Introduction
Maintaining a constant core body temperature of 37°C is essential for survival, through maintaining optimal organ, tissue and cellular function. Tight temperature regulation is achieved through a complex, integrated system of thermogenesis and heat loss. Different levels of hypothermia for defined periods of time can be protective. Temperature management during and after cardiac surgery, as well as after accidental hypothermia can have major effects on both immediate and long-term outcome. Through knowledge of the mechanisms of temperature regulation, we can understand its influence of on different vital organ functions in modern cardiac anaesthesia. We here describe mechanisms of thermoregulation, the protective effect of induced hypothermia, detailed techniques and risks of temperature changes during cardiac surgeries .
Figure 1. .
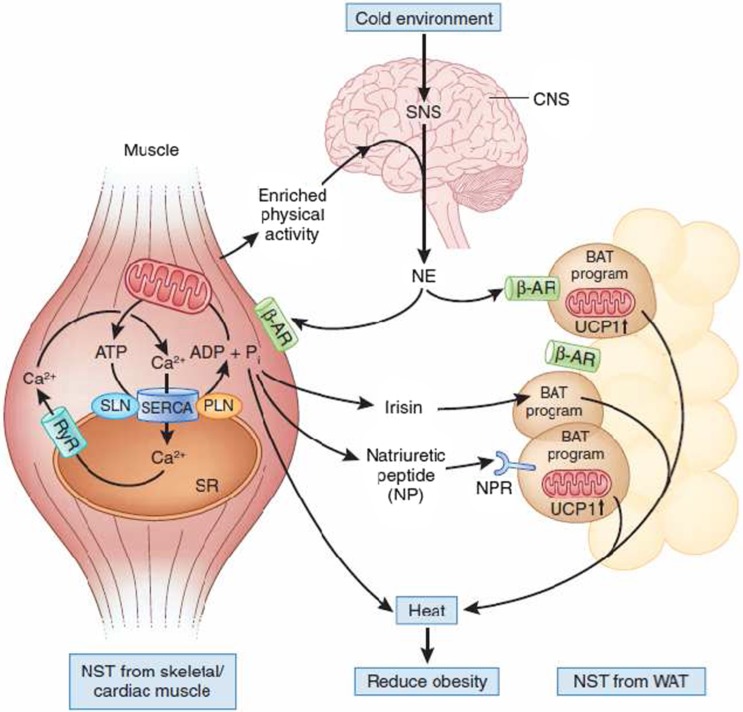
Nonshivering thermogenesis (NST) from muscle and brown adipose tissue (BAT).
Thermoregulation
Thermoregulation involves an extremely sophisticated system of balancing heat production by several organs, and heat loss. Heat production may be classified into shivering and non-shivering components, with each component playing a more dominant role during different physiological and pathological conditions. The different mechanisms of thermoregulation are summarized in Table 1.
Table 1 .
Response of different organs to changes in temperature.
| Effectors | Response to low temperature | Response to high temperature |
| Smooth muscles in arterioles in the skin. | Muscles contract causing vasoconstriction. Less heat is carried from the core to the surface of the body, maintaining core temperature. Extremities can turn blue and feel cold, and can even be damaged (frostbite). | Muscles relax causing vasodilation. More heat is carried from the core to the surface, where it is lost by convection and radiation (conduction is generally low, except when in water). Skin turns red. |
| Sweat glands | No sweat produced. | Glands secrete sweat onto surface of skin, where it evaporates. Since water has a high latent heat of evaporation, it takes heat from the body. High humidity, and tight clothing made of manmade fibres reduce the ability of the sweat to evaporate and causes discomfort in hot weather. Transpiration from trees has a dramatic cooling effect on surrounding air temperature. |
| Erector pili muscles in skin (attached to skin hairs) | Muscles contract, raising skin hairs and trapping an insulating layer of warm air next to the skin. Not very effective in humans, other than causing “goosebumps”. | Muscles relax, lowering the skin hairs and allowing air to circulate over the skin, encouraging convection and evaporation. |
| Skeletal muscles | Shivering: Muscles contract and relax repeatedly, generating heat by friction and from metabolic reactions respiration is only 40% efficient: 60% of increased respiration thus generates heat). | No shivering. |
| Adrenal and thyroid glands | Glands secrete adrenaline and thyroxine, which generate heat and increase the metabolic rate in different tissues, especially the liver. | Glands stop secreting adrenaline and thyroxine. |
| Behaviour | Curling up, huddling, finding Stretching out, finding shade, shelter, putting on more clothes. | Stretching out, finding shade, swimming, removing clothes. |
| Nonshivering thermogenesis (NST) from muscle and brown adipose tissue (BAT) | The cold environment centrally activates the sympathetic nervous system (SNS), which releases norepinephrine (NE) to activate the Ca2+ pump SERCA, which is under control of sarcolipin (SLN) and phospholamban (PLN) in muscle. Sarcolipin uncouples SERCA-mediated ATP hydrolysis from ‘work’ (that is, Ca2+ pumping), resulting in the liberation of energy in the form of heat. Simultaneously, brown adipocyte formation is stimulated in white adipose tissue (WAT) by the SNS and by irisin and naturietic peptide, which are secreted by skeletal and cardiac muscle, respectively. This results in an increase of the uncoupling protein 1 (UCP1) in the mitochondria, which induces heat production. A program of enriched physical activity is also proposed to increase brown adipocyte formation in WAT via the SNS. Accordingly, the concerted action of a cold environment and physical activity generates heat from muscle and white fat to reduce systemic obesity. b-AR, b-androgenic receptor; RyR, ryanodine receptor10 (Figure 1). |
Heat loss occurs primarily from the skin of a patient to the environment through several processes, including; radiation, conduction, convection and evaporation. Of these processes, radiation is the most significant and accounts for approximately 60% of total heat loss. Radiation is emitted in the form of infrared rays. Heat from core body tissues is transported in blood to subcutaneous vessels, where heat is lost to the environment through radiation. Radiation is the major source of heat loss in most surgical patients.
Conduction refers to loss of kinetic energy from molecular motion in skin tissues to surrounding air. Water absorbs far more conducted heat than air, and this accounts for more rapid hypothermia during accidental drowning, as well as the efficacy of water baths to cool hyperthermic patients. For this to be effective, warmed air or water must be moved away from the skin surface by currents in a process called convection. This accounts for the cooling effect of wind and laminar air flow in many surgical suites. Conduction and convection account for ∼15% of body heat loss.
Approximately 22% of heat loss occurs by evaporation, as energy in the form of heat is consumed during the vaporization of water. Water evaporates from the body even when the body is not sweating, but mechanisms that enhance sweating increase evaporation. As long as the skin temperature is greater than its surroundings, radiation and conduction provide heat loss. At very high environmental temperatures, these processes cannot work and evaporation is the only manner in which heat can be dissipated. This generally is not the case in clinical settings.
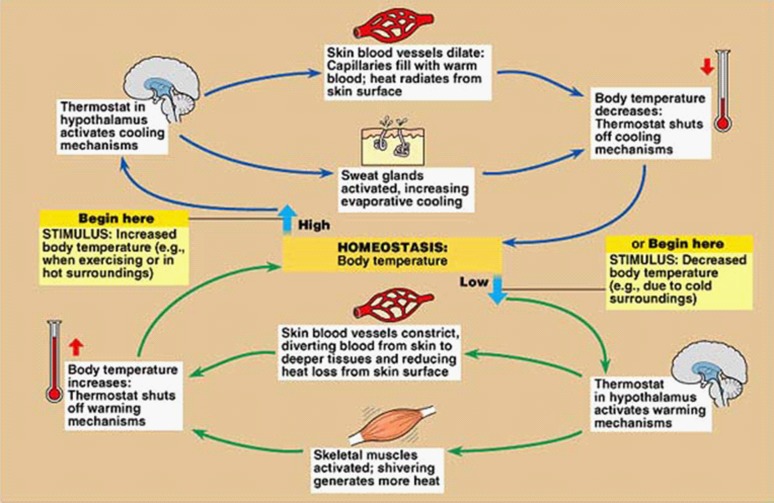
Skin temperature rises and falls with the temperature of a patient's surroundings. However, the core temperature remains relatively constant. This is due to a remarkable thermoregulatory system that is conventionally organized into three components: afferent sensing, central control and efferent responses (Figure 2). 1
Figure 2. .
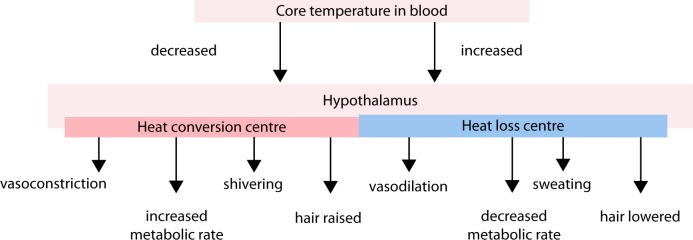
Cerebral temperature control.
Afferent sensing and central control:
Some integration and temperature regulation may occur at the spinal cord level. However, the hypothalamus is the primary control center for thermoregulatory as it integrates most afferent input and coordinates the various efferent outputs required to maintain a normothermic level.
Efferent response:
As temperature receptors transmit information to the hypothalamus, this information is integrated and compared with threshold settings. Values above or below these thresholds determine the generated efferent response. Efferent outputs from the hypothalamus regulate body temperature by altering subcutaneous blood flows, sweating, skeletal muscle tone and overall metabolic activity. Heat loss is promoted by vasodilatation and sweating, while heat is conserved by inhibiting these processes. Production of heat (thermogenesis) is promoted by shivering and increases the overall metabolic rate (Figure 3).
Figure 3. .
Temperature homeostasis.
Temperature inputs to the hypothalamus are integrated and compared with threshold temperatures that trigger appropriate thermoregulatory responses. Normally these responses are initiated at as little as 0.1°C above and below normal body temperature of 37 °C. Therefore, the difference between temperatures that initiate sweating versus those initiating vasoconstriction is only 0.2°C. This is defined as the ‘interthreshold range’ and represents the narrow range at which the body does not initiate thermoregulatory efforts.
