Abstract
The development of the modern era of bioengineering and the advances in our understanding of the cardiovascular system have been intertwined over the past one-half century. This is true of bioengineering as an area for research in universities. Bioengineering is ultimately the beginning of a new engineering discipline, as well as a new discipline in the medical device industry.
Historical perspective
As discussed by both Citron and Nerem 1 and also Bergman and Nerem, 2 the medical device industry evolved in the second half of the 20th century. First, there was kidney dialysis, a technology accredited to the pioneering work of a Dutch physician, Willem Kolff. Beyond the treatment of chronic kidney failure, Kolff's technologies formed the foundation to the membrane oxygenator. In turn, this led to cardiopulmonary bypass being performed safely for extended periods of time. There followed the development of prosthetic heart valves with the first implant being performed in 1952. This was followed by the first successful open heart surgery in 1953.
Clinically-practical electrical stimulation therapies for cardiac rhythm disorders began to emerge in the 1950s. In 1958, Dr. C. Walton Lillehei collaborated with Earl Bakken, an electrical engineer, in the use of the world's first transistorized battery-powered cardiac pacemaker that was externally powered. However, a few years later, in 1960, William Chardack implanted the first pacemaker that was completely internal, i.e. within the body. This was possible because of the mercury/zinc battery that had been designed by Wilson Greatbach, an engineer who passed away in 2011. This was the start of the medical device/implant industry. This industry grew in the last 50 years to become a major industry with $200 billion in annual sales, with a workforce of 300,000, and an industry that invests seven percent of revenues back into R&D.
Bioengineering in universities has developed in parallel to the development of the medical device/implant industry. Back in the 1950s and 1960s, bioengineering involved the application of the traditional engineering disciplines to problems in medicine and biology. However, this all began to change in the 1970s with the establishment of academic departments, called either biomedical engineering or bioengineering. This development grew slowly until the 1990s when there was an acceleration in the formation of such academic units. This was due to many factors; chief among these was the impact of the biological revolution and the significant investments made by the Whitaker Foundation. Worldwide, there are now more than 100 departments of this type, and the field of biomedical engineering/bioengineering is now recognized as being its own academic discipline, an engineering discipline based on the science of biology.
Cardiovascular research and orthopaedic research have been two major areas of focus in the evolving discipline of bioengineering. Three areas of cardiovascular research in bioengineering will be discussed in this article. Each section focuses on a certain area of cardiovascular research in which this author has been personally involved.
Hemodynamics and atherosclerosis
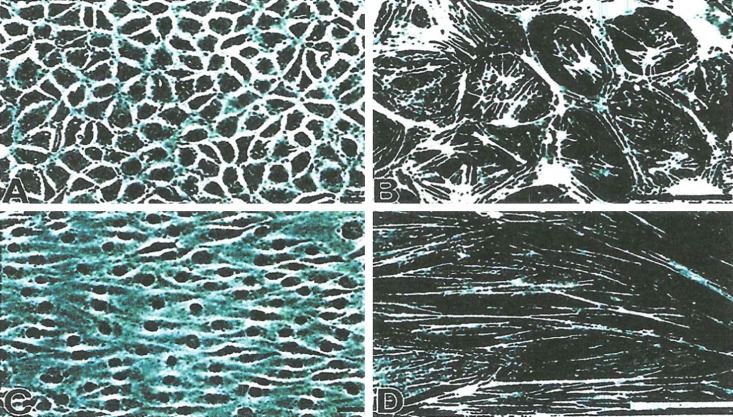
An important research area in the historical development of bioengineering is the role of hemodynamics in the disease atherosclerosis, particularly in the early atherogenic stage of the disease. In the 1960s, there was already evidence that the pattern of atherogenesis appeared to correlate with the pattern of blood flow. As studies developed, it became clear that there was greater predilection of the disease in low-shear stress regions. The vascular endothelium, which was in contact with the flowing blood and the associated shear stress environment, became a focus on the effects of the hemodynamic environment on vascular endothelial biology. 3 These studies included the effects of flow, the effects of cyclic stress and in some cases even pressure; however, the major focus was on the study of the influence of flow and the associated shear stress environment. Much of this research over the years has been done in vitro with cultured vascular endothelial cells exposed to a variety of flow environments either using a parallel-plate flow chamber 4 or a cone-plate device. 5 The flow environments studied have included steady laminar flow, a purely oscillating flow, and pulsatile type flows, either with a reversing or a non-reversing waveform. 6,7 Although a key indicator of the influence of flow is the change in morphology, as illustrated in Figure 1, and the reorganization of the cytoskeletal network, 8 there are also changes in gene expression and protein expression. For a flow environment where there is a non-zero mean flow component, vascular endothelial cells elongate and align their major axis parallel to the direction of flow. There is reorganization of F-actin, such that the fibers also are aligned with the flow; and if the actin cytoskeleton is disrupted, then the vascular endothelial cells do not elongate and align. This thus indicates that the change in morphology is due to a reorganization of the F-actin. Interestingly, there is no morphological change (no elongation nor alignment of the vascular endothelial cells) for a purely oscillatory flow. 9 With respect to gene expression, some genes are upregulated and some downregulated. Obviously, much has been learned about vascular endothelial biology from these in vitro studies and later confirmed with in vivo studies using a variety of animal models.
