs. Due to technical issues, this service will initially be from dawn to sunset, but steps are underway to make it another component of the 24/7 service.
Qatar Nationwide Primary-PCI Program
There are many centers and cities with successful primary-PCI programs in the world, such as the citywide primary-PCI in Montreal [32]. The experience with primary-PCI in the UK is worth examining. In 2004, the UK had no strategy of primary-PCI. A survey in 2005 concluded that primary-PCI is feasible in most geographical areas, and that the best DBTs could be achieved if all STEMI patients were directed to facilities with primary-PCI. In 2011, it is estimated that over 90% of all STEMI patients in England who received re-perfusion therapy, had primary-PCI compared to only 46% in 2008 [33]. However, most countries have multiple local or regional programs run under different districts or health authorities. The primary-PCI program in Qatar will be the first to have a unified nationwide primary-PCI program in the world.
The program in Qatar aims to be an important link between successful clinical primary-PCI service and world-class research program in the field of acute management of AMI, especially STEMI.
Relation of Heart Hospital with other Hospitals and EMS Service (The Geography of Primary-PCI Service in Qatar)
Until the recent opening of the state-of-the-art Heart Hospital, the Department of Cardiology and all of its facilities, including the catheterization laboratory were parts of Hamad General Hospital (HGH). For years, patients with chest pain from all over the country, as well as ambulances transporting such patients, traditionally went to the Emergency department in the General Hospital. While patients still go to HGH, the cardiology service has now moved to the new Heart Centre which is situated about a mile from the General Hospital. We have therefore adopted strategies and protocols to assure fast identification and transport of STEMI patients who land in the general HGH into the catheterization laboratory in the new Heart Centre. The Heart Hospital is a state-of-the art tertiary referral cardiac center, with a total of 120 beds. This includes fully monitored critical care beds situated in two cardiology and one cardiac surgery intensive care units. There are three interventional cardiac catheterization laboratories and one hybrid cath lab. The latter is placed within the premises of the surgical suites.
Compliance with Target Door to Balloon Time
Krumholz et al [34] identified nine common themes that were associated with the success of any primary-PCI program. The most important are the setting of explicit and realistic goals to reducing D2B time and motivated and highly respected clinical leaders who push for improvement, as well as equally committed collaborative multi-disciplinary teams and non-blaming data feedback. As an initial target, we should aim to achieve 100% compliance with a DBT of < 90 minutes, but as the program matures, we should target a DBT of < 60 minutes. So far and except for one month, the mean DBT over the last 8 months has consistently been less than the international standard of 90 minutes.
Delay in DBT often can occur from predictable factors, such as waiting for cardiac markers, but it can also result from the most unexpected steps, such as simply delay in taking or reading the 12-lead ECG. Therefore, setting clear targets with regular audits, with appropriate corrective steps, are essential. Furthermore, achieving a target is not the end; we have to make sure that things do not slip back unnoticed. Therefore, a regular audit and mock primary-PCI codes will be conducted periodically as a means of quality control.
Primary-PCI should not be viewed separately from other equally important targets such as 100% compliance with dual platelet therapy, administration of beta-blockers, angiotensin converting enzyme inhibitors (or angiotensin receptor blockers), and statin therapy, and risk factor modification, both in hospital and during follow up [35]. A successful primary-PCI that is not followed by good compliance to dual anti-platelet therapy can lead to devastating stent thrombosis. Similarly, primary-PCI that is not followed by appropriate secondary prevention measures and lifestyle modification will not achieve optimal long-term results..
Primary-PCI Team Activation
The primary-PCI team can potentially be activated from 4 critical points in the country where STEMI patients are potentially identified: from the ambulance, from the ERs, from general practitioner offices and, less commonly, from inpatients already admitted in the Heart Centre or HGH. The latter are the easiest to deal with. Plans were therefore put in place to facilitate the communication from former 3 points to the primary-PCI team. The most important of these is the setup of a dedicated central telephone line that is manned 24/7 to ensure the activation of the primary-PCI team by a single phone call from any of the 3 points mentioned above. The one phone-call system is important so that the first medic to make the diagnosis can activate the primary-PCI team without delay.
Both the emergency physicians and the cardiologists in the ER will have the privilege to activate the primary-PCI team once STEMI is identified. Pain management and initial stabilization should not delay the immediate activation of the primary-PCI teams and fast transfer of the patient to the catheterization laboratory.