General anesthesia and thermogenesis:
Causes for inadvertent hypothermia include patients' exposure to a cold environment and the inability to initiate behavior responses. Volatile anesthetics, propofol, and older opioids such as morphine and meperidine promote heat loss through vasodilation. This process is compounded further by the fact that these drugs, as well as fentanyl and its derivatives, directly impair hypothalamic thermoregulation in a dose-dependent manner. Opioids also depress overall sympathetic outflow, which further inhibits any attempts at thermoregulation. The depressant effect on the hypothalamus results in an elevated threshold for heat response, along with a diminished threshold for cold response such as vasoconstriction and shivering. Therefore, opioids widen the normal interthreshold range from ∼0.2°C to as much as 4°C, 2 and patients are unable to adjust to cold environments and heat loss resulting from vasodilation. 1 It is notable that nitrous oxide depresses thermoregulation to a lesser extent than equipotent concentrations of the volatiles, and midazolam has minimal or no influence. 3 This should be true for other benzodiazepines as well. 2,3
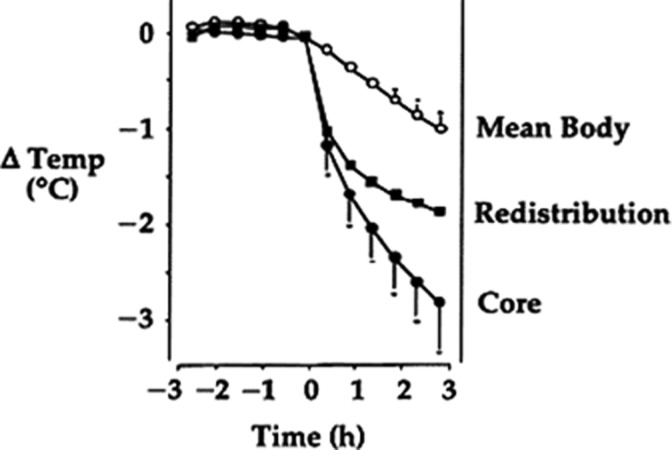
Following induction of general anesthesia, the decline in body temperature occurs in three phases. The greatest decline occurs during the first half hour or phase 1. Normally body heat is maintained in an unevenly distributed manner; the temperature of core tissues is 2°C to 4°C greater than skin temperature. Following anesthesia induction, however, vasodilation combined with a lowered cold threshold in the hypothalamus allows a redistribution of body heat from core tissues to skin, where heat is lost primarily through radiation. Phase 2 commences after approximately 1 h, as core temperature decreases at a slower rate and proceeds in a linear manner as heat lost from the body exceeds heat production. Finally, after 3 to 5 h, phase 3 commences, as equilibrium is reached where heat loss is matched by heat production and thermoregulated vasoconstriction commences to function (Figure 4). 1–3
Figure 4. .
Temperature drop during anesthesia.
Perioperative temperature monitoring:
Temperature monitoring devices vary according to the type of transducer used and the site to be monitored. The most commonly used transducers are thermistors and thermocouples. A more recent development is the use of monitors that emit infrared to measure temperature; these monitors are commonly found in aural canal thermometers, which are often referred to as tympanic membrane thermometers. Liquid crystal sensors also can be used to measure skin temperature.
Core temperature is the best single indicator of body temperature. Therefore, all noncore temperature-monitoring sites need to be judged by their ability to accurately assess core temperature. Core temperature monitoring is appropriate for most patients undergoing general anesthesia, to facilitate detection and treatment of fever, malignant hyperthermia, and hypothermia.
Monitoring sites:
1- Pulmonary artery catheter (PAC):
PAC thermistor is located at the tip of the distal end allowing measurement of central blood temperature. Although it is the gold standard for core temperature measurement, 4 it is not used routinely due to numerous drawbacks including invasiveness and cost effectiveness.
2- Oesophageal temperature:
This is usually monitored with a thermistor or thermocouple that is incorporated into an esophageal stethoscope. Oesophageal temperature accurately reflects core temperature in almost all conditions. These readings, however, can be artificially affected during general anesthesia by the use of humidified gases if the probe is not inserted far enough. 5,6 The optimal position for the sensor in adults is approximately 45 cm from the nose, which is 12 to 16 cm distal from where the heart and breath sounds are heard best. 7 More proximal positioning can result in falsely decreased temperatures as a result of the proximity to the trachea and the impact of cold, dry gases on the site. 6 Esophageal temperature probes are used frequently for their ease of placement and relatively minimal risk, and because the site is reliable.
3- Nasopharyngeal temperature:
Nasopharyngeal temperature can be measured with an esophageal probe positioned above the palate, and it is close to brain and core temperature. 8
4- Tympanic membrane temperature:
Because the eardrum is close to the carotid artery and the hypothalamus, tympanic membrane temperature is a reliable measure of core temperature and often is used as a reference for other sites. This measurement requires that a transducer be placed in contact with the tympanic membrane.
5- Bladder temperature:
Bladder temperature is measured with a Foley catheter and attached temperature thermistor or thermocouple. Although bladder temperature is a close approximation of core temperature, the accuracy of this site decreases with low urine output and during surgical procedures of the lower abdomen. 9
6- Rectal temperature:
Rectal temperature measurement is another site that approximates core temperature, but these readings may be affected by the presence of stool and of bacteria that generate heat. 10 Consequently, rectal temperature tends to exceed core temperature. Rectal and bladder temperatures lag behind other central monitoring sites during conditions in which the temperature changes rapidly such as cardiopulmonary bypass surgery.
7- Skin temperature:
Intraoperative skin temperature monitoring is confounded by several factors; Core-to-peripheral redistribution which may be seen on anesthetic induction, thermoregulatory changes in vasomotor tone triggered when sufficient core hypothermia initiates intraoperative cutaneous vasoconstriction which can lead to a reduction in skin blood flow and temperature, and finally changes in ambient temperature.
8- Axillary temperature:
Axillary temperatures are relatively close to core temperature and may be a reasonable choice in selected patients. However, its accuracy is questionable unless the probe is positioned carefully over the axillary artery and the arms positioned at the patient's side. 11
Hypothermia as a cytoprotective strategy
Hypothermia had demonstrated potential benefits in myocardial infarction, organ transplantation, cardiopulmonary bypass (CPB), spinal cord injury (SCI), intestinal ischemia, and neonatal hypoxic ischemia. 12 Understanding how hypothermia can be of benefit is not simple as its action is mediated through complex systemic and cellular changes; moreover, such changes can vary widely depending on underlying pathological and metabolic condition. However, examples from nature have indicated that physiological hypothermia could be observed in species that normally hibernate. In these animals, body temperature and metabolism drops in a manner as that observed when intentional hypothermia is applied. 12
Hypothermia can be classified based on the depth of cooling from a normal body temperature of 37-38°C: mild hypothermia (32-35°C), moderate hypothermia (28-32°C) and deep hypothermia ( < 28°C). While deep hypothermia has been embraced by various surgical subspecialties, the neuroprotective effect of mild to moderate hypothermia appears to be similar to deep. 13 Thus, less drastic decreases in body temperature have been favored by other disciplines, as it is easier to cool nonsurgical patients to these target temperatures, and the risks of medical complications such as infection, arrhythmia, hypokalemia, coagulopathies and even heart failure are lower. 13
Historical perspective of therapeutic hypothermia
Therapeutic hypothermia was first used in 1960 in patients with acute neurological insults. The publication “Management of the comatose patient” 14 was released in 1965. This early experience was instrumental in the evolution of the current recommendation by the American Heart Association and the International Liaison Committee on Resuscitation. 15 In addition, in 1964, the historic “first ABCs of resuscitation” recommended that hypothermia could be used in patients who remain comatose after successful restoration of spontaneous circulation. 16 This recommendation is still followed to the present day. 15
However, therapeutic hypothermia began to fall out of favor in the 1980s. This resulted, in part, from overzealous application in some patients, who were treated for durations longer than a week and at temperatures in the moderate (28°C to 32°C) rather than mild (33°C to 35°C) range which resulted in complications. 17
Cytoprotective mechanisms of induced hypothermia
There are many mechanisms by which hypothermia may be cytoprotective. Earlier literature has focused on its ability to preserve metabolic stores and thus render the tissue or organism in a state of ‘suspended animation’. 18 Suspended animation is defined as therapeutic induction of a state of tolerance to temporary complete systemic ischemia.
The objectives of suspended animation include:
a) To help save victims of temporarily uncontrollable exsanguination, followed by delayed resuscitation;
b) To help save some non-traumatic cases of sudden death, seemingly non-resuscitable before definite repair; and
c) To enable performing selected (elective) surgical procedures, which are only feasible in a bloodless field.
Hypothermia has generally been viewed as a means by which protein synthesis can be suppressed. 19 However, the widely held notion that hypothermia protects because lower temperatures slow metabolism is not completely accurate. Recent research has shown that hypothermia can alter a plethora of cell death and cell survival pathways including gene regulation resulting in the inhibition of apoptosis and inflammation 19 and the up-regulation of anti-apoptotic 20 or trophic factors. 21
In some systems, hypothermia has been shown to up-regulate a family of ‘cold shock proteins’ at temperatures between 25-33°C. This up-regulation could potentially regulate cell survival at a proximal point in the cell death pathways. These cold shock proteins include cold inducible RNA binding protein (CIRP) and RNA-binding motif protein 3 (RBM3). CIRP has been speculated to protect and restore native RNA conformations during stress, and protects against apoptosis by up-regulating extracellular signal-regulated kinase (ERK), which is involved in a cell survival pathway. 22 RBM3 may also protect cells from death by acting in a manner similar to the X-linked inhibitor of apoptosis (XIAP). 23 Approximately 5% of the oxygen and glucose consumption is reduced per degree centigrade cooling of the brain. Hypothermia is also thought to couple both the brain energy and blood flow to a lower rate for cerebral tissue. 24 Injury triggers the release of excitatory amino acids (probably a specific phenomenon in brain). Glutamate is now recognized to potentiate brain injury by binding to its ionotropic receptors and allow entry of toxic levels of calcium. A key mechanism in hypothermic brain protection is to prevent this toxic increase. 25–27
Activation of peripheral leukocytes and brain resident microglia also occurs after brain injury, and mild hypothermia has been shown by multiple investigators to inhibit this activation and thus provide protection against ischemic injury. 28,29 Suppression of this activation could be explained by the observation that hypothermia inhibits the pro-inflammatory transcription factor NFκB. 30–32 Anti-apoptotic effects of hypothermia have also been documented by several investigators. Hypothermia decreases numbers of apoptotic cells 28,33,34 and reduces expression of pro-apoptotic genes 35 while increasing anti-apoptotic genes such as Bcl-2. 36,37
Hypothermia for neuroprotection:
Prior work has shown that the extent of neuroprotection by hypothermia is not related to the amount of cooling, but the time when cooling is begun and its duration. 38 Similar amounts of neuroprotection have been observed where cooling to 30-34°C and that observed at 25°C. 13 Clinically, cooling to 35°C appears to be as beneficial as 33°C while causing fewer complications. 39 Timing of the initiation of cooling is also important, in that the likelihood of a better outcome seems to depend on earlier initiation. 40 Prolonged cooling (24-48 h) is essential for long term and robust protection. 41,42
In such context, hypothermia had been used in various clinical situations aiming for neuroprotection:
1- Cardiac arrest:
Clinical studies now show that mild hypothermia improves neurological outcome from cardiac arrest. 20 A multi-center clinical trial in Europe studied 274 patients with cardiac arrest due to ventricular fibrillation, and showed that cooling to 32°C to 34°C over a period of 24 h had a significant protection. As a consequence of therapeutic hypothermia, the 6 month mortality was reduced from 76/138 (normothermia) to 56/137 (hypothermia, P < 0.05). The cooled patients also had improved neurological outcome 6 months later, compared to those who were not cooled. 43 Another clinical trial in Australia included 77 patients who had cardiac arrest and randomly treated patients with either hypothermia (33°C within 2 h after the return of spontaneous circulation and maintained for 12 h) or remained normothermic. In this second study, 49% of the patients who received hypothermia survived with a good neurological outcome, while only 26% of the patients survived in the normothermia group (P < 0.05). 44
2- Brain trauma:
Hypothermia has been used in brain trauma for many years, and results of several small clinical trials have been published. 45,46 One study showed modest but transient benefit in patients with head trauma, provided the Glasgow Coma Scale (GCS) was between 5–7, 47 but further studies in adults are ongoing to clarify whether earlier intervention might result in a favorable outcome.