Figure 1. .
In vitro images of morphology and F-actin for bovine aortic endothelial cells for static conditions (A and B) and after 24 hours of laminar shear stress (C and D) with flow left to right; scale of F-actin images a factor of ten different from the morphology images.
Interestingly, some life scientists studying atherosclerosis in the 1970s did not believe that the physical forces associated with the hemodynamic environment could have any influence at all on cell behavior and the cellular processes involved in the initiation of the disease. Today, the concept of an important role for hemodynamics is widely accepted. In fact, it now is clear that the function of a cell is determined by the signals associated with the microenvironment in which the cell resides. This “symphony” of signals, what I call Nature's Orchestra, is made up of the soluble molecules to which the cell is exposed, the other cells which are in contact, the substrate to which it is adhered (extracellular matrix and/or some type of synthetic material), and the mechanical environment in which the cell resides, i.e. the physical forces to which it is exposed.
One may speculate that the reason much was learned about vascular endothelial function from in vitro studies was because these monolayer studies mimicked the fact that the vascular endothelium is a monolayer in vivo. As the environment in cell culture is not physiologic, there have been efforts to engineer a more physiologic in vitro environment. 10 This has included using a co-culture of vascular endothelial cells with vascular smooth muscle cells. 11 It also included using a three dimensional architecture. It is now clear that smooth muscle cells in a three-dimensional environment have different characteristics than such cells in a two-dimensional environment. 12
Even with the advances made, much due to the involvement of engineers in the study of vascular biology, there is more to be done. This includes continuing to develop in vitro models that are more physiologic and better at simulating in vivo environments. It should also be noted that, although there were many engineers who learned biology to be leaders in this field, there were also life scientists who became more like engineers in their approach in the study of vascular biology. Thus, the bioengineering community that emerged from studies in this area has been very much an interdisciplinary one, and this provided the foundation for the emergence of bioengineering as a discipline in its own right, a discipline based on the science of biology, a discipline in which biology and engineering are very much integrated.
Heart valve engineering
Heart valves engineering is another area, in which engineers have been involved and which has fostered the growth of bioengineering. Initially, much of the engineering effort was focused on the development of improved prosthetic valves to be used as defective valve replacements. This certainly was important to the emerging medical device/implant industry in the 1970s and 1980s, and it was an area in which university researchers became very active. A key issue was to optimize the fluid mechanical characteristics of such heart valves implants. 13 Engineers have begun to make a contribution to the area of heart valves engineering in another way, in order to learn more about the basic biology of heart valves, a tissue that resides in an extremely dynamic mechanical environment. Our knowledge about the biology of blood vessels has increased considerably over the last few decades; this was largely driven by studies aimed at achieving a better understanding of the biology and pathobiology associated with atherosclerosis. However, the same situation is not paralleled in the area of heart valves. Although there were clinical problems associated with heart valves, there seemingly was not satisfactory motivation to study the basic biology of heart valves. However, this has changed now. Several bioengineering laboratories are participating in this, and the area of heart valves has became an important part of bioengineering research.
