Abstract
Self-assembly is a powerful synthetic tool that has enabled chemists to construct numerous, structurally complex, supermolecules of various shapes, functionality, and dimensions from relatively simple precursors. Metal-organic polyhedra (MOPs) are an emerging family of self-assembled supermolecules that have intriguing structures and tailored functionality. During the last decade, research in this area have rapidly evolved and interest is now directed towards fine tuning and tailoring such structures targeting applications in sensing, catalysis, and most recently, in the biomedical field. Three examples of MOPs of interest showing promising potentials for biomedical applications are described.
Introduction
Self-assembly is defined by George Whitesides as “a process where pre-designed components assemble in a determined structure without the intervention of human operators”. 1 This broad but carefully stated definition calls for three essential characteristics for a process to be termed self-assembly. This includes utilization of carefully designed building blocks where information encoded within the building blocks will dictate a specific way of their interaction, generating determined structures of higher complexity and by default functionality, as compared to the simpler building blocks, and finally the process will commensurate without intervention of a human operator. Working with chemical self-assembled systems entails close familiarity with the concepts and tools of supramolecular chemistry. The term supramolecular chemistry was coined by Jean-Marie Lehn who shared the 1987 Nobel Prize in chemistry with Donald J. Cram and Charles J. Pederson for “their development and use of molecules with structure-specific interactions of high specificity”. 2
In supramolecular chemistry, chemistry beyond the molecule as defined by Lehn, 3 strong emphasis is given to intermolecular forces dominated by reversible, non-covalent interactions that range from Van der Waals, dipole-induced dipole, dipole-dipole, hydrogen and halogen bonding to even, more recently utilized, coordination bonds. Intermolecular interactions of the types mentioned here involve molecular recognition processes that can be designed, and further tailored, to dictate specific modes of binding to construct particular ordered structures.
Why self-assembly?
Self-assembly is an efficient synthetic pathway to construct relatively complex, multi-component systems that would be extremely difficult to attain otherwise. In step-wise syntheses, the reaction intermediates are commonly isolated and further purified prior to further elaboration. This enables synthetic chemists to devise synthetic pathways utilizing what is commonly referred to as a retrosynthetic approach, armed with an arsenal of reagents, protecting groups, established synthesis conditions, and rigorous procedures developed over the last decades.
In contrast, self-assembly is not amenable to retrosynthetic analysis as the reaction intermediates are present transiently and no isolation/purification steps are carried out. Although this imposes considerable challenges to successfully construct chemical species, it simultaneously provides an unmatched powerful tool in construction of complex, multi-component systems in high yield and with minimal intervention from the chemist.