The primary-PCI team will consist of a “core” team of those who must attend all primary-PCI procedures, and a “support” team, which will be called when needed. The core team includes a consultant interventional cardiologist, a cardiology fellow or specialist, an anesthesia specialist, anesthesia technician, two cath lab nurses and one cath lab technician. The support team includes consultant cardiac anesthetist, consultant cardiac surgeon, and perfusionist (pump technician).
Another difficulty that hinders faster DBT is the fact that many of the catheterization laboratory staff live away from the hospital, and sometimes a long distance, with the potential of busy traffic. Therefore, assembling the primary-PCI team under these circumstances can be a challenge. We have changed this. The core team will be resident on site and one of the cath lab staff will always be in the catheterization laboratory itself, while the others are stationed nearby, in the Hamad Medical City accommodation, which is less than half mile away.
General Implementation Plan
Moving a country with its hospitals and EMS service to full 24/7 primary-PCI compliance is a huge task. One advantage of fibrinolytic therapy is its simplicity that makes it suitable to administer even in a pre-hospital setting, such as in the ambulance. For primary-PCI to be successful, it requires a large number of logistic arrangements, protocols and highly trained, motivated and dedicated staff. However, Qatar is ideal for implementing such a program from many aspects. Qatar has one health authority (the Supreme Council of Health), one major teaching heart hospital and one EMS service. Furthermore, the infrastructure and communication technology is fairly advanced.
We have begun working with our colleagues in the EMS service and the ER at HGH, as well as in the Heart Hospital and catheterization laboratory. Intense and frank discussions included cardiologists, nurses, technicians, ER staff and members of the national EMS, as well as the cardiac surgeons and anesthetists. Many meetings were carried out leading to crucial decisions and mutual understandings to create a common culture and an implementation plan (Figure 3). It took some time, but it was certainly time well spent. Amongst the important changes that we undertook with our colleagues in the ER are those dealing with the initial triage and handling of patients with STEMI. The ER in HGH is very busy and receives over 1000 patients a day. Therefore it is easy for patients with STEMI not to draw immediate attention, especially if they have an atypical presentation or not displaying obvious hemodynamic instability. Amid this large flow of patients, STEMI patients may have to wait for a while, as they are mixed in with less urgent patients, such as those with typical chest pain, stable heart failure, atrial fibrillation, and benign syncope. Delays could also be due to late acquisition or reading of the 12-lead ECG, which will, in turn, delay recognition of STEMI and activation of primary-PCI teams. Moreover, activation of the primary-PCI team has been delayed in the past by waiting for the results of cardiac makers, and sometimes even the chest x-ray to rule out dissecting aneurysms.
Figure 3. .
Implementation plan for the nationwide primary-PCI program in Qatar to reduce pre-hospital delay and facilitate door-to-balloon time.
We have studied the potential causes of delay in the ER, and we have now developed a system whereby ER physicians and cardiologists will keep a high index of suspicion looking for STEMI patients, especially, but not limited to, patients presenting with chest pain or symptoms suggestive of ACS. The aim is to have 12 lead-ECG recorded and read within 10 minutes of arrival with immediate activation of the primary-PCI team, unless the patient needs stabilization before transfer. Those with suspicious, but inconclusive ST-elevation should have a repeat ECG in another 10 minutes. This does not mean less attention to other patients, but it does mean that STEMI patients are tagged to activate the primary-PCI team immediately and transfer from the ER to the catheterization laboratory.
The ER processing of STEMI patients has also been reviewed. The long path of clerical registration, routine nursing procedures, waiting for laboratory results, slow ordering of essential first-line medications such as aspirin, clopidogrel and beta blockers from the pharmacy have all been scrutinized. Although this pace may be permissible in many other conditions, it was certainly against the fundamental time constraints of primary-PCI.
To facilitate all these processes, we have taken several practical steps. We have devised pre-packed boxes, wherein everything that the patient needs is put together in one kit. This includes pre-filled forms for laboratory tests, medications and consent forms. The medications are made available onsite. The recent introduction of third generation P2Y12 inhibitors (prasugrel and ticagrelor) are also available, given their special features, especially their faster action, but also bearing in mind their contraindications in elderly patients and in those with previous stroke.