3- Neonatal hypoxic encephalopathy:
Clinical trials of therapeutic hypothermia in neonates with hypoxic encephalopathy also suggest a benefit in this patient population. A recently published study in 2008, Total Body Hypothermia for Neonatal Encephalopathy Trial (TOBY), showed similar benefits in newborns with perinatal asphyxia that received whole body cooling within a similar timeframe. 48 The primary outcome at 18 months of age showed that cooled infants had better survival and neurological outcomes than those that were not cooled. However, defining the optimal candidate for hypothermia has yet to be defined, and subsequent studies are ongoing.
4- Ischemic stroke:
Experimental stroke studies have shown the neuroprotective effects of hypothermia when cooling was applied in the range of 24°C to 33°C, 49 with temperatures in the mild to moderate range being better tolerated. The timing of cooling is also important, in that hypothermia should be initiated within 2–3 h of ischemia onset.
A significant challenge in applying hypothermia to stroke patients is that unlike other neurological conditions, stroke patients are generally awake and do not tolerate cooling. Like that encountered in the cardiac arrest and brain injury studies, attaining target temperature and maintaining it, especially in adult humans, is challenging.
5- Hemorrhagic stroke:
Models of ICH are frequently studied where bacterial collagenase was instilled into the striata to cause hemorrhage due to proteolytic disruption of the basal lamina, or autologous blood is directly injected into the brain. 50 Unlike that observed in ischemic brain ischemia models, studies of therapeutic hypothermia in brain hemorrhage are less consistent. While a few studies have shown beneficial effects of hypothermia in ICH, others have not. 22 A reason why the response of hemorrhagic stroke is so varied, is that cooling can also affect endogenous coagulant and thrombolytic systems, and may predispose to bleeding with potential worsening.
Hypothermia for Cardiac procedures:
Cardiopulmonary bypass (CPB), with its potential for creating temperature fluctuations, imposes enormous challenges to cerebral oxygenation and perfusion. Two physiologic principles, functional and structural cerebral metabolic rate of oxygen (CMRO2), are vital to understanding the importance of temperature in patients undergoing CPB. 51 In humans, the CMRO2 is approximately 20% at basal metabolism: approximately 3.5 mL/100g− 1/min− 1. Of the total CMRO2, 60% is used to support cerebral electrophysiological function (functional CMRO2), and 40% is used for maintenance of cellular integrity (structural CMRO2). Temperature is the only agent known to affect both functional and structural CMRO2; total CMRO2 decreases 6%–7% per degree centigrade reduction in temperature. Anesthetic drugs, on the other hand, alter only functional CMRO2. 52
Intraoperative hypothermia is used to varying degrees in surgical procedures requiring CPB or circulatory arrest. Relatively small degrees of hypothermia, e.g., 2–3°C, can offer significant brain protection. 53,54 The unique and beneficial effects of more profound hypothermia consist of reduction in both functional and structural metabolic rates, making it superior to any neuroprotective drugs.
In addition to the effects on metabolism, other mechanisms of therapeutic hypothermia include suppression of free radicals, inhibition of destructive enzymatic reactions, reduction in metabolic requirements in low-flow regions, and inhibition of the biosynthesis, release, and uptake of excitatory neurotransmitters. 44,55,56 Through these mechanisms, hypothermia provides a favorable balance between oxygen supply and demand, slows the onset of ischemic depolarization, decreases the release of ischemic-induced intracellular calcium influx, and suppresses nitric oxide synthase activity.
Cerebral blood flow (CBF) decreases as temperature decreases during CPB 57–59 with the electroencephalogram tracing becoming isoelectric at approximately 18°C. The use of IV anesthetic drugs, thiopental in particular, reduces cerebral metabolic requirements responsible for brain function and synaptic activity, conditions achieved during the isoelectric electroencephalogram state. 60
Hypothermia is widely used in open cardiac surgery not only to protect against perioperative brain ischemia that could potentially develop 61 but also the myocardium. Following coronary artery occlusion, myocardial tissue consequently endures oxygen and energy depletion and thus cell death if not rescued. For several decades, cardiac surgeons have been using hypothermia to protect the non-working heart during open heart surgeries. 62 In the ischemic working heart, mild hypothermia (34°C) preserves microvascular flow and maintains cardiac output. 21 In addition to maintaining myocardial contractility after ischemia, studies in a rabbit model of acute myocardial infarction showed that regional myocardial hypothermia (32°C), initiated 10 min before reperfusion and maintained for 2 h, significantly improved the reflow, reduced the “no-flow” phenomenon, myocardial necrosis and infarct size. 63 Like the brain, timing of hypothermia is a major concern in order to rescue the heart from ischemia. Several studies have shown salutary effects of hypothermia in cardioprotection when cooling was initiated prior to the onset of ischemia. 64 Post insult hypothermia has also been shown to benefit experimental myocardial ischemia (MI). Whether initiated before or after ischemia onset, mild hypothermia resulted in protection provided the target temperature (left atrial temperature 2-2.5°C lower than normal) was reached at the time of coronary artery reperfusion resulted in a marked protection. 65
Possible mechanisms of protection seem to parallel those described for brain ischemia. Studies have shown correlations between protection and reduced energy demand, 66 delayed ATP depletion, 67 induced HSP70 expression 68 decreased apoptotic cell death through reduced expression of tumor suppressor gene p53 translational product 66 and improved blood flow to the myocardial microvasculature. 69
Clinical application of hypothermia for cardioprotection has largely been used in the intraoperative setting; however, some smaller studies have been conducted in patients with acute myocardial infarction. Considerations to an extent specific to the heart include compromised cardiac function with cooling.
Other indications for hypothermia:
1) Spinal cord injury (SCI):
Mild hypothermia also decreased astrocyte and capillary proliferation which could affect regeneration of axons. However, laboratory studies seem to mostly suggest that hypothermia improves axon recovery. 70 Studies to define optimal cooling methods, depth and duration of cooling, timing of cooling and ideal candidates for cooling are still needed.
2) Hepatic encephalopathy:
Severe liver failure may increase toxic metabolite accumulation in the body with portal blood being shunted into the systemic circulation. Mild hypothermia can improve detrimental effects of liver failure by improving ammonia metabolism, suppressing inflammation, normalizing brain osmolarity and cerebral blood flow. 71
3) Hypotensive bleeding trauma patients:
Severe bleeding is a frequent cause of hypotension and shock in trauma patients. Since hypotension does not develop until loss of more than 30-40% blood volume, these injuries are often fatal and must be managed rapidly to avoid death. Mild to moderate hypothermia decreases heart rate and increases systemic vascular resistance while maintaining stroke volume and blood pressure. Hence, hypothermia decreases cardiac metabolic demands while sustaining cardiac output and myocardial perfusion. 72 In a model of uncontrolled hemorrhage, mild to moderate hypothermia induced by surface cooling delayed the onset of cardiac arrest and significantly improved survival in rats. 73–76
Potential risks associated with therapeutic hypothermia
Combination of anesthetic induced impairment of thermoregulatory control and exposure to a cool operating room environment makes most surgical patients hypothermic. Several prospective, randomized trials have demonstrated various hypothermia-induced complications.
1- Myocardial ischemia:
Evidence connecting perioperative hypothermia with myocardial complications was initially based on a retrospective analysis of data collected prospectively for a different purpose. 77 These data indicated that patients becoming hypothermic were more likely to experience myocardial ischemia and ventricular arrhythmias.
The mechanism by which mild hypothermia triggers myocardial events remains unclear. Cold-induced hypertension in the elderly is associated with a three-fold increase in plasma norepinephrine concentrations, 78 which may augment cardiac irritability and facilitate development of ventricular arrhythmias. Hypothermia also causes hypertension in elderly patients and those at high risk for cardiac complications. 79
2- Coagulopathy:
Surgeons have long suspected that hypothermia produces coagulopathy and increases perioperative blood loss. Other surgeons believed that hypothermia “thickened the blood” and reduced bleeding. Schmied and colleagues 80 showed that mild hypothermia increases blood loss. In their study, patients were randomly assigned to normothermia or mild hypothermia during elective primary hip arthroplasty. Just 1.6°C core hypothermia increased blood loss by 500 ml (30%) and significantly augmented allogeneic transfusion requirement. The same group subsequently confirmed the haemostatic benefits of maintaining intraoperative normothermia in a retrospective analysis. 81 In contrast, another study of blood loss during hip arthroplasty failed to identify temperature dependence to blood loss. 82
Three general mechanisms contribute to temperature-related coagulation disorders: platelet function, clotting factor enzyme function, and fibrinolytic activity.