One example is the heart valve team from Georgia Tech and Emory University School of Medicine in Atlanta, Georgia. This team includes the laboratories of Professors Hanjoong Jo and Ajit Yoganathan, and Robert M. Nerem. An issue being investigated is whether there is any difference between endothelial cells on the different sides of the aortic valve leaflets, i.e. the ventricularis side as compared to the fibrosa side. Whereas the hemodynamic environment on the ventricularis side may be characterized as a unidirectional, time varying laminar flow, on the fibrosa side it is a reversing pulsatile flow (Figure 2). The question thus is whether there are differences between these two sides? If so, are these differences due to the very different hemodynamic environments on each side, or alternatively due to fundamental differences between the endothelial cells? Is the difference between the endothelial cells on the two sides genetic or is it environmental? We found over 700 genes downregulated and over 300 genes upregulated by oscillatory flow as compared to steady laminar flow. However, no significant difference has been found when fibrosa side and ventricularis side endothelial cells are exposed to the same shear stress conditions. There is no side-dependency and no apparent difference other than differences in their respective hemodynamic environment. 14 Also, miRNA array analysis yielded 30 shear-sensitive miRNAs and three side-specific miRNAs. Moreover, miRNA validation confirmed four of 17 shear-sensitive and one of three side-dependent miRNAs. Although there is clearly much more to do, we are slowly beginning to better understand the biology of heart valves.
Figure 2. .

Illustration of difference in the hemodynamic environment for the fibrosa side versus the ventricularis side of the aortic heart valve and the influence on calcification and sclerosis.
The above is only an example; however, the biology of heart valves including their biomechanical properties has become a major topic with several sessions at virtually every bioengineering conference. It thus has become very much a part of the evolution of bioengineering as a field and the intertwining of this field with cardiovascular research.
Tissue engineering and regenerative medicine
Tissue engineering is another research area where bioengineering has had a significant involvement, and thus has been intertwined with the growth of this new engineering discipline. It was only in 1987 that the term “tissue engineering” was created; and it was in 1988 that a conference called “tissue engineering” was first held at Lake Tahoe, California. The focus was on fabricating replacement tissues and organs outside of the body using cells and scaffolds for later implantation into the body. 15 This field of engineering was driven largely by clinicians and engineers. However, a much broader interdisciplinary effort evolved in the 1990s as stem cells received more interest. Furthermore, the field of tissue engineering broadened into what now is called regenerative medicine and includes, in addition to replacement, also repair and regeneration. 16 Clinical targets being pursued include targets in the cardiovascular system. Chief among these are the development of a small-diameter blood vessel substitute for the use in coronary bypass surgery, repair of a damaged myocardial wall following a heart attack and the development of a valvular substitute for use in defective heart valve replacement.
The tissue engineering of a heart valve substitute is in fact what has stimulated much of the interest in the biology of heart valves. 17 Furthermore, the pediatric population is one of the main targeted patient populations. A young child that has a heart valve with a congenital defect will need a larger sized replacement every few years. If one had a replacement valve made of living cells that would grow over time as the child grows, then only a single surgery would be needed. To date there has been some success with the two major efforts of the laboratories of John Mayer in Boston Sacks et al. 18 and Simon Hoerstrup in Zurich, Switzerland, 19 with both laboratories having engineers as part of their teams. The successful tissue engineering of a heart valve requires a combination of the right cells, a scaffold to provide the initial architecture, and the signals necessary to drive the process. Although none of the current efforts has progressed to human studies yet, large animal experiments have been conducted. Furthermore, from this one can see that there is a real role for engineering.
The tissue engineering of a small-diameter blood vessel substitute in many ways may be viewed as one of the field's “holy grails”. 20 This is because there are many patients who need the coronary bypass procedure but do not have native vessels available for use. Here again success depends on the right combination of cells, a scaffold either biologic or a synthetic material, and the necessary signals. In this area some progress has been made, and at least three efforts have been able to move into clinical trials. First is the work of Shinoka and colleagues, who reported in 2001 the first clinical use of a tissue-engineered blood vessel (TEVB), based on an autologous cell-seeded biodegradable scaffold, to repair cardiac defects in the low-pressure circulation of children. 36 Although the cells initially were taken from excised tissue and cultured in vitro, later studies were performed with cells isolated from the bone marrow and then directly seeded into the scaffold in the operating room. 21 This approach was used in more than 40 patients with considerable success. 22 Dr. Shinoka moved to Yale University, where he received FDA clearance to start a clinical trial. a
Another important effort has been that of Dr. Laura Niklason and her co-workers. This was based on the 1999 report of the use of a tubular synthetic, biodegradable scaffold composed of polyglycolic acid and seeded with smooth muscle cells as a vascular graft. 23 In a recently published study by by Niklason's startup company, Humacyte, 24 the TEBVs showed good patency in both coronary and carotid bypass models. Perhaps the most important achievement in that study was to produce a small diameter human TEBV with a burst pressure in excess of 3000 mmHg. These vessels, which were produced in 10 weeks in a pulsating bioreactor and decellularized, showed better compliance than that of a human saphenous vein, but still considerably less than that of human arteries.