Another aspect that we had to address was the chain of communication and the order to transfer STEMI patients. The chain was found to have unnecessary steps that caused long delays. Classically, upon identifying STEMI, the ER physician would call the cardiology specialist who after confirming the STEMI would then call the consultant on call (who may not be an interventionalist). It was not until the interventional cardiologist approved the transfer, that the catheterization laboratory team was activated and the patient transferred. Furthermore, there was not a simple dedicated number to call to activate the primary-PCI team and therefore one may find it necessary to call several numbers before the “go-head” is given to transfer the patient. All of these constituted potentials for delay and have now been addressed. It is now possible, not only for the cardiology specialist to activate the primary-PCI team, but the ER physicians have also been empowered to activate the primary-PCI team if they are confident about the diagnosis of STEMI. We have also now dedicated a central hotline number specifically for primary-PCI. Contacting this number will be enough to set the whole procedure into motion.
We also found that the existing protocols necessitated that the EMS had several hurdles in the handover of the patient to the ER staff in the Heart Hospital, creating additional delays for the necessary registration, obtaining vital signs and being seen by the nurses and cardiologists, before the patient is transferred to the catheterization laboratory. It is now possible for STEMI patients transferred from another facility to go directly to the catheterization laboratory without any unnecessary transit in the ER. The EMS and the ER staff have been well informed to facilitate this process. Clear wall signs have now been displayed to guide EMS to the route to the catheterization laboratory.
Finally, with regard the ambulance transfer of STEMI patients, it was customary for the ambulance to be drawn from a pool of vehicles and therefore delay was often inevitable, as the first available vehicle may be a long distance away. The National Command Centre (NCC) in charge of ambulance dispatch did not have a clear plan on how to prioritize the transfer of STEMI patients or where to send them. Priority in such cases was often governed by the presence of hemodynamic or electrical instability, rather than the type of AMI. After discussion with our colleagues in the EMS, we now have a dedicated ambulance stationed in the vicinity of the ER department ready to transfer cardiac patients, especially those with STEMI, to the Heart Hospital, and we have requested two more. In this manner, we now have the autonomy to transfer STEMI patients at our discretion, without being held in a queue. We acknowledge, however, that the NCC must be informed of the movement of all ambulances at all times.
Phases to Implementation Plan
-
[1]
Preparation Phase. This was outlined in the previous sections and includes setting protocols for fast recognition and transfer of STEMI patients to the catheterization laboratory for timely primary-PCI. However, the program could be expanded in future to include other indications and patients who benefit from 24/7 cath lab access.
-
[2]
Clinical Pilot Phase. A two to three month period will be needed to coordinate all the multi-disciplinary services, from EMS, to the ERs and to the cardiac catheterization laboratory. This is mainly to emphasize the speed while maintaining quality, discover pitfalls to correct and improve all procedures into a seamless flow in optimal time scales. Regular audit and mock STEMI codes will be two quality control tools that will used in this phase.
-
[3]
Active Research Phase. The aim in this phase is twofold. Firstly, we will establish a comprehensive research database that will include not only details of clinical presentation, risk profile and clinical course of patients, but also to document various time intervals and technical details such as TIMI flow at presentation and end of procedure, size of and number of vessels affected, types of stents, and use of support devices, such as thrombectomy and embolization protection devices. Outcome data will be an important part of the database.
Secondly, in addition to comprehensive database, we will aim to conduct specific pioneering research projects and clinical trials into the cutting edge issues in primary-PCI, either as a single center or in collaboration with of other regional or international centers. To ensure completeness of any research data and follow up, each patient will be tagged to the cardiology specialist who received the patient and to the consultant who performed the primary-PCI procedure.