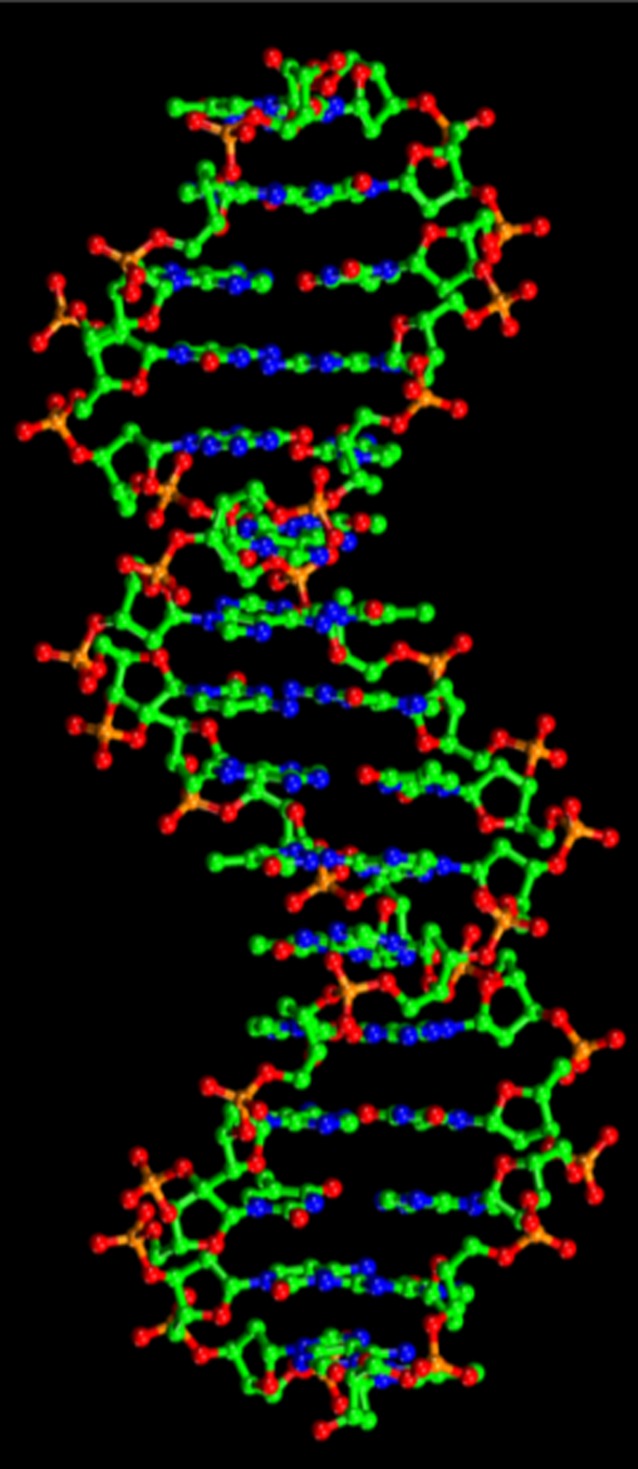
Merging the powerful tools of synthetic chemistry, the knowledge of types and nature of intermolecular interactions, and the underlying principles of crystal engineering and supramolecular chemistry open the doors for constructing tailor-made structures for demanding applications. Examples of self-assembled systems are numerous of which the self-assembled DNA double helix and the lipid bi-layer membranes in cell walls are outstanding examples. In the DNA double helix, Figure 1, specific and complimentary hydrogen bond interactions between the base pairs dictates very specific mode of binding that results almost invariably into the observed structure.
Figure 1. .
Self-assembled double helix DNA supermolecule.
It is because of the reversible nature of hydrogen bond interactions that pairing and un-pairing of the two DNA molecules are feasible, providing room for correction of mismatches. In the lipid bilayer membrane, reversible intermolecular interactions between the phospholipids molecules derive their self-assembly into the bilayer structure. It is again due to the reversible nature of such intermolecular interactions that the lipid bi-layer membranes express their remarkable physical and physiological properties.
A relatively recently developed family of self-assembled supermolecules is the metal-organic polyhedra (MOPs). An MOP is typically composed of organic molecules coordinating metal ions. The organic molecules commonly referred to as the linkers, contain functional groups like carboxylic acids, phenols, or heterocycles (e.g. pyridine, imidazole, pyrimidine, etc) acting as Lewis-bases towards wide range of metal ions, acting as Lewis-acids.
As synthetic chemistry is capable of delivering an ever-increasing number of designer organic linkers, and as the number of metal ions available for utilization from the periodic table is relatively large, the number of structures accessible from rational reaction permutations is nearly innumerable. However, design strategies that rely on rational and judicial selection of reaction components, conditions, etc can be devised to guide the synthesis of targeted structures.
Metal-Organic Polyhedra (MOPs)
The early examples of self-assembled supermolecules containing organic and inorganic parts connected through coordination bonds came to existence through the works of Fujita et al. 4 and Stang et al. 5 in 1993 and 1994, respectively. Both authors reported independently the synthesis and characterization of self-assembled macrocyclic complexes composed of four metal ions –Pd(II) or Pt(II)–where two of the available coordination sites on each metal ion are occupied by capping phosphine or amine ligands and the other two sites coordinated to nitrogen-donor organic linkers. The earlier organic linkers were commonly selected as linear ditopic linkers (containing two opposing nitrogen atoms that can coordinate metal ions). It is due to geometric complimentarity between linear linkers and the available coordination sites around each metal ion (oriented at 90° angle) that the 8-component system (4 linkers and 4 metal ions) can self-assemble into a supermolecule with a square-like geometry, Figure 2.
Figure 2. .
Syntheses of the self-assembled Pt(II) or Pd(II) macrocycles.