Hypothermia and platelet function:
Platelet numbers remains normal during mild hypothermia. However, Valeri and colleagues 83 demonstrated that mild perioperative hypothermia seriously impaired platelet function. Inhibition was a strictly local phenomenon: bleeding time was comparably increased by systemic or local hypothermia. Subsequent work indicated that the defect resulted from reduced release of thromboxane A2. 84,85
Hypothermia and clotting factor enzyme function:
One feature of hypothermic coagulopathy is that standard coagulation tests, including the prothrombin time and the partial thromboplastin times, remain normal. 86 The reason is that the tests are normally performed at 37°C, regardless of what the patient's temperature is. These sometimes are prolonged by hypothermia when they are performed at the patient's actual core temperature. Rohrer et al demonstrated that series of enzymatic reactions of the coagulation cascade are strongly inhibited by hypothermia, as demonstrated by the dramatic prolongation of prothrombin time and partial thromboplastin time tests at hypothermic deviations from normal temperature in a situation where factor levels were all known to be normal. 87
Hypothermia and fibrinolytic activity:
Fibrin is a major structural element in formed clots but is subject to degradation by plasmin, the activated enzymatic form of plasminogen. The conversion of plasminogen to plasmin is at the core of the fibrinolytic mechanism. Preliminary data suggest that fibrinolysis remain normal during mild hypothermia but is significantly increased during hyperthermia, suggesting that hypothermia-induced coagulopathy does not result from excessive clot lysis. The corresponding effects of thermal disturbances on plasminogen activator are yet to be determined, but thromboelastographic data suggest that hypothermia impairs clot formation rather than facilitates clot degeneration. 88–90
3- Wound infection and healing:
Hypothermia primarily impairs wound infection and healing through thermoregulatory vasoconstriction and impairment of immune function. Hypothermia triggers thermoregulatory vasoconstriction, 91 vasoconstriction decreases subcutaneous oxygen tension and incidence of wound infection correlates with subcutaneous oxygen tension. 92 Evidence indicating that mild core hypothermia directly impairs immune function, including T-cell—mediated antibody production and nonspecific oxidative bacterial elimination by neutrophils, was demonstrated over 30 years ago. 93–95
4- Thermal discomfort:
It had been indicated that feeling cold in the immediate postoperative period is the worst part for patients during hospitalization, with some patients even rating the sensation as worse than surgical pain. 96,97
5- Other consequences:
Although their clinical significance remains trivial, there are several consequences associated with hypothermia: Hypokalemia, 98,99 increased cardiotoxicity of bupivacaine, 100 mild affection of somatosensory evoked potentials, 101 and obliteration of the oxymeter signal by sufficient vasoconstriction. 102
Pharmacokinetics and pharmacodynamics with hypothermia:
The enzymes that moderate organ function and metabolize most drugs are highly temperature-sensitive. It is therefore not surprising that drug metabolism is temperature-dependent. Curiously however, the pharmacokinetics of only few anesthetic drugs had been evaluated. Hypothermia also alters the pharmacodynamics of various drugs, especially volatile anesthetics.
Muscle relaxants:
The duration of action of vecuronium bromide is doubled in patients with a 2°C reduction in core temperature. 103 Hypothermic prolongation of vecuronium action results from a pharmacokinetic effect, as pharmacodynamics of muscle relaxants are essentially unchanged by mild hypothermia. 104
Volatile anesthetics:
The tissue solubility of volatile anesthetics increases with hypothermia. At a given steady-state plasma partial pressure, body anesthetic content increases at subnormal temperatures. This does not alter anesthetic potency, because potency is determined by partial pressure rather than anesthetic concentration. However, it may slow recovery from anesthesia because larger amounts of anesthetic eventually need to be exhaled. 96
Intravenous anesthetics:
During a constant infusion of propofol, plasma concentration is approximately 30% greater than normal when individuals are 3°C hypothermic. The increase apparently results from a reduced intercompartmental clearance between the central and peripheral compartments. Hypothermia also increases steady state plasma concentrations of fentanyl by approximately 5%/°C. The effects of mild hypothermia on the metabolism and pharmacodynamics of most other drugs has yet to be reported. Evidence suggests that hypothermia was associated with delayed discharge of adult patients from the postanesthesia care unit. 105
Temperature management during cardiac surgery
Intraoperative temperature regimen is usually planned preoperatively by the anesthesiologist, surgeon and the perfusionist. Selecting and understanding the impact of the temperature regimen (normothermia, or mild or moderate or severe hypothermia) is usually related to the type of the cardiac surgery (using CPB circulatory arrest or beating heart surgery).
Temperature monitoring during cardiopulmonary bypass:
Cardiopulmonary bypass constitutes a challenging situation for monitoring temperature because of the rapid and extraordinary degree of heat transferred through the bypass circuit during heating and cooling. The core compartment undergoes the fastest temperature changes because of the rapid rate of blood reinfused into the mediastinal organs. The thermal changes from the core compartment then are dissipated slowly to the periphery because of the slower nature of heat passing through tissue as opposed to the cardiopulmonary bypass circuit.
During bypass, core-monitoring sites are useful for temperature monitoring. However, the pulmonary artery catheter may not give an accurate temperature reading because of diminished flow in the central circulation. Bladder and rectal temperatures often are considered intermediate temperature monitoring sites during bypass, because they lag behind the core sites but change faster than peripheral tissues. During cardiac surgery, bladder temperature equals rectal temperature when urine flow is low, but equals the pulmonary artery temperature when urine flow is high. 106 Because urine flow is such an important determinant of temperature in the cardiac surgery, it is best to consider both core and intermediate sites when judging adequacy of rewarming or cooling. Skin temperature, although unrelated to core temperature, it may be helpful in evaluating heat distribution between compartments at the end of bypass. 107
Management of temperature during cardiopulmonary bypass:
The clinical technique of temperature management during extracorporeal circulation (ECC) in open heart surgery is divided into three groups according to the nasopharyneal temperature: mild hypothermia group (32-35°C), moderate hypothermia group (26-31°C) and deep hypothermia group (25°C).
In hypothermic CPB, patients are cooled to 31-32°C after the beginning of CPB. Rewarming begins 10-15 min before release of aortic cross-clamp. The gradient between heat-exchanger and nasopharynx during rewarming will be maintained at 2-3°C. ln normothermic CPB, patients are kept at normothermia throughout the procedure (>36°C).
Hypothermic versus normothermic CPB:
Hypothermic CPB had become an established practice for adult cardiac surgery by the late 1960s, and constituted the largest part of the surgical practice at most institutions until the reintroduction of warm CPB in early 1990s. 108 A systematic review of benefits and risks of maintaining normothermia during CPB among adults undergoing cardiac surgery had been published 109 and demonstrated no benefit of hypothermia during CPB in regard to mortality, risk of stroke, cognitive decline, atrial fibrillation, use of inotropic support or intra-aortic balloon pump, myocardial infarction, all cause infections, and acute kidney injury after cardiac surgery were comparable. Moreover, hypothermic bypass was associated with an increased risk of allogeneic red blood cells, fresh frozen plasma, and platelet transfusion. The authors concluded that “maintaining normothermia during cardiopulmonary bypass in adult cardiac surgery is as safe as that of hypothermic surgery, and associated with a reduced risk of allogeneic blood transfusion”. 109 This systemic review supports earlier trials in such field where normothermic CPB and warm cardioplegia were concomitantly used during adult cardiac surgery. 110–112
On the other hand, arguments for hypothermic CPB techniques are still favored due to its effectiveness in reducing O2 demand and in increasing ischemic tolerance: these arguments have been established a long time ago. 62,113 Moreover, other argued that the reduction in metabolic rate associated with hypothermia would also allow for the use of reduced CPB flows. 51 Finally, as hypothermia requires haemodilution as hypothermia with whole blood prime results in hypertension during CPB, the introduction of hypothermia is of crucial importance in decreasing strain on blood bank resources. 114
Total aortic arch replacement (TARCH) is generally performed with hypothermic circulatory arrest (HCA) at 15-22°C plus selective cerebral perfusion (SCP) in an attempt to minimize neuropsychological morbidities associated with this type of surgery. However, the cooling and rewarming phases of HCA are time-consuming, and the complications due to prolonged cardiopulmonary bypass (CPB) remain a serious problem. Moreover, there is great controversy regarding optimal perfusion temperature and flow for SCP and the safe time limit for HCA.
In a recent trial, the safety and efficacy of TARCH with deep HCA (at the lowest rectal temperatures of 20-25°C) was compared with TARCH with tepid HCA (32 °C), retrospectively. Twenty-seven patients (group C) who underwent TARCH with deep hypothermia at the lowest rectal temperatures of 20-25°C were compared with 23 patients (group W), who underwent TARCH with 32°C tepid hypothermia. Circulatory arrest time, cardiopulmonary bypass time, operating time, amount of blood transfused and postoperative neurological complications were significantly reduced in group W compared with group C. 115
Temperature management during off pump surgeries:
In OPCAB surgery, a patient's temperature is influenced by the same environmental sources of heat loss that many other non-CPB surgical patients encounter. Additionally, because of an open thorax and extremities exposed for vascular conduit harvest, maintaining normothermia can be difficult.
Although the optimal temperature during OPCAB is not clearly known, efforts to maintain normothermia are generally instituted and normothermia has been considered a goal, particularly to aid in the timely extubation of these patients. These efforts include maintaining an elevated ambient operating room temperature, warming ventilated gases and intravenous fluids, water blankets, and forcing warm convective air over non exposed portions of the body.
Potential benefits of maintaining normothermia had been thoroughly discussed and can be summarized as: maintenance of normothermia, rather than hypothermia, may facilitate early tracheal extubation. Hypothermia alters the distribution and decreases the metabolism of most drugs, including anesthetic drugs and muscle relaxants, thus prolonging recovery. Postoperative shivering increases metabolic rate and potentially lead to myocardial ischemia; coagulopathies, increased incidence of surgical wound infection, and perioperative cardiac morbidity are other potential risk factors. 116
Normothermia is proved to be associated with better cardiac and vascular conditions, a lower cardiac injury rate, and a lower inflammatory response. The close correlation between the increased interleukin-6 and troponin-I levels indicates a potential deleterious effect of lowered temperature on the patient's outcome. 117 During off-pump coronary artery bypass grafting, hypothermia increases vasoconstriction, myocardial after load, coagulopathy and postoperative bleeding. 118
Woo and colleagues 118 demonstrated that during off-pump coronary artery bypass grafting, hypothermia increases vasoconstriction, myocardial after load, coagulopathy and postoperative bleeding. On the other hand, a prospective randomized study to evaluate the effect of fluid warming using Hotline™ system during off-pump coronary artery bypass (OPCAB) surgery demonstrated no significant differences between the 2 thermal management groups in hemodynamic parameters, serum catecholamine concentrations, duration of intensive care unit stay, or duration of ward stay though warming IV fluids with Hotline™ system was capable in preventing hypothermia. 119
Again, it is obvious that the optimal temperature during OPCAB is not known. Efforts to maintain normothermia are generally appreciated, however no insights regarding whether temperature management during such procedure can positively influence patient outcome.