Primary-PCI continues to attract research in many areas. Amongst the recent publications, the amount of ST-segment resolution and its relation to outcome parameters, pre-hospital GPIIb/IIIa inhibitors, optimal anti-thrombotic therapy and the use of optical coherence tomography (OCT) are some of the active areas of research. Primary-PCI in diabetic patients, in females and in the young are also vital issues for Qatar. Others include the debate of complete versus culprit lesion only revascularization during primary-PCI, as well as thrombus aspiration and other thrombectomy devices. An important area of research is the “no reflow” phenomenon, which has rightly been called the “Achilles heel” of primary-PCI. Angiographically, no-reflow is defined as less than grade TIMI-2 flow at the end of the primary-PCI procedure, despite vessel patency and the absence of dissection, spasm, or distal macroembolization. It is thought to reflect micro-vascular dysfunction and is more common in diabetic patients. The incidence was reported differently between 12 to 25% [36]. It was 4–7% in CADILLAC and PAMI studies [5]. These were reports of the angiographically visible no-reflow. However, if one looks at the no-reflow phenomenon at the level of the micro-vascular circulation, then the incidence could be much higher. Imaging studies have indicated a significantly higher incidence at around 30–40% using contrast enhanced magnetic resonance [37]
Competency for Individual Operators and the Centre
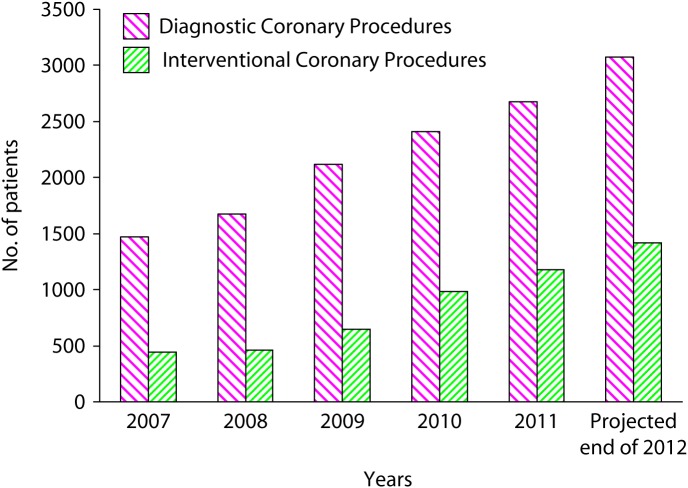
The annual statistics from our centre for both diagnostic and interventional coronary procedures have been increasing steadily over the years. The number of diagnostic procedures have increased from 1470 in 2007, to 2200 in 2009 and are projected to exceed 3000 by the end of 2012. The total number of PCIs has also increased from 443 in 2007, to 646 in 2009 and 1176 in 2011 and is expected to exceed 1400 by the end of 2012 (Figure 4). The minimum required for centers to justify performing coronary interventions is 400 PCIs per year. Centers performing less than 400 are considered low volume, while those performing more than 800 per year are labeled high volume. We perform nearly double the latter number per year. In a recent study [38] nearly half of all registered US centers have a PCI volume of less than 400 and thus are considered low volume.
Figure 4. .
Diagnostic and interventional coronary procedures at Heart Hospital, Hamad Medical Corporation, 2007–2012.
In our centre, the average volume per operator is approximately 150 procedures per year, which is double the international requirement per interventional operator. The guidelines set a minimum for each of the operators of 75 PCI per year (that is all PCI procedures per year, not only primary-PCI). These are well within the volumes performed in Qatar. A gross estimate of 1000 STEMI per million population was suggested as a working average incidence in Europe. While this may be applicable to high incidence countries like Russia, an incidence of 500–600 per million is thought to be more realistic for most of Europe [39]. The same ratio will give around 1000 to 1200 in Qatar. While we have a robust data on all MI patients admitted to our centre, we do not have a precise prevalence of STEMI in the population. However, given the figures of fibrinolytic therapy, we predict at least 600 primary-PCI per year. However, as our awareness program makes an impact, the figure may rise.
The incidence of STEMI in various countries appears to differ greatly, and there is evidence that it may be declining in some countries. In the US a study for the incidence of STEMI showed a decline from 121 to 77 per 100,000 population in between the years 1997 and 2005 [40]. Considering the Qatar population of 1.8 million, we should expect around 1200 patients per year. We do not have precise data on the incidence of STEMI in the whole of Qatar, but our centre receives the majority of patients with diagnosed AMI. Statistics of STEMI from our tertiary referral centre shows an annual figure to be at least 600 patients per year, which is lower than the estimated number of 1200. However, those estimates are based on US data which may not apply to Qatar, but they do raise a legitimate question of how many undiagnosed cases of STEMI occur in the community. Traditionally, the majority of patients with STEMI received fibrinolytic therapy, but primary-PCI has frequently been used for a considerable number of years in our centre for high risk patients, such as those in cardiogenic shock, large anterior AMI, compromised LV function and in patients with history of recent MI, especially if they had recent intervention and stent placement. In the last 3 years, most patients presenting with STEMI during the working hours were directed to primary-PCI whenever possible, again giving priority to high risk patients. This is in addition to “rescue PCI” for failed fibrinolysis.