The intriguing symmetry of the resulted square-like supermolecules and the almost quantitative yield of the syntheses, along with the relatively straightforward reaction conditions, stimulated many research groups to explore this area more actively. It was clear from those seminal publications that skepticism towards the ability to generate such beautiful assemblies with relative ease is no longer well-founded. Such skepticism was rooted in the chemists' perception of the many ways such molecular or ionic components can come together to yield a large number of possible structures in absence of directing forces. Although such “directing forces” were not fully defined in the early days, it is becoming clearer to scientists in the field that a plethora of complex factors in subtle balance holds the key to realize one of chemists' most sought dreams, to make molecules to order and at will. Those directing forces can be very broadly classified into two categories: 1) information encoding at the molecular level and 2) realization of proper reaction conditions.
Maintaining structural and functional complimentarity between the building blocks, along with placing interaction sites on each of the reacting species at correct relative disposition, is the first and foremost design element. This step, if done properly, ensures feasibility to construct the targeted metal-organic polyhedra (MOP), given that a proper set of experimental reaction conditions is formulated. Reaction conditions of interest include; mixing stoichiometry, reaction temperature and time, solvent system, ionic strength and concentration, addition of weak acids or bases, nature of counterions, among others. It becomes evident from the large number of reaction conditions that need to be explored and then optimized that a trial-and-error process is essential to arrive at a proper reaction setup for each system. Although initial screening trials can be time and effort consuming, relying on good chemist's intuition can cut down the number of trials to manageable size and accumulation of knowledge over the past decade have contributed positively towards this goal.
One fascinating example of such MOPs is the MOP constructed from 24 Cu(II) ions and 24 isophthalate ions, Figure 3. 6 This MOP (known as nanoball or cuboctahedron) is constructed through coordination interactions between Cu(II) ions and carboxylate groups on benzene rings. The 120° angle between two carboxylate groups on each benzene ring of the isophthalate dictate the orientation between two coordination clusters in a way that results in formation of a ball-like structure with c.a. 2.6 nm outer diameter. Experimentally, the nanoball can successfully be constructed by heating at 85°C for 12 h a mixture of copper nitrate and isopthalic acid in 1:1 ratio in N, N'-dimethylformamide as the major solvent and methanol as a co-solvent. 7
Figure 3. .
Synthesis of the self-assembled Cu(II) nanoball from Cu(II) and isopthalate ions. Cu (green), O (red), C (gray), H (white). Yellow sphere represents guest-accessible void inside the nanoball (∼1 nm diameter).
Several research groups started to explore the potential for MOPs in various applications soon after chemists were able to generate isostructural variants of the same MOP, 8 those sharing same underlying structure but with larger dimensions and/or with peripheral functionalities. Among the applications of special interest are applications in bio-nanotechnology. One of the earliest examples was presented in 2008 when Kim et al. 10 reported a synthetic ion channel based on a tailor-made MOP, the nanoball bearing 24 saturated hydrocarbon arms (C12 chains), Figure 4. 9 The lipophilic arms protruding from the MOP surface facilitated its incorporation in-between a model lipid bi-layer membrane. Due to the voids inside the nanoball that ion conductivity was observed. Furthermore, the authors reported that the recorded behavior in terms of the ion transport activity (Li+>> Na+ > K+ > Rb+ > Cs+) following Eisenman sequence XI. The authors suggested 10 that one possible explanation is that binding of the cations to the synthetic channel (the MOP) is more crucial than dehydration of the cations in the ion transport process.
Figure 4. .
Self-assembled MOP with 24 lipophilic, saturated aliphatic, chains decorating its surface (to the left is crystal structure of the MOP). 5 This MOP with diameter of ∼5 nm was utilized as synthetic ion channel when situated in-between a lipid bi-layer membrane. 8
In 2007 Fujita and co-workers 11 reported a fascinating example of the molecular recognition process involving MOP supermolecule (a supermolecule being composed of a number of smaller molecular and/or ionic species held together through non-covalent interactions). In this example, the authors synthesized a MOP from Pd(II) ions and pyridine-containing linkers where the MOP surface was uniformly functionalized by 24 saccharide molecules, Figure 5. Molecular recognition between the saccharide-functionalized MOPs and saccharide-binding proteins induced cross-linking of the soluble proteins and formation of aggregates followed. The authors studied the interactions of the family of saccharide functionalized MOPs with concanavalin A (ConA), a well-studied lectin from Canavalia ensiformis. ConA is known to selectively recognize α-mannopyranoside and α-glucopyranoside at its four binding sites. In accordance, the MOP bearing 24 α-mannopyranoside units at its periphery served as a cross-linker of ConA to form aggregates in solution, Figure 6.