Active thermal manipulations
It is well known that initial 0.5–1.5°C reduction in core temperature is difficult to prevent because it results from redistribution of heat from the central thermal compartment to cooler peripheral tissues. 120 Even the most effective clinical warmers do not prevent hypothermia during the first hour of anesthesia. 120,121 Although redistribution cannot effectively be treated, it can be prevented. Redistribution results when anesthetic induced vasodilatation allows heat to flow peripherally down the normal temperature gradient. Skin surface warming before induction of anesthesia does not significantly alter core temperature (which remains well regulated), but it does increase body heat content. When peripheral tissue temperature is sufficiently increased, little redistribution hypothermia occurs. 122,123
Airway heating and humidification:
Simple thermodynamic calculations indicate that less than 10% of metabolic heat production is lost via the respiratory tract. Because little heat is lost via respiration, even active airway heating and humidification minimally influence core temperature. 124
Consequently, airway heating and humidification are even less effective than usual in patients in most need of effective warming. Airway heating and humidification are more effective in infants and children than in adults, 125 but cutaneous warming also is more effective in these patients and transfers more than ten times as much heat. Hygroscopic condenser humidifiers and heat-and-moisture exchanging filters (“artificial noses”) retain substantial amounts of moisture and heat within the respiratory system. In terms of preventing heat loss, these passive devices are about half as good as active systems, however their costs are much affordable compared to other active systems.
Warm intravenous fluids:
It is not possible to warm patients by administering heated fluids, because the fluids cannot (much) exceed body temperature. On the other hand, heat loss resulting from cold IV fluids becomes significant when large amounts of crystalloid solution or blood are administered. One unit of refrigerated blood or one litre of crystalloid solution administered at room temperature decreases mean body temperature more than 0.25°C. Fluid warmers minimize these losses and should be used when large amounts of IV fluid or blood are administered. Most warmers allow fluid to warm in the tubing between the heater and the patient.
Cutaneous warming:
Operating room temperature is the most critical factor influencing heat loss, because it determines the rate at which metabolic heat is lost by radiation and convection from the skin and by evaporation from within surgical incisions. Consequently, increasing room temperature is one way to minimize heat loss. However, room temperatures exceeding 23°C are generally required to maintain normothermia in patients undergoing all but the smallest procedures 126 as most operating room personnel find such temperatures uncomfortably warm. Infants may require ambient temperatures exceeding 26°C to maintain normothermia. Such temperatures are sufficiently high to impair performance of operating room personnel.
The easiest method of decreasing cutaneous heat loss is to apply passive insulation to the skin surface. Insulators readily available in most operating rooms include cotton blankets, surgical drapes, plastic sheeting, and reflective composites (space blankets). A single layer of insulators reduces heat loss by approximately 30 percent; moreover, there are no clinically important differences among the insulation types. 127 The reduction in heat loss from all commonly used passive insulators is similar because the layer of still air trapping beneath the covering provides most of the insulation. Consequently, adding additional layers of insulation further reduces heat loss only slightly. 128 These data indicate that simply adding additional layers of passive insulation or warming the insulation before application usually is insufficient in patients who become hypothermic while covered with a single layer of insulation.
Cutaneous heat loss is roughly proportional to surface area throughout the body. 129 Consequently, the amount of skin covered is more important than which surfaces are insulated. Passive insulation alone rarely is sufficient to maintain normothermia in patients undergoing large operations. Active warming is required in those cases. Because about 90% of metabolic heat is lost via the skin surface, only cutaneous warming transfers sufficient heat to prevent hypothermia. Consequently, for intraoperative use, circulating-water and forced-air devices are the two major systems requiring consideration.
Studies consistently report that circulating-water mattresses are nearly ineffective. 130 Circulating water is more effective, and safer, when placed over patients rather than under them, and in that position, it can almost completely eliminate metabolic heat loss. 131
The forced air warming device is the most effective method to maintain temperature during most surgical procedures. 120,132,133 These devices are able to maintain normothermia even in patients undergoing large surgical procedures, and if employed in the intraoperative period, they increase central temperature by almost 0.75°C/hour. 120
Malignant hyperthermia in cardiac surgery
The underlying mechanism for malignant hyperthermia (MH) is uncontrolled release of calcium from the sarcoplasmic reticulum triggered by volatile anesthetics and depolarizing muscle relaxants. 134 This exaggerated intracellular calcium release in MH-susceptible skeletal muscle leads to large increases in aerobic and anaerobic metabolism as the muscle cells attempt to re-establish homeostasis by sequestering unbound calcium. 135 However, in MH-susceptible muscle, the rise in calcium caused by the triggering agents overwhelms the cellular capacity to re-establish homeostasis. The pathologically enhanced intracellular calcium rise eventually reaches the threshold levels for myofibrillar contraction and muscular rigidity begins. This leads to increased oxygen consumption and increased carbon dioxide production. Heat is generated with rising lactic acid levels. Rhabdomyolysis ensues with leakage of muscle cell contents into the circulation. 134–136
In case an MH episode is suspected, all triggering agents should be discontinued immediately and anesthesia should be changed to total intravenous anesthesia. 137 Help should be called as early as possible. Hyperventilation with a high fresh gas flow (>10 l/min) should be started and treatment with a bolus of dantrolene at 2.5 mg/kg initiated. 138 The initial bolus of dantrolene (2.5 mg/kg) should be repeated until clinical signs and acidosis subside. 139 Cooling measures need to be started (e.g., placing ice packs on the groin, axillae and neck) and maintained until the temperature of the patient is below 38.5°C. 135 Urine output of at least 2 ml/kg/h should be targeted. 136
There are only a few reports of surgery with CPB and MH. Those reports suggest the use of non-triggering agents and careful management of temperature changes in the intra- and postoperative period, specifically during rewarming. 140,141 In patients with a presumptive history of MH undergoing cardiac surgery, normothermic CPB and off-pump surgery are alternatives that should be considered individually.
Conclusion:
Temperature is a physiologic variable that can be manipulated to suit the requirements of a particular management strategy according to patients' preoperative risk factors. Because of its profound physiologic and pathophysiologic implications, temperature is a crucial homeostatic variable, particularly in the setting of cardiac surgery during which significant changes in temperature can occur.
A debate has emerged in recently published studies about the optimum cardiopulmonary bypass temperature for good outcome (normothermic vs. hypothermic). The ideal temperature for CPB is probably an indeterminate value that varies with the physiological goals. The choice of CPB temperature will always be a compromise between competing goals.
To date, there has been a lack of evidence regarding the optimal temperature management strategy during cardiopulmonary bypass. Thus, temperature management strategies during CPB rely primarily on personal or institutional preference, rather than a solid scientific basis.
References
- [1].Guyton ACHJ. Textbook of Medical Physiology. 11 ed. Philadelphia: Elsevier Inc; 2006. [Google Scholar]
- [2].Sessler DI. Mild perioperative hypothermia. N Engl J Med. 1997;336:1730–1737. doi: 10.1056/NEJM199706123362407. [DOI] [PubMed] [Google Scholar]
- [3]. DI S. Temperature monitoring RD M, ed. Miller's Anesthesia 6th ed. Philadelphia: Elsevier, Churchill Livingstone; 2005. 1571 1597 [Google Scholar]
- [4].De Witte J, Sessler DI. Perioperative shivering: physiology and pharmacology. Anesthesiology. 2002;96:467–484. doi: 10.1097/00000542-200202000-00036. [DOI] [PubMed] [Google Scholar]
- [5].Siegel MN, Gravenstein N. Passive warming of airway gases (artificial nose) improves accuracy of esophageal temperature monitoring. J Clin Monit. 1990;6:89–92. doi: 10.1007/BF02828283. [DOI] [PubMed] [Google Scholar]
- [6].Bissonnette B, Sessler DI, LaFlamme P. Intraoperative temperature monitoring sites in infants and children and the effect of inspired gas warming on esophageal temperature. Anesth Analg. 1989;69:192–196. [PubMed] [Google Scholar]
- [7].Erickson RS. The continuing question of how best to measure body temperature. Crit Care Med. 1999;27:2307–2310. doi: 10.1097/00003246-199910000-00051. [DOI] [PubMed] [Google Scholar]