The third promising approach has as its foundation in the research conducted in 1990s by the laboratory of Dr. Francois Auger in Quebec, Canada. The innovation here was to create sheets from the matrix secreted by cells, with these “self-assembled” sheets then rolled into the many distinct layers that compose a natural blood vessel. This method was used to construct the first tissue-engineered human blood vessel that was truly biological and that displayed physiological mechanical properties. 25 In 2000, Cytograft Tissue Engineering, Inc. was founded with the purpose of developing this new technology and bringing it to the clinic. The company aimed to simplify the complex in vitro model that was developed in Auger's laboratory, while retaining its many biological advantages. This was done by eliminating the medial layer of SMCs. The argument for eliminating this layer was based on contractility having little effect on the patency of a TEBV substitute. However, by eliminating the medial layer, the mechanical properties of this TEBV substitute were significantly altered. Preliminary results from initial human trials of this type of graft were reported a few years ago. 26 This trial involved the use of the graft as an arterio-venous (AV) shunt for hemodialysis access. Biomechanical testing indicated an average burst pressure in excess of 3000 mmHg. Furthermore, production of the graft proved to be reproducible. More recently an expanded study was published. 27,28 Cytograft is focusing its clinical trials in Europe and Asia, and the Phase III clinical studies for hemodialysis access employs both autologous and allogeneic grafts. Of interest is that the new allogeneic model has the potential of reducing the overall production time to 3 weeks. Furthermore, it allows 1000 of grafts to be fabricated from a single master cell line.
The more recent approaches of Niklason and her co-workers, L'Heureux and the Cytograft team involve creating a biological scaffold made up of extracellular matrix components. Still, a major issue is cells' source. If one is to use an autologous cell approach, then the concept of a TEBV off-the-shelf availability is not possible to implement. On the other hand, the use of an allogeneic cell approach could lead to off-the-shelf availability. Such approach might use allogeneic smooth muscle cells and/or fibroblasts. However, one must again emphasize that, if allogeneic endothelial cells are to be used, then one must incorporate some type of immune-suppressive strategy. Alternatively one could possibly recruit endothelial cells from the patient into a non-endothelialized TEBV which has been implanted. For all the progress in the last decade, it still appears that we are a few years away from having a TEBV substitute achieving FDA approval.
Another important area is that of the heart itself 29 and the use of a cell-based therapy for myocardial repair. Although there have been a variety of studies, they have not been particularily encouraging. This is because there has been only modest improvements in left ventricular function no matter what cell type is used. Also, cell engraftment has been poor, and it is very unlikely that significant repair/regeneration actually occurred. The fact that the use of different cell types leads to very similar results suggests that it is a paracrine effect, not one of cell replacement. A review of this area was published in 2011. 30 One important question is “what is the best method for delivering the cells to effect cardiac repair”? This is an area where engineers can contribute with one example being the work of Simpson et al. 31
A critical issue in tissue engineering and regenerative medicine is that of cell source. Because of this, the field of stem cell technology has taken on an important role in the field of tissue engineering and regenerative medicine. 32 Engineers are also contributing to the advances being made in this field. 33 Here again there have been studies of the role of physical forces, in this case in the modulation of stem cell behavior. One example is the use of flow and the associated shear stress to influence the differentiation of mouse embryonic stem cells to endothelial cells. 34
There is an emerging tissue engineering and regenerative medicine industry. 35 In the translation of the benchtop research to patient therapies, there is a real role for engineers. There also is a recognition that there is a need for the further development of bioprocessing systems for stem cell biomanufacturing. These systems will need to provide for the scaleup in cell numbers needed for a patient therapy and also the systematic assessment of the cell population required to provide for quality control and thus regulatory approval.