Figure 5. .

Self-assembly of M12L24 complexes with 24 saccharide moieties at the periphery. When combined with lectins, they form aggregates because of the cluster effect of the saccharides on the spheres. Reprinted with permission from (N. Kamiya; M. Tominaga; S. Sato; M. Fujita. J. Am. Chem. Soc. 2007, 129, 3816–3817). Copyright (2007) American Chemical Society.
Figure 6. .

Turbidity changes, monitored by the absorbance at 500 nm, on addition of MOPs 2a and 2b to (a) ConA. A large excess of (a) α-methyl mannopyranoside or (b) α -galactose was added as an inhibitor at 20 min. Reprinted with permission from (N. Kamiya; M. Tominaga; S. Sato; M. Fujita. J. Am. Chem. Soc. 2007, 129, 3816–3817). Copyright (2007) American Chemical Society.
Another example of surface-functionalized MOP capable of molecular recognition of nucleic bases was later reported in 2010. 12 In this report, well-defined, perfectly monodisperse short DNA strands on the surface of a self-assembled coordination MOP was presented, Figure 7. The MOP served as a nanoparticle template to control the number, spacing, and alignment of the peripheral DNA strands. The investigated system was deoxythymine (T) based MOP that showed specific binding to the complimentary nucleotide deoxyadenosine (A), Figure 8. The binding of MOP (T) nucleotides to the added (A) was monitored in solution through measurement of changes in proton chemical shifts using solution NMR spectroscopy.
Figure 7. .
Self-assembly of DNA-displaying coordination nanospheres. (a) Structures of ligands 1a − c. (b) Self-assembly of DNA-conjugated molecular sphere 2c from 12 Pd(II) ions and 24 1c ligands. Ligands 1a and 1b also give the corresponding M12L24 spheres (consisting of 12 Pd(II) ions and 24 ligands). Reprinted with permission from (T. Kikuchi; S. Sato; M. Fujita. J. Am. Chem. Soc. 2010, 132, 15930–15932). Copyright (2010) American Chemical Society.
Figure 8. .
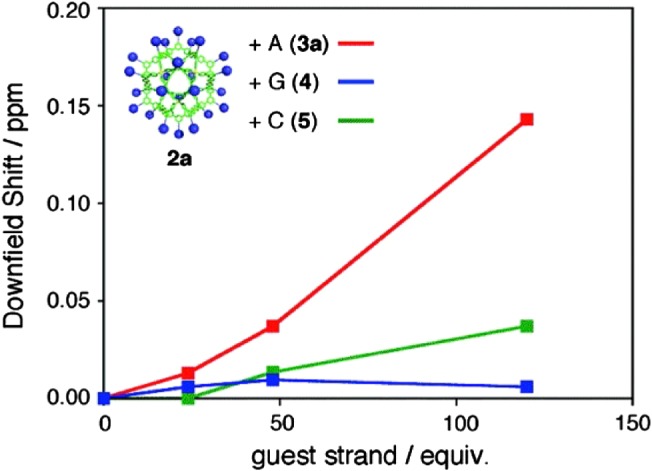
The values of downfield shift of thymine proton signals of 2a after the addition of mono-A (3a), G (4), C(5) (red line: 2a+3a; blue line: 2a+4; green line: 2a+5) ([2] = 0.167 mM, DMSO-d6/CDCl3 = 1:4, 500 MHz, 300 K). Reprinted with permission from (T. Kikuchi; S. Sato; M. Fujita. J. Am. Chem. Soc. 2010, 132, 15930–15932). Copyright (2010) American Chemical Society.