Qatar is a rapidly growing country and the population of both nationals and expatriates is increasing exponentially. This is likely to increase the annual incidence of all cardiac admissions, including STEMI. Considering the number of STEMI received per year, the expected average number of primary-PCI procedures per operator will be around 65 per year, which is nearly equal to the guideline requirements for all PCI procedures per operator per year.
Cost Effectiveness of Primary-PCI
One must emphasize from the outset that the main measure for cost effectiveness of primary-PCI is in the patient's lower morbidity, better survival and quality of life. In addition, there is an important preservation of LV function. In addition, primary-PCI reduces the length of hospital stay and eliminates the cost of fibrinolytic therapy. Several studies have demonstrated better cost-effectiveness of primary-PCI over fibrinolysis [41]. Although a recent study from the UK concluded that the cost difference between the two modalities was nearly neutral, there was still a significant reduction in subsequent events, re-admission, as well as in the length of hospital stay (8.5 versus 4 days; p < 0.001) [42].
To understand how the primary-PCI program will reduce the hospital stay for patients with STEMI in our centre, some explanation is required. In our centre, the majority if not all patients with MI, including STEMI, receive coronary angiography. In fact, we have the largest percentage of coronary angiography following MI in the region [43] and many are done during the same admission. However, even if this is possible, it will mean delay of discharge, thus contributing to bed shortage. If there is a shortage of beds, some patients are discharged with a scheduled readmission at a later date for elective day-care coronary angiography. Those who prove in need of PCI will have it done on site only if a bed is secured to allow for an overnight stay after the PCI. If a bed is not available, then they get discharged after the angiography procedure to be re-scheduled yet again, for elective PCI at a later date. Recently we have adopted more radial artery approaches, which may ease the problem in future. However, it is clear that doing primary-PCI for STEMI patients from the start will reduce all of these delays, reduce repeated admissions and ease the demand on much needed beds.
When primary-PCI is successful, the average stay of STEMI patients will be reduced by at least 2 days. Early, or even next day, discharge of STEMI patients who received primary-PCI has been shown to be feasible and safe, at least in uncomplicated patients [44]. Given a conservative estimate of at least 600 STEMI patients we receive annually, there will be a saving of more than 1200 acute bed-days per year just from the shorter stay during the initial hospitalization. This translates to more than 3 extra beds to be available every day, year round. Furthermore, the reduced need for re-admission because of recurrent symptoms has also been shown to be another advantage of primary-PCI over fibrinolytic therapy. Patients in the latter group are more likely to need re-admission because of incomplete recanalization or re-occlusion after fibrinolysis [45]. We estimate that 20% of all patients with STEMI who receive fibrinolysis undergo coronary angiography and PCI during the initial admission for different reasons, such as failed fibrinolysis (rescue PCI), post-MI angina, evidence of large infarction with reduced LV function or other complications. This leaves nearly 500 out of the 600 STEMI patients to be re-admitted for day-care coronary angiography. At least half of the latter (250 patients) prove in need of PCI. These will either be done ad hoc and transferred from the day care unit to the intensive care wards to be formally admitted, or given an appointment for another admission for elective PCI, which also translates to more bed demand. Given these estimates, the annual number of beds needed for re-admission is 500 bed-days in day-care unit for coronary angiography and another 500 bed-days per year for the subsequent intervention, thus a total of 1000 bed-days per year for all re-admissions for coronary angiography and PCI.
Adding all the above savings together, adopting primary-PCI for all STEMI patients will provide 2000 acute bed-days per year. As explained, this is the result of shorter initial hospital stay (saving of 1000 bed-days), and reduced need for re-admission (saving of 1000 bed-days). A significant number of savings given the shortage in critical beds in a fast growing population, and a very welcome strategy that can be used toward providing beds for much needed elective admissions for other procedures, let alone providing beds for acute admissions other than STEMI itself. The annual fiscal saving resulting from the combination of unused fibrinolytic therapy, shorter hospital stay and reduced need for the re-admission will certainly be significant. However, as stated earlier, this is the least important of all savings offered by primary-PCI compared to fibrinolytic therapy, as the most important outcome is improved quality of patient care.
Conclusions
While fibrinolytic therapy is still a standard therapy for patients with STEMI, primary-PCI, performed timely, is currently the reperfusion strategy of choice. Setting a comprehensive primary-PCI program is a major task. We have taken major steps to set up strategies, protocols, to set up the first nationwide, 24/7 primary PCI. The volume of interventional procedures performed per year in our centre, as well as the massive experience in performing centre makes it well qualified to take this leading role. This clinical and research program could be a model that may be adopted in other countries to improve outcomes of patients with STEMI.