Concluding discussion
Over the past half century, bioengineering has emerged as an engineering discipline in its own right. At the same time, there have been major advances in our understanding of cardiovascular disease and in the development of therapeutic approaches. As described here, there has been an intertwining of these advances; and although one might argue that cardiovascular research would have advanced without the involvement of bioengineers, at the same time engineers have made major contributions.
The participation and contribution of bioengineers to cardiovascular research has been illustrated here using three specific areas: hemodynamics and atherosclerosis, heart valve engineering, and tissue engineering. These are areas in which the biomechanical aspects of the problem proved to be important. This was true in terms of the role of flow and the associated shear stress in the development of atherosclerosis, the fluid mechanic characteristics of prosthetic heart valves and more recently side-specific differences in the function of valvular endothelial cells, and in the development of innovative therapies using tissue engineering and regenerative medicine approaches.
There have been other contributions by bioengineers that have led to advancements in our understanding of the cardiovascular system and disease processes. This includes imaging, which engineers have contributed to the advancement of technologies that range from ultrasound to computerized tomography to magnetic resonance imaging to position emission tomography. This resulted in lower usage of surgical biopsy as a diagnostic. Engineers also have contributed to our understanding of the electrophysiological characteristics of the heart and to cardiac rhythm therapies. After all, it was only over half century ago that critical contributions of engineers led to the implantable pacemaker and to the establishment of the medical device industry.
The story told here, however, has been based on biomechanics and its role in various aspects of the cardiovascular system. Furthermore, it may be argued that it was individuals with an engineering background that recognized the importance of biomechanics in biology and in physiology. As noted earlier, the mechanical environment to which cells are exposed is part of the symphony of signals that orchestrates function, both normal and pathological. The result is that biomechanics has not only contributed to our understanding of the cardiovascular system, but it also has contributed to the establishment of the discipline of bioengineering. Looking into the future, there will be the continuing involvement of bioengineers including biomechanicians in cardiovascular research, and with this there will be continuing advances in our understanding of the cardiovascular system, including the basic biology and pathobiological aspects of disease as well as the development of new therapeutic approaches.
Footnotes
References
- [1].Citron P, Nerem RM. Bioengineering: 25 years of progress, but still only a beginning. Technol Soc. 2004;26(2–3):415–431. [Google Scholar]
- [2].Bergman RM, Nerem RM. The cardiovascular technology industry: past, present, and future. Cardiovasc Eng Technol. 2010;1(1):19–24. [Google Scholar]
- [3].Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Levesque MJ, Nerem RM. The elongation and orientation of cultured endothelial cells in response to shear stress. ASME J Biomech Eng. 1985;107(4):341–347. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- [5].Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. ASME J Biomech Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- [6].Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. 2010;298:H367. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Girard PR, Nerem RM. Shear stress modulates endothelial cell morphology and F-actin organization through the regulation of focal adhesion-associated proteins. J Cell Physiol. 1995;163:179–193. doi: 10.1002/jcp.1041630121. [DOI] [PubMed] [Google Scholar]
- [9].DeKeulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- [10].Johnson TL, Barabino GA, Nerem RM. Engineering more physiologic in vitro models for the study of vascular biology. Prog Pediatr Cardiol. 2006;21:201–210. [Google Scholar]
- [11].Ziegler T, Alexander RW, Nerem RM. An endothelial cell-smooth muscle cell co-culture model for use in the investigation of flow effects on vascular biology. Ann Biomed Eng. 1995;23:216–225. doi: 10.1007/BF02584424. [DOI] [PubMed] [Google Scholar]
- [12].Stegemann JP, Nerem RM. Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two- and three- dimensional culture. Exp Cell Res. 2003;283:146–155. doi: 10.1016/s0014-4827(02)00041-1. [DOI] [PubMed] [Google Scholar]