Conclusion
Although practical utilization of self-assembled MOPs is still in its infancy, interest is growing in exploring their potentials as novel materials to address some particular challenges in biomedical applications. The wide versatility of accessible MOPs starting from a wide range of organic linkers and metal ions along with the arsenal of synthetic pathways that chemists can utilize to construct made-to-order supermolecules calls for collaborative efforts from chemists, molecular biologists and clinicians to fully explore and exploit such material in current demanding applications.
References
- [1].Whitesides GM. Self-assembly at all scales. Science. 2002;295(5564):2418–2421. doi: 10.1126/science.1070821. Available at: http://dx.doi.org/10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- [2]. The Nobel Prize in Chemistry 1987. Nobelprize.org. Retrieved 28 Aug 2012 http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1987/
- [3].Lehn J-M, Atwood JL, Davies JED, MacNicol DD, Vogtel F, editors. Comprehensive Supramolecular Chemistry. Oxford: Pergamon; 1996. [Google Scholar]
- [4].Fujita M, Yazaki J, Kuramochi T, Ogura K. Self-assembly of a Macrocyclic dinuclear Pd(II)-phosphine complex. Bull Chem Soc Jpn. 1993;66(6):1837–1839. Available at: http://dx.doi.org/10.1246/bcsj.66.1837. [Google Scholar]
- [5].Stang PJ, Cao DH. Transition metal based cationic molecular boxes. Self-assembly of macrocyclic platinum(II) and palladium(II) tetranuclear complexes. J Am Chem Soc. 1994;116(11):4981–4982. Available at: http://dx.doi.org/10.1021/ja00090a051. [Google Scholar]
- [6].Eddaoudi M, Kim J, Wachter JB, Chae HK, O Keeffe M, Yaghi OM. Porous metal − organic polyhedra: 25 Å cuboctahedron constructed from 12 Cu2(CO2)4 paddle-wheel building blocks. J Am Chem Soc. 2001;123(18):4368–4369. doi: 10.1021/ja0104352. Available at: http://dx.doi.org/10.1021/ja0104352. [DOI] [PubMed] [Google Scholar]
- [7].Moulton B, Lu J, Mondal A, Zaworotko MJ. Nanoballs: nanoscale faceted polyhedra with large windows and cavities. Chem Commun. 2001;(9):863–864. Available at: http://dx.doi.org/10.1039/b10,2714j. [Google Scholar]
- [8].Tranchemontagne D, Ni Z, O'Keeffe M, Yaghi O. Reticular chemistry of metal–organic polyhedra. Angewandte Chem Int Ed. 2008;47(28):5136–5147. doi: 10.1002/anie.200705008. Available at: http://dx.doi.org/10.1002/anie.200705008. [DOI] [PubMed] [Google Scholar]
- [9].Furukawa H, Kim J, Plass KE, Yaghi OM. Crystal Structure, dissolution, and deposition of a 5 nm functionalized metal − organic great rhombicuboctahedron. J Am Chem Soc. 2006;128(26):8398–8399. doi: 10.1021/ja062491e. Available at: http://dx.doi.org/10.1021/ja062491e. [DOI] [PubMed] [Google Scholar]
- [10].Jung M, Kim H, Baek K, Kim K. Synthetic ion channel based on metal–organic polyhedra. Angewandte Chem Int Ed. 2008;47(31):5755–5757. doi: 10.1002/anie.200802240. Available at: http://dx.doi.org/10.1002/anie.200802240. [DOI] [PubMed] [Google Scholar]
- [11].Kamiya N, Tominaga M, Sato S, Fujita M. Saccharide-coated M12L24 molecular spheres that form aggregates by multi-interaction with proteins. J Am Chem Soc. 2007;129(13):3816–3817. doi: 10.1021/ja0693082. Available at: http://dx.doi.org/10.1021/ja0693082. [DOI] [PubMed] [Google Scholar]
- [12].Kikuchi T, Sato S, Fujita M. Well-defined DNA Nanoparticles templated by self-assembled M12L24 molecular spheres and binding of complementary oligonucleotides. J Am Chem Soc. 2010;132(45):15930–15932. doi: 10.1021/ja108334g. Available at: http://dx.doi.org/10.1021/ja108334g. [DOI] [PubMed] [Google Scholar]