References
- [1].Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–372. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- [2]. Fibrinolytic Therapy Trialists' (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomized trials of more than 1000 patients Lancet 1994. 343 311 322 [PubMed] [Google Scholar]
- [3]. The GUSTO Angiographic Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction N Engl J Med 1993. 329 1615 1622 [DOI] [PubMed] [Google Scholar]
- [4].Simes RJ, Topol EJ, Holmes DR, Jr, et al. for the GUSTO-I Investigators Link between the angiographic substudy and mortality outcomes in a large randomized trial of myocardial reperfusion: importance of early and complete infarct artery reperfusion. Circulation. 1995;91:1923–1928. doi: 10.1161/01.cir.91.7.1923. [DOI] [PubMed] [Google Scholar]
- [5].Mehta RH, Harjai KJ, Cox D, et al. Primary Angioplasty in Myocardial Infarction (PAMI) Investigators Clinical and angiographic correlates and outcomes of suboptimal coronary flow inpatients with acute myocardial infarction undergoing primary percutaneous coronary intervention. J Am Coll Cardiol. 2003;42:1739–1746. doi: 10.1016/j.jacc.2003.07.012. [DOI] [PubMed] [Google Scholar]
- [6].Grines CL, Browne KF, Marco J, et al. for the Primary Angioplasty in Myocardial Infarction Study Group A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. N Engl J Med. 1993;328:673–679. doi: 10.1056/NEJM199303113281001. [DOI] [PubMed] [Google Scholar]
- [7]. Ross AM, Coyne KS, Moreyra E, et al. ,. Extended mortality benefit of early postinfarction reperfusion. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries Trial Circulation 1998. 97 1549 1556 [DOI] [PubMed] [Google Scholar]
- [8].Zijlstra F, de Boer MJ, Hoorntje JC, Reiffers S, Reiber JH, Suryapranata H. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med. 1993;328:680–684. doi: 10.1056/NEJM199303113281002. [DOI] [PubMed] [Google Scholar]
- [9].Mayer JM, Merx W, Dörr R, Lanbertz H, Bethge C, Effert S. Successful treatment of acute myocardial infarction shock by combined percutaneous transluminal coronary recanalization (PTCR) and percutaneous transluminal coronary angioplasty (PTCA) Am Heart J. 1982;103:132–138. doi: 10.1016/0002-8703(82)90540-3. [DOI] [PubMed] [Google Scholar]
- [10].Zahn R, Schiele R, Schneider S, et al. Primary angioplasty versus intravenous thrombolysis in acute myocardial infarction: can we define subgroups of patients benefiting most from primary angioplasty? Results from the pooled data of the Maximal Individual Therapy in Acute Myocardial Infarction Registry and the Myocardial Infarction Registry. J Am Coll Cardiol. 2001;37:1827–1835. doi: 10.1016/s0735-1097(01)01264-5. [DOI] [PubMed] [Google Scholar]
- [11].Berger PB, Ellis SG, Holmes DR, Jr, et al. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction: results from the Global Use of Strategies to Open Occluded Arteries in Acute Coronary Syndromes (GUSTO-IIb) Trial. Circulation. 1999;100:14–20. doi: 10.1161/01.cir.100.1.14. [DOI] [PubMed] [Google Scholar]
- [12].Cannon CP, Antman EM, Walls R, Braunwald E. Time as an adjunctive agent to thrombolytic therapy. J Thromb Thrombolys. 1994;1:27–34. doi: 10.1007/BF01061992. [DOI] [PubMed] [Google Scholar]
- [13].De Luca G, Biondi-Zoccai G, Marino P. Transferring patients with ST-segment elevation myocardial infarction for mechanical reperfusion: a meta-regression analysis of randomized trials. Ann Emerg Med. 2008;52:665–676. doi: 10.1016/j.annemergmed.2008.08.033. [DOI] [PubMed] [Google Scholar]
- [14].Busk M, Maeng M, Rasmussen K, et al., DANAMI-2 Investigators The Danish multicentre randomized study of fibrinolytic therapy vs. primary angioplasty in acute myocardial infarction (the DANAMI-2 trial): outcome after 3 years follow-up. Eur Heart J. 2008;29:1259–1266. doi: 10.1093/eurheartj/ehm392. [DOI] [PubMed] [Google Scholar]