- [8].Whitby JD, Dunkin LJ. Cerebral, oesophageal and nasopharyngeal temperatures. Br J Anaesth. 1971;43:673–676. doi: 10.1093/bja/43.7.673. [DOI] [PubMed] [Google Scholar]
- [9].Webb GE. Comparison of esophageal and tympanic temperature monitoring during cardiopulmonary bypass. Anesth Analg. 1973;52:729–733. [PubMed] [Google Scholar]
- [10].Wallace CT, Marks WE, Jr, Adkins WY, Mahaffey JE. Perforation of the tympanic membrane, a complication of tympanic thermometry during anesthesia. Anesthesiology. 1974;41:290–291. doi: 10.1097/00000542-197409000-00015. [DOI] [PubMed] [Google Scholar]
- [11].Hooper VD, Andrews JO. Accuracy of noninvasive core temperature measurement in acutely ill adults: the state of the science. Biol Res Nurs. 2006;8:24–34. doi: 10.1177/1099800406289151. [DOI] [PubMed] [Google Scholar]
- [12].Johansson BW. The hibernator heart—nature's model of resistance to ventricular fibrillation. Cardiovasc Res. 1996;31:826–832. doi: 10.1016/0008-6363(95)00192-1. [DOI] [PubMed] [Google Scholar]
- [13].Huh PW, Belayev L, Zhao W, Koch S, Busto R, Ginsberg MD. Comparative neuroprotective efficacy of prolonged moderate intraischemic and postischemic hypothermia in focal cerebral ischemia. J Neurosurg. 2000;92:91–99. doi: 10.3171/jns.2000.92.1.0091. [DOI] [PubMed] [Google Scholar]
- [14].Rosomoff HL, Safar P. Management of the comatose patient. Clin Anesth. 1965;1:244–258. [PubMed] [Google Scholar]
- [15].Storm C, Schefold JC, Nibbe L, Martens F, Krueger A, Oppert M, Joerres A, Hasper D. Therapeutic hypothermia after cardiac arrest—the implementation of the ILCOR guidelines in clinical routine is possible! Crit Care. 2006;10:425. doi: 10.1186/cc5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Safar P. Community-Wide Cardiopulmonary Resuscitation. J Iowa Med Soc. 1964;54:629–635. [PubMed] [Google Scholar]
- [17].Bohn DJ, Biggar WD, Smith CR, Conn AW, Barker GA. Influence of hypothermia, barbiturate therapy, and intracranial pressure monitoring on morbidity and mortality after near-drowning. Crit Care Med. 1986;14:529–534. doi: 10.1097/00003246-198606000-00002. [DOI] [PubMed] [Google Scholar]
- [18].Bellamy R, Safar P, Tisherman SA, Basford R, Bruttig SP, Capone A, Dubick MA, Ernster L, Hattler BG, Jr, Hochachka P, Klain M, Kochanek PM, Kofke WA, Lancaster JR, McGowan FX, Jr, Oeltgen PR, Severinghaus JW, Taylor MJ, Zar H. Suspended animation for delayed resuscitation. Crit Care Med. 1996;24:S24–S47. [PubMed] [Google Scholar]
- [19].Liu L, Yenari MA. Therapeutic hypothermia: neuroprotective mechanisms. Front Biosci. 2007;12:816–825. doi: 10.2741/2104. [DOI] [PubMed] [Google Scholar]
- [20].Slikker W, 3rd, Desai VG, Duhart H, Feuers R, Imam SZ. Hypothermia enhances bcl-2 expression and protects against oxidative stress-induced cell death in Chinese hamster ovary cells. Free Radic Biol Med. 2001;31:405–411. doi: 10.1016/s0891-5849(01)00593-7. [DOI] [PubMed] [Google Scholar]
- [21].Dae MW, Gao DW, Sessler DI, Chair K, Stillson CA. Effect of endovascular cooling on myocardial temperature, infarct size, and cardiac output in human-sized pigs. Am J Physiol Heart Circ Physiol. 2002;282:H1584–H1591. doi: 10.1152/ajpheart.00980.2001. [DOI] [PubMed] [Google Scholar]
- [22].MacLellan CL, Clark DL, Silasi G, Colbourne F. Use of prolonged hypothermia to treat ischemic and hemorrhagic stroke. J Neurotrauma. 2009;26:313–323. doi: 10.1089/neu.2008.0580. [DOI] [PubMed] [Google Scholar]
- [23].Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk RG. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat Cell Biol. 1999;1:190–192. doi: 10.1038/11109. [DOI] [PubMed] [Google Scholar]
- [24].Sakoh M, Gjedde A. Neuroprotection in hypothermia linked to redistribution of oxygen in brain. Am J Physiol Heart Circ Physiol. 2003;285:H17–H25. doi: 10.1152/ajpheart.01112.2002. [DOI] [PubMed] [Google Scholar]
- [25].Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke. 1989;20:904–910. doi: 10.1161/01.str.20.7.904. [DOI] [PubMed] [Google Scholar]
- [26].Baker AJ, Zornow MH, Grafe MR, Scheller MS, Skilling SR, Smullin DH, Larson AA. Hypothermia prevents ischemia-induced increases in hippocampal glycine concentrations in rabbits. Stroke. 1991;22:666–673. doi: 10.1161/01.str.22.5.666. [DOI] [PubMed] [Google Scholar]
- [27].Huang R, Shuaib A, Hertz L. Glutamate uptake and glutamate content in primary cultures of mouse astrocytes during anoxia, substrate deprivation and simulated ischemia under normothermic and hypothermic conditions. Brain Res. 1993;618:346–351. doi: 10.1016/0006-8993(93)91289-5. [DOI] [PubMed] [Google Scholar]
- [28].Inamasu J, Suga S, Sato S, Horiguchi T, Akaji K, Mayanagi K, Kawase T. Postischemic hypothermia attenuates apoptotic cell death in transient focal ischemia in rats. Acta Neurochir Suppl. 2000;76:525–527. doi: 10.1007/978-3-7091-6346-7_110. [DOI] [PubMed] [Google Scholar]
- [29].Wang GJ, Deng HY, Maier CM, Sun GH, Yenari MA. Mild hypothermia reduces ICAM-1 expression, neutrophil infiltration and microglia/monocyte accumulation following experimental stroke. Neuroscience. 2002;114:1081–1090. doi: 10.1016/s0306-4522(02)00350-0. [DOI] [PubMed] [Google Scholar]
- [30].Han HS, Karabiyikoglu M, Kelly S, Sobel RA, Yenari MA. Mild hypothermia inhibits nuclear factor-kappaB translocation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:589–598. doi: 10.1097/01.WCB.0000059566.39780.8D. [DOI] [PubMed] [Google Scholar]
- [31].Yenari MA, Han HS. Influence of hypothermia on post-ischemic inflammation: role of nuclear factor kappa B (NFkappaB) Neurochem Int. 2006;49:164–169. doi: 10.1016/j.neuint.2006.03.016. [DOI] [PubMed] [Google Scholar]
- [32].Webster CM, Kelly S, Koike MA, Chock VY, Giffard RG, Yenari MA. Inflammation and NFkappaB activation is decreased by hypothermia following global cerebral ischemia. Neurobiol Dis. 2009;33:301–312. doi: 10.1016/j.nbd.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ohmura A, Nakajima W, Ishida A, Yasuoka N, Kawamura M, Miura S, Takada G. Prolonged hypothermia protects neonatal rat brain against hypoxic-ischemia by reducing both apoptosis and necrosis. Brain Dev. 2005;27:517–526. doi: 10.1016/j.braindev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- [34].Sahuquillo J, Vilalta A. Cooling the injured brain: how does moderate hypothermia influence the pathophysiology of traumatic brain injury. Curr Pharm Des. 2007;13:2310–2322. doi: 10.2174/138161207781368756. [DOI] [PubMed] [Google Scholar]
- [35].Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- [36].Prakasa Babu P, Yoshida Y, Su M, Segura M, Kawamura S, Yasui N. Immunohistochemical expression of Bcl-2, Bax and cytochrome c following focal cerebral ischemia and effect of hypothermia in rat. Neurosci Lett. 2000;291:196–200. doi: 10.1016/s0304-3940(00)01404-x. [DOI] [PubMed] [Google Scholar]
- [37].Hu WW, Du Y, Li C, Song YJ, Zhang GY. Neuroprotection of hypothermia against neuronal death in rat hippocampus through inhibiting the increased assembly of GluR6-PSD95-MLK3 signaling module induced by cerebral ischemia/reperfusion. Hippocampus. 2008;18:386–397. doi: 10.1002/hipo.20402. [DOI] [PubMed] [Google Scholar]
- [38].Tang XN, Liu L, Yenari MA. Combination therapy with hypothermia for treatment of cerebral ischemia. J Neurotrauma. 2009;26:325–331. doi: 10.1089/neu.2008.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tokutomi T, Miyagi T, Takeuchi Y, Karukaya T, Katsuki H, Shigemori M. Effect of 35 degrees C hypothermia on intracranial pressure and clinical outcome in patients with severe traumatic brain injury. J Trauma. 2009;66:166–173. doi: 10.1097/TA.0b013e318157dbec. [DOI] [PubMed] [Google Scholar]
- [40].Maier CM, Sun GH, Kunis D, Yenari MA, Steinberg GK. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. J Neurosurg. 2001;94:90–96. doi: 10.3171/jns.2001.94.1.0090. [DOI] [PubMed] [Google Scholar]
- [41].Colbourne F, Li H, Buchan AM. Indefatigable CA1 sector neuroprotection with mild hypothermia induced 6 hours after severe forebrain ischemia in rats. J Cereb Blood Flow Metab. 1999;19:742–749. doi: 10.1097/00004647-199907000-00003. [DOI] [PubMed] [Google Scholar]
- [42].Colbourne F, Corbett D, Zhao Z, Yang J, Buchan AM. Prolonged but delayed postischemic hypothermia: a long-term outcome study in the rat middle cerebral artery occlusion model. J Cereb Blood Flow Metab. 2000;20:1702–1708. doi: 10.1097/00004647-200012000-00009. [DOI] [PubMed] [Google Scholar]
- [43].Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- [44].Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- [45].Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database Syst Rev. 2009:CD001048. doi: 10.1002/14651858.CD001048.pub3. [DOI] [PubMed] [Google Scholar]
- [46].Alderson P, Gadkary C, Signorini DF. Therapeutic hypothermia for head injury. Cochrane Database Syst Rev. 2004:CD001048. doi: 10.1002/14651858.CD001048.pub2. [DOI] [PubMed] [Google Scholar]
- [47].Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, Wisniewski SR, DeKosky ST. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336:540–546. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- [48].Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P, TOBY Study Group Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- [49].Krieger DW, Yenari MA. Therapeutic hypothermia for acute ischemic stroke: what do laboratory studies teach us? Stroke. 2004;35:1482–1489. doi: 10.1161/01.STR.0000126118.44249.5c. [DOI] [PubMed] [Google Scholar]
- [50].Strbian D, Durukan A, Tatlisumak T. Rodent models of hemorrhagic stroke. Curr Pharm Des. 2008;14:352–358. doi: 10.2174/138161208783497723. [DOI] [PubMed] [Google Scholar]