- [13].Dasi LP, Simon HA, Sucosky P, Yoganatha AP. Fluid mechanics of artificial heart valves. Clin Exp Pharmacol Physiol. 2009;36(2):225–237. doi: 10.1111/j.1440-1681.2008.05099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Holliday CJ, Ankeny RF, Jo H, Nerem RM. Discovery of shear-and side-specific mRNAs and miRNAs in human aortic valvular cells. AJP Heart Circ Physiol. 2011;301(3):H856–H867. doi: 10.1152/ajpheart.00117.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nerem RM, Sambanis A. Tissue engineering: from biology to biological substitute. Tissue Eng. 1995;1:3–13. doi: 10.1089/ten.1995.1.3. [DOI] [PubMed] [Google Scholar]
- [16].Badylak SF, Nerem RM. Progress in tissue engineering and regenerative medicine. Proc Natl Acad Sci USA. 2010;107(8):3285–3286. doi: 10.1073/pnas.1000256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Butcher J, Nerem RM. Valvular endotelial cells and the mechanoregulation of valvular pathology. Phil Trans R Soc Lond Part B. 2007;362(1484):1445–1457. doi: 10.1098/rstb.2007.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sacks MS, Schoen FJ, Mayer JE. Bioengineering challenges for heart valve tissue engineering. Ann Rev Biomed Eng. 2009;11:289–313. doi: 10.1146/annurev-bioeng-061008-124903. [DOI] [PubMed] [Google Scholar]
- [19].Schmidt D, Dikman PE, Driessen-Mol A, Stenger R, Mariani C, Puolakka A, Rissanen M, Deichmann T, Odermatt B, Weber B, Emmert MY, Zund G, Baaijens F, Hoerstrup S. Minimally-invasive implantation of living tissue engineered heart valves: a comprehensive approach from autologous vascular cells to stem cells. J Am Cell Cardiol. 2010;56(6):510–520. doi: 10.1016/j.jacc.2010.04.024. [DOI] [PubMed] [Google Scholar]
- [20].Nerem RM, Ensley AE. The tissue engineering of blood vessels and the heart. Am J Transplant. 2004;4(Supplement 6):36–42. doi: 10.1111/j.1600-6135.2004.0343.x. [DOI] [PubMed] [Google Scholar]
- [21].Shin'oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- [22].Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, Shinoka T. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139(2):431–436. doi: 10.1016/j.jtcvs.2009.09.057. [DOI] [PubMed] [Google Scholar]
- [23].Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro . Science. 1999;284(5413):489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- [24].Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, Dibernardo L, Hensley MT, Carter R, Williams TP, Prichard HL, Dey MS, Begeleman KG, Niklason LE. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3(68):1–11. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- [25].L'Heureux N, Paquet S, Labbe R, Germain L, Auger F. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12(1):47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- [26].L'Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NA, Kyles AE, Gregory CR, Hoyt G, Robbins RC, McAllister TN. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12(3):361–365. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Garrido S, McAllister T, Dusserre N, Marini A, L'Heureux N. Haemodialysis access via tissue-engineered vascular graft. Lancet. 2009;374(9685):20. [Google Scholar]
- [28].McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, Antonelli J, Kocher A, Zembala M, Cierpka L, de la Fuente LM, L'Heureux N. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373(9673):1440–1446. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- [29].Yacoub MY, Nerem RM. Introduction: bioengineering of the heart. Philos Trans R Soc Lond Part B. 2007;362(1484):1253–1255. doi: 10.1098/rstb.2007.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Malliaras K, Kreke M, Marban E. The stuttering progress of cell therapy for heart disease. Clin Pharmacol Ther. 2011;90(4):532–541. doi: 10.1038/clpt.2011.175. [DOI] [PubMed] [Google Scholar]
- [31].Simpson D, Liu H, Hwang T, Fan M, Nerem RM, Dudley SC. A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007;25:2350–2357. doi: 10.1634/stemcells.2007-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vats A, Bielby RC, Tolley NS, Nerem R, Polak JM. Stem cells. Lancet. 2005;366:592–602. doi: 10.1016/S0140-6736(05)66879-1. [DOI] [PubMed] [Google Scholar]
- [33].McCloskey KE, Lyons I, Rao RR, Stice SL, Nerem RM. Purified and proliferating endothelial cells derived and expanded in vitro from embryonic stem cells. Endothelium. 2003;10:329–336. doi: 10.1080/10623320390272325. [DOI] [PubMed] [Google Scholar]
- [34].Ahsan T, Nerem RM. Fluid shear stress promotes an endothelial phenotype during early differentiation of embryonic stem cells. Tissue Eng Part A. 2010;16(11):3547–3553. doi: 10.1089/ten.tea.2010.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nerem RM. Regenerative medicine: the emergence of an industry. J R Soc Interface. 2010;7:S771–S775. doi: 10.1098/rsif.2010.0348.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shin'oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344(7):532–533. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]