- [15].Widimsky P, Bilkova D, Penicka M, et al., PRAGUE Study Group Investigators Long-term outcomes of patients with acute myocardial infarction presenting to hospitals without catheterization laboratory and randomized to immediate thrombolysis or interhospital transport for primary percutaneous coronary intervention: five years' follow-up of the PRAGUE-2 Trial. Eur Heart J. 2007;28:679–684. doi: 10.1093/eurheartj/ehl535. [DOI] [PubMed] [Google Scholar]
- [16].Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- [17].Timmer JR, Ottervanger JP, de Boer MJ, et al., Primary Coronary Angioplasty vs Thrombolysis-2 Trialists Collaborators Group Primary percutaneous coronary intervention compared with fibrinolysis for myocardial infarction in diabetes mellitus: results from the Primary Coronary Angioplasty vs Thrombolysis-2 trial. Arch Intern Med. 2007;167:1353–1359. doi: 10.1001/archinte.167.13.1353. [DOI] [PubMed] [Google Scholar]
- [18].Boersma E, Maas AC, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet. 1996;348:771–775. doi: 10.1016/S0140-6736(96)02514-7. [DOI] [PubMed] [Google Scholar]
- [19].Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- [20].Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation. Eur Heart J. 2008;29:2909–2945. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- [21].Kushner FG, Hand M, Smith SC, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) J Am Coll Cardiol. 2009;54:2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- [22].Gurwitz JH, McLaughlin TJ, Willison DJ, et al. Delayed hospital presentation in patients who have had acute myocardial infarction. Ann Intern Med. 1997;126:593–599. doi: 10.7326/0003-4819-126-8-199704150-00001. [DOI] [PubMed] [Google Scholar]
- [23].McGinn AP, Rosamond WD, Goff DC, Jr, Taylor HA, Miles JS, Chambless L. Trends in prehospital delay time and use of emergency medical services for acute myocardial infarction: experience in 4 US communities from 1987–2000. Am Heart J. 2005;150:392–400. doi: 10.1016/j.ahj.2005.03.064. [DOI] [PubMed] [Google Scholar]
- [24].Cannon CP, Gibson CM, Lambrew CT, et al. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA. 2000;283:2941–2947. doi: 10.1001/jama.283.22.2941. [DOI] [PubMed] [Google Scholar]
- [25].Terkelsen CJ, Christiansen EH, Sørensen JT, et al. Primary PCI as the preferred reperfusion therapy in STEMI: it is a matter of time. Heart. 2009;95:362–369. doi: 10.1136/hrt.2007.139493. [DOI] [PubMed] [Google Scholar]
- [26].Lambert L, Brown K, Segal E, Brophy J, Rodes-Cabau J, Bogaty P. Association between timeliness of reperfusion therapy and clinical outcomes in ST-elevation myocardial infarction. JAMA. 2010;303:2148–2155. doi: 10.1001/jama.2010.712. [DOI] [PubMed] [Google Scholar]
- [27].Brodie BR, Stone GW, Morice MC, et al. Importance of time to reperfusion on outcomes with primary coronary angioplasty for acute myocardial infarction:results from the Stent Primary Angioplasty in Myocardial Infarction Trial. Am J Cardiol. 2001;88:1085–1090. doi: 10.1016/s0002-9149(01)02039-2. [DOI] [PubMed] [Google Scholar]
- [28].Brodie BR, Gersh BJ, Stuckey T, et al. When Is door-to-balloon time critical? Analysis from the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) and CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) Trials. J Am Coll Cardiol. 2010;56:407–413. doi: 10.1016/j.jacc.2010.04.020. [DOI] [PubMed] [Google Scholar]
- [29].Brodie BR, Hansen C, Stuckey TD, et al. Door-to-balloon time with primary percutaneous coronary intervention for acute myocardial infarction impacts late cardiac mortality in high-risk patients and patients presenting early after the onset of symptoms. J Am Coll Cardiol. 2006;47:289–295. doi: 10.1016/j.jacc.2005.08.065. [DOI] [PubMed] [Google Scholar]
- [30].De Luca G, Suryapranata H, Zijlstra F, et al., ZWOLLE Myocardial Infarction Study Group Symptom-onset-to-balloon time and mortality in patients with acute myocardial infarction treated by primary angioplasty. J Am Coll Cardiol. 2003;42:991–997. doi: 10.1016/s0735-1097(03)00919-7. [DOI] [PubMed] [Google Scholar]