- [51].Sealy WC, Brown IW, Jr, Young WG., Jr A report on the use of both extracorporeal circulation and hypothermia for open heart surgery. Ann Surg. 1958;147:603–613. doi: 10.1097/00000658-195805000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Michenfelder JD, Milde JH. The relationship among canine brain temperature, metabolism, and function during hypothermia. Anesthesiology. 1991;75:130–136. doi: 10.1097/00000542-199107000-00021. [DOI] [PubMed] [Google Scholar]
- [53].Hicks SD, DeFranco DB, Callaway CW. Hypothermia during reperfusion after asphyxial cardiac arrest improves functional recovery and selectively alters stress-induced protein expression. J Cereb Blood Flow Metab. 2000;20:520–530. doi: 10.1097/00004647-200003000-00011. [DOI] [PubMed] [Google Scholar]
- [54].Yager JY, Asselin J. Effect of mild hypothermia on cerebral energy metabolism during the evolution of hypoxic-ischemic brain damage in the immature rat. Stroke. 1996;27:919–925. doi: 10.1161/01.str.27.5.919. discussion 26. [DOI] [PubMed] [Google Scholar]
- [55].Safar P. Mild hypothermia in resuscitation: a historical perspective. Ann Emerg Med. 2003;41:887–888. doi: 10.1067/mem.2003.215. author reply 8. [DOI] [PubMed] [Google Scholar]
- [56].Safar PJ, Kochanek PM. Therapeutic hypothermia after cardiac arrest. N Engl J Med. 2002;346:612–613. doi: 10.1056/NEJM200202213460811. [DOI] [PubMed] [Google Scholar]
- [57].Govier AV, Reves JG, McKay RD, Karp RB, Zorn GL, Morawetz RB, Smith LR, Adams M, Freeman AM. Factors and their influence on regional cerebral blood flow during nonpulsatile cardiopulmonary bypass. Ann Thorac Surg. 1984;38:592–600. doi: 10.1016/s0003-4975(10)62316-8. [DOI] [PubMed] [Google Scholar]
- [58].Cook DJ, Oliver WC, Jr, Orszulak TA, Daly RC, Bryce RD. Cardiopulmonary bypass temperature, hematocrit, and cerebral oxygen delivery in humans. Ann Thorac Surg. 1995;60:1671–1677. doi: 10.1016/0003-4975(95)00648-6. [DOI] [PubMed] [Google Scholar]
- [59].Cook DJ, Oliver WC, Jr, Orszulak TA, Daly RC. A prospective, randomized comparison of cerebral venous oxygen saturation during normothermic and hypothermic cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1994;107:1020–1028. discussion 8–9. [PubMed] [Google Scholar]
- [60].Klementavicius R, Nemoto EM, Yonas H. The Q10 ratio for basal cerebral metabolic rate for oxygen in rats. J Neurosurg. 1996;85:482–487. doi: 10.3171/jns.1996.85.3.0482. [DOI] [PubMed] [Google Scholar]
- [61].Grocott HP, Yoshitani K. Neuroprotection during cardiac surgery. J Anesth. 2007;21:367–377. doi: 10.1007/s00540-007-0514-1. [DOI] [PubMed] [Google Scholar]
- [62].Bigelow WG, Lindsay WK, Greenwood WF. Hypothermia; its possible role in cardiac surgery: an investigation of factors governing survival in dogs at low body temperatures. Ann Surg. 1950;132:849–866. doi: 10.1097/00000658-195011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hale SL, Dae MW, Kloner RA. Hypothermia during reperfusion limits ‘no-reflow’ injury in a rabbit model of acute myocardial infarction. Cardiovasc Res. 2003;59:715–722. doi: 10.1016/s0008-6363(03)00456-5. [DOI] [PubMed] [Google Scholar]
- [64].Hale SL, Kloner RA. Myocardial temperature in acute myocardial infarction: protection with mild regional hypothermia. Am J Physiol. 1997;273:H220–H227. doi: 10.1152/ajpheart.1997.273.1.H220. [DOI] [PubMed] [Google Scholar]
- [65].Kanemoto S, Matsubara M, Noma M, Leshnower BG, Parish LM, Jackson BM, Hinmon R, Hamamoto H, Gorman JH, 3rd, Gorman RC. Mild hypothermia to limit myocardial ischemia-reperfusion injury: importance of timing. Ann Thorac Surg. 2009;87:157–163. doi: 10.1016/j.athoracsur.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ning XH, Chi EY, Buroker NE, Chen SH, Xu CS, Tien YT, Hyyti OM, Ge M, Portman MA. Moderate hypothermia (30 degrees C) maintains myocardial integrity and modifies response of cell survival proteins after reperfusion. Am J Physiol Heart Circ Physiol. 2007;293:H2119–H2128. doi: 10.1152/ajpheart.00123.2007. [DOI] [PubMed] [Google Scholar]
- [67].Ruiz-Meana M, Garcia-Dorado D, Pina P, Inserte J, Agullo L, Soler-Soler J. Cariporide preserves mitochondrial proton gradient and delays ATP depletion in cardiomyocytes during ischemic conditions. Am J Physiol Heart Circ Physiol. 2003;285:H999–H1006. doi: 10.1152/ajpheart.00035.2003. [DOI] [PubMed] [Google Scholar]
- [68].Ning XH, Xu CS, Portman MA. Mitochondrial protein and HSP70 signaling after ischemia in hypothermic-adapted hearts augmented with glucose. Am J Physiol. 1999;277:R11–R17. doi: 10.1152/ajpregu.1999.277.1.R11. [DOI] [PubMed] [Google Scholar]
- [69].Hamamoto H, Leshnower BG, Parish LM, Sakamoto H, Kanemoto S, Hinmon R, Miyamoto S, Gorman JH, 3rd, Gorman RC. Regional heterogeneity of myocardial reperfusion injury: effect of mild hypothermia. Ann Thorac Surg. 2009;87:164–171. doi: 10.1016/j.athoracsur.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lo TP, Jr, Cho KS, Garg MS, Lynch MP, Marcillo AE, Koivisto DL, Stagg M, Abril RM, Patel S, Dietrich WD, Pearse DD. Systemic hypothermia improves histological and functional outcome after cervical spinal cord contusion in rats. J Comp Neurol. 2009;514:433–448. doi: 10.1002/cne.22014. [DOI] [PubMed] [Google Scholar]
- [71].Vaquero J, Blei AT. Mild hypothermia for acute liver failure: a review of mechanisms of action. J Clin Gastroenterol. 2005;39:S147–S157. doi: 10.1097/01.mcg.0000155515.94843.55. [DOI] [PubMed] [Google Scholar]
- [72].Meyer DM, Horton JW. Effect of moderate hypothermia in the treatment of canine hemorrhagic shock. Ann Surg. 1988;207:462–469. doi: 10.1097/00000658-198804000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kim SH, Stezoski SW, Safar P, Tisherman SA. Hypothermia, but not 100% oxygen breathing, prolongs survival time during lethal uncontrolled hemorrhagic shock in rats. J Trauma. 1998;44:485–491. doi: 10.1097/00005373-199803000-00010. [DOI] [PubMed] [Google Scholar]
- [74].Takasu A, Norio H, Sakamoto T, Okada Y. Mild hypothermia prolongs the survival time during uncontrolled hemorrhagic shock in rats. Resuscitation. 2002;54:303–309. doi: 10.1016/s0300-9572(02)00148-x. [DOI] [PubMed] [Google Scholar]
- [75].Wu X, Kochanek PM, Cochran K, Nozari A, Henchir J, Stezoski SW, Wagner R, Wisniewski S, Tisherman SA. Mild hypothermia improves survival after prolonged, traumatic hemorrhagic shock in pigs. J Trauma. 2005;59:291–299. doi: 10.1097/01.ta.0000179445.76729.2c. discussion 9–301. [DOI] [PubMed] [Google Scholar]
- [76].Alam HB, Bowyer MW, Koustova E, Gushchin V, Anderson D, Stanton K, Kreishman P, Cryer CM, Hancock T, Rhee P. Learning and memory is preserved after induced asanguineous hyperkalemic hypothermic arrest in a swine model of traumatic exsanguination. Surgery. 2002;132:278–288. doi: 10.1067/msy.2002.125787. [DOI] [PubMed] [Google Scholar]
- [77].Frank SM, Beattie C, Christopherson R, Norris EJ, Perler BA, Williams GM, Gottlieb SO. Unintentional hypothermia is associated with postoperative myocardial ischemia. The Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology. 1993;78:468–476. doi: 10.1097/00000542-199303000-00010. [DOI] [PubMed] [Google Scholar]
- [78].Frank SM, el-Gamal N, Raja SN, Wu PK. alpha-Adrenoceptor mechanisms of thermoregulation during cold challenge in humans. Clin Sci (Lond) 1996;91:627–631. doi: 10.1042/cs0910627. [DOI] [PubMed] [Google Scholar]
- [79].Frank SM, Higgins MS, Breslow MJ, Fleisher LA, Gorman RB, Sitzmann JV, Raff H, Beattie C. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia. A randomized clinical trial. Anesthesiology. 1995;82:83–93. doi: 10.1097/00000542-199501000-00012. [DOI] [PubMed] [Google Scholar]
- [80].Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–292. doi: 10.1016/s0140-6736(96)90466-3. [DOI] [PubMed] [Google Scholar]
- [81].Schmied H, Schiferer A, Sessler DI, Meznik C. The effects of red-cell scavenging, hemodilution, and active warming on allogenic blood requirements in patients undergoing hip or knee arthroplasty. Anesth Analg. 1998;86:387–391. doi: 10.1097/00000539-199802000-00032. [DOI] [PubMed] [Google Scholar]
- [82].Johansson T, Lisander B, Ivarsson I. Mild hypothermia does not increase blood loss during total hip arthroplasty. Acta Anaesthesiol Scand. 1999;43:1005–1010. doi: 10.1034/j.1399-6576.1999.431006.x. [DOI] [PubMed] [Google Scholar]
- [83].Valeri CR, Feingold H, Cassidy G, Ragno G, Khuri S, Altschule MD. Hypothermia-induced reversible platelet dysfunction. Ann Surg. 1987;205:175–181. doi: 10.1097/00000658-198702000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Valeri CR, Khabbaz K, Khuri SF, Marquardt C, Ragno G, Feingold H, Gray AD, Axford T. Effect of skin temperature on platelet function in patients undergoing extracorporeal bypass. J Thorac Cardiovasc Surg. 1992;104:108–116. [PubMed] [Google Scholar]
- [85].Khuri SF, Wolfe JA, Josa M, Axford TC, Szymanski I, Assousa S, Ragno G, Patel M, Silverman A, Park M. Hematologic changes during and after cardiopulmonary bypass and their relationship to the bleeding time and nonsurgical blood loss. J Thorac Cardiovasc Surg. 1992;104:94–107. [PubMed] [Google Scholar]
- [86].Bunker JP, Goldstein R. Coagulation during hypothermia in man. Proc Soc Exp Biol Med. 1958;97:199–202. doi: 10.3181/00379727-97-23689. [DOI] [PubMed] [Google Scholar]
- [87].Rohrer MJ, Natale AM. Effect of hypothermia on the coagulation cascade. Crit Care Med. 1992;20:1402–1405. doi: 10.1097/00003246-199210000-00007. [DOI] [PubMed] [Google Scholar]
- [88].Kettner SC, Kozek SA, Groetzner JP, Gonano C, Schellongowski A, Kucera M, Zimpfer M. Effects of hypothermia on thrombelastography in patients undergoing cardiopulmonary bypass. Br J Anaesth. 1998;80:313–317. doi: 10.1093/bja/80.3.313. [DOI] [PubMed] [Google Scholar]