- [31].De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223–1225. doi: 10.1161/01.CIR.0000121424.76486.20. [DOI] [PubMed] [Google Scholar]
- [32].Le May MR, So DY, Dionne R, et al. A citywide protocol for primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2008;358:231–240. doi: 10.1056/NEJMoa073102. [DOI] [PubMed] [Google Scholar]
- [33].Carter A, Wood S, Goodacre S, et al. Evaluation of workforce and organizational issues in establishing primary angioplasty in England. J Health Serv Res Policy. 2010;1:6–13. doi: 10.1258/jhsrp.2009.009019. [DOI] [PubMed] [Google Scholar]
- [34].Krumholz HM, Bradley EH, Nallamothu BK, et al. A campaign to improve the timeliness of primary percutaneous coronary intervention: Door-to-Balloon: an alliance for quality. J Am Coll Cardiol Interv. 2008;1:97–104. doi: 10.1016/j.jcin.2007.10.006. [DOI] [PubMed] [Google Scholar]
- [35].Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACC secondary prevention and risk reduction therapy for patients with coronary and other vascular disease: update. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- [36].Piana RN, Paik GY, Moscucci M, et al. Incidence and treatment of ‘no-reflow’ after percutaneous coronary intervention. Circulation. 1994;89:2514–2518. doi: 10.1161/01.cir.89.6.2514. [DOI] [PubMed] [Google Scholar]
- [37].Tarantini G, Cacciavillani L, Corbetti F, et al. Duration of ischemia is a major determinant of transmurality and severe microvascular obstruction after primary angioplasty: a study performed with contrast-enhanced magnetic resonance. J Am Coll Cardiol. 2005;46:1229–1235. doi: 10.1016/j.jacc.2005.06.054. [DOI] [PubMed] [Google Scholar]
- [38].Dehmer GJ, Weaver D, Roe MT, et al. A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 Through June 2011. J Am Coll Cardiol. doi: 10.1016/j.jacc.2012.08.966. 2012 Oct 5. [Epub ahead of print] doi: 10.1016/j.jacc.2012.08.966. [DOI] [PubMed] [Google Scholar]
- [39].Di Mario C, Syrseloudis D, James S, Viceconte N, Wijns W. STEMI guidelines: from formulation to implementation. Eurointervention. 2012;8(Supplement P):11–17. doi: 10.4244/EIJV8SPA4. [DOI] [PubMed] [Google Scholar]
- [40].McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;1:40–47. doi: 10.1016/j.amjmed.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wailoo A, Goodacre S, Sampson F, et al. Primary angioplasty versus thrombolysis for acute ST-elevation myocardial infarction: an economic analysis of the National Infarct Angioplasty project. Heart. 2010;96:668–672. doi: 10.1136/hrt.2009.167130. [DOI] [PubMed] [Google Scholar]
- [42].Morgan KP, Leahy MG, Butts JN, Beatt KJ. The cost effectiveness of primary angioplasty compared to thrombolysis in the real world: one year results from West London. EuroIntervention. 2010;6:596–603. doi: 10.4244/EIJV6I5A100. [DOI] [PubMed] [Google Scholar]
- [43].Al Suwaidi J, Al Habib K, Asaad N, et al. Immediate and one-year outcome of patients presenting with acute coronary syndrome complicated by stroke: findings from the 2nd Gulf Registry of Acute Coronary Events (Gulf RACE-2) BMC Cardiovasc Dis. 2012;12:64. doi: 10.1186/1471-2261-12-64. http://www.biomedcentral.com/1471-2261/12/64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jirmár R, Widimský P, Capek J, Hlinomaz O, Groch L. Next day discharge after successful primary angioplasty for acute ST elevation myocardial infarction. An open randomized study “Prague-5”. Int Heart J. 2008;6:653–659. doi: 10.1536/ihj.49.653. [DOI] [PubMed] [Google Scholar]
- [45]. Veen G, de Boer MJ, Zijlstra F, Verheugt FW. Improvement in three-month angiographic outcome suggested after primary angioplasty or acute myocardial infarction (Zwolle trial) compared with successful thrombolysis (APRICOT trial). Antithrombotics in the Prevention of Reocclusion In COronary Thrombolysis Am J Cardiol. 199984 763 767 [DOI] [PubMed] [Google Scholar]