- [89].Ramaker AJ, Meyer P, van der Meer J, Struys MM, Lisman T, van Oeveren W, Hendriks HG. Effects of acidosis, alkalosis, hyperthermia and hypothermia on haemostasis: results of point-of-care testing with the thromboelastography analyser. Blood Coagul Fibrinolysis. 2009;20:436–439. doi: 10.1097/MBC.0b013e32832dc327. [DOI] [PubMed] [Google Scholar]
- [90].Rundgren M, Engstrom M. A thromboelastometric evaluation of the effects of hypothermia on the coagulation system. Anesth Analg. 2008;107:1465–1468. doi: 10.1213/ane.0b013e31817ee955. [DOI] [PubMed] [Google Scholar]
- [91].Sessler DI, Olofsson CI, Rubinstein EH. The thermoregulatory threshold in humans during nitrous oxide-fentanyl anesthesia. Anesthesiology. 1988;69:357–364. doi: 10.1097/00000542-198809000-00012. [DOI] [PubMed] [Google Scholar]
- [92].Hopf HW, Hunt TK, West JM, Blomquist P, Goodson WH, 3rd, Jensen JA, Jonsson K, Paty PB, Rabkin JM, Upton RA, von Smitten K, Whitney JD. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997–1004. doi: 10.1001/archsurg.1997.01430330063010. discussion 5. [DOI] [PubMed] [Google Scholar]
- [93].Saririan K, Nickerson DA. Enhancement of murine in vitro antibody formation by hyperthermia. Cell Immunol. 1982;74:306–312. doi: 10.1016/0008-8749(82)90031-4. [DOI] [PubMed] [Google Scholar]
- [94].Farkas LG, Bannantyne RM, James JS, Umamaheswaran B. Effect of two different climates on severely burned rats infected with Pseudomonas aeruginosa. Eur Surg Res. 1974;6:295–300. doi: 10.1159/000127732. [DOI] [PubMed] [Google Scholar]
- [95].van Oss CJ, Absolom DR, Moore LL, Park BH, Humbert JR. Effect of temperature on the chemotaxis, phagocytic engulfment, digestion and O2 consumption of human polymorphonuclear leukocytes. J Reticuloendothel Soc. 1980;27:561–565. [PubMed] [Google Scholar]
- [96].Sessler DI, Rubinstein EH, Moayeri A. Physiologic responses to mild perianesthetic hypothermia in humans. Anesthesiology. 1991;75:594–610. doi: 10.1097/00000542-199110000-00009. [DOI] [PubMed] [Google Scholar]
- [97].Kurz A, Sessler DI, Narzt E, Bekar A, Lenhardt R, Huemer G, Lackner F. Postoperative hemodynamic and thermoregulatory consequences of intraoperative core hypothermia. J Clin Anesth. 1995;7:359–366. doi: 10.1016/0952-8180(95)00028-g. [DOI] [PubMed] [Google Scholar]
- [98].Boelhouwer RU, Bruining HA, Ong GL. Correlations of serum potassium fluctuations with body temperature after major surgery. Crit Care Med. 1987;15:310–312. doi: 10.1097/00003246-198704000-00006. [DOI] [PubMed] [Google Scholar]
- [99].Bruining HA, Boelhouwer RU. Acute transient hypokalemia and body temperature. Lancet. 1982;2:1283–1284. doi: 10.1016/s0140-6736(82)90142-8. [DOI] [PubMed] [Google Scholar]
- [100].Freysz M, Timour Q, Mazze RI, Bertrix L, Cohen S, Samii K, Faucon G. Potentiation by mild hypothermia of ventricular conduction disturbances and reentrant arrhythmias induced by bupivacaine in dogs. Anesthesiology. 1989;70:799–804. doi: 10.1097/00000542-198905000-00016. [DOI] [PubMed] [Google Scholar]
- [101].Reynolds PC, Antoine JA, Bettencourt J, Starck TW. Regional hypothermia affects somatosensory evoked potentials. Anesth Analg. 1991;73:653–656. doi: 10.1213/00000539-199111000-00026. [DOI] [PubMed] [Google Scholar]
- [102].Paulus DA, Monroe MC. Cool fingers and pulse oximetry. Anesthesiology. 1989;71:168–169. doi: 10.1097/00000542-198907000-00034. [DOI] [PubMed] [Google Scholar]
- [103].Heier T, Caldwell JE, Sessler DI, Miller RD. Mild intraoperative hypothermia increases duration of action and spontaneous recovery of vecuronium blockade during nitrous oxide-isoflurane anesthesia in humans. Anesthesiology. 1991;74:815–819. doi: 10.1097/00000542-199105000-00003. [DOI] [PubMed] [Google Scholar]
- [104].Heier T, Caldwell JE, Sharma ML, Gruenke LD, Miller RD. Mild intraoperative hypothermia does not change the pharmacodynamics (concentration-effect relationship) of vecuronium in humans. Anesth Analg. 1994;78:973–977. doi: 10.1213/00000539-199405000-00024. [DOI] [PubMed] [Google Scholar]
- [105].Lenhardt R, Marker E, Goll V, Tschernich H, Kurz A, Sessler DI, Narzt E, Lackner F. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997;87:1318–1323. doi: 10.1097/00000542-199712000-00009. [DOI] [PubMed] [Google Scholar]
- [106].Rajek A, Lenhardt R, Sessler DI, Grabenwöger M, Kastner J, Mares P, Jantsch U, Gruber E. Tissue heat content and distribution during and after cardiopulmonary bypass at 17 degrees C. Anesth Analg. 1999;88:1220–1225. doi: 10.1097/00000539-199906000-00006. [DOI] [PubMed] [Google Scholar]
- [107].Rajek A, Lenhardt R, Sessler DI, Kurz A, Laufer G, Christensen R, Matsukawa T, Hiesmayr M. Tissue heat content and distribution during and after cardiopulmonary bypass at 31 degrees C and 27 degrees C. Anesthesiology. 1998;88:1511–1518. doi: 10.1097/00000542-199806000-00015. [DOI] [PubMed] [Google Scholar]
- [108].Barber PA, Hach S, Tippett LJ, Ross L, Merry AF, Milsom P. Cerebral ischemic lesions on diffusion-weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39:1427–1433. doi: 10.1161/STROKEAHA.107.502989. [DOI] [PubMed] [Google Scholar]
- [109].Ho KM, Tan JA. Benefits and risks of maintaining normothermia during cardiopulmonary bypass in adult cardiac surgery: a systematic review. Cardiovasc Ther. 2009;29(4):260–279. doi: 10.1111/j.1755-5922.2009.00114.x. [DOI] [PubMed] [Google Scholar]
- [110].Partington MT, Acar C, Buckberg GD, Julia PL. Studies of retrograde cardioplegia. II. Advantages of antegrade/retrograde cardioplegia to optimize distribution in jeopardized myocardium. J Thorac Cardiovasc Surg. 1989;97:613–622. [PubMed] [Google Scholar]
- [111].Buckberg GD, Brazier JR, Nelson RL, Goldstein SM, McConnell DH, Cooper N. Studies of the effects of hypothermia on regional myocardial blood flow and metabolism during cardiopulmonary bypass. I. The adequately perfused beating, fibrillating, and arrested heart. J Thorac Cardiovasc Surg. 1977;73:87–94. [PubMed] [Google Scholar]
- [112].Lichtenstein SV, Ashe KA, el Dalati H, Cusimano RJ, Panos A, Slutsky AS. Warm heart surgery. J Thorac Cardiovasc Surg. 1991;101:269–274. [PubMed] [Google Scholar]
- [113].Bigelow WG, Lindsay WK, Harrison RC, Gordon RA, Greenwood WF. Oxygen transport and utilization in dogs at low body temperatures. Am J Physiol. 1950;160:125–137. doi: 10.1152/ajplegacy.1949.160.1.125. [DOI] [PubMed] [Google Scholar]
- [114].Moffitt EA, Sessler AD, Molnar GD, McGoon DC. Normothermia versus hypothermia for whole-body perfusion: effects on myocardial and body metabolism. Anesth Analg. 1971;50:505–516. [PubMed] [Google Scholar]
- [115].Watanabe G, Ohtake H, Tomita S, Yamaguchi S, Kimura K, Yashiki N. Tepid hypothermic (32°C) circulatory arrest for total aortic arch replacement: a paradigm shift from profound hypothermic surgery. Interact CardioVasc Thorac Surg. 2011;12:952–955. doi: 10.1510/icvts.2010.250605. [DOI] [PubMed] [Google Scholar]
- [116].Leslie K, Sessler DI. The implications of hypothermia for early tracheal extubation following cardiac surgery. J Cardiothorac Vasc Anesth. 1998;12:30–34. discussion 41–44. [PubMed] [Google Scholar]
- [117].Nesher N, Uretzky G, Insler S, Nataf P, Frolkis I, Pineau E, Cantoni E, Bolotin G, Vardi M, Pevni D, Lev-Ran O, Sharony R, Weinbroum AA. Thermo-wrap technology preserves normothermia better than routine thermal care in patients undergoing off-pump coronary artery bypass and is associated with lower immune response and lesser myocardial damage. J Thorac Cardiovasc Surg. 2005;129:1371–1378. doi: 10.1016/j.jtcvs.2004.08.021. [DOI] [PubMed] [Google Scholar]
- [118].Woo YJ, Atluri P, Grand TJ, Hsu VM, Cheung A. Active thermoregulation improves outcome of off-pump coronary artery bypass. Asian Cardiovasc Thorac Ann. 2005;13:157–160. doi: 10.1177/021849230501300213. [DOI] [PubMed] [Google Scholar]
- [119].Jeong SM, Hahm KD, Jeong YB, Yang HS, Choi IC. Warming of intravenous fluids prevents hypothermia during off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2008;22:67–70. doi: 10.1053/j.jvca.2007.04.003. [DOI] [PubMed] [Google Scholar]
- [120].Hynson JM, Sessler DI. Intraoperative warming therapies: a comparison of three devices. J Clin Anesth. 1992;4:194–199. doi: 10.1016/0952-8180(92)90064-8. [DOI] [PubMed] [Google Scholar]
- [121].Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab. 2007;27:1879–1894. doi: 10.1038/sj.jcbfm.9600540. [DOI] [PubMed] [Google Scholar]
- [122].Hynson JM, Sessler DI, Moayeri A, McGuire J, Schroeder M. The effects of preinduction warming on temperature and blood pressure during propofol/nitrous oxide anesthesia. Anesthesiology. 1993;79:219–228. doi: 10.1097/00000542-199308000-00005. discussion 21A–22A. [DOI] [PubMed] [Google Scholar]
- [123].Just B, Trevien V, Delva E, Lienhart A. Prevention of intraoperative hypothermia by preoperative skin-surface warming. Anesthesiology. 1993;79:214–218. doi: 10.1097/00000542-199308000-00004. [DOI] [PubMed] [Google Scholar]
- [124].Deriaz H, Fiez N, Lienhart A. [Effect of hygrophobic filter or heated humidifier on peroperative hypothermia] Ann Fr Anesth Reanim. 1992;11:145–149. doi: 10.1016/s0750-7658(05)80005-x. [DOI] [PubMed] [Google Scholar]
- [125].Bissonnette B, Sessler DI. Passive or active inspired gas humidification increases thermal steady-state temperatures in anesthetized infants. Anesth Analg. 1989;69:783–787. [PubMed] [Google Scholar]
- [126].Morris RH. Operating room temperature and the anesthetized, paralyzed patient. Arch Surg. 1971;102:95–97. doi: 10.1001/archsurg.1971.01350020005002. [DOI] [PubMed] [Google Scholar]
- [127].Sessler DI, McGuire J, Sessler AM. Perioperative thermal insulation. Anesthesiology. 1991;74:875–879. doi: 10.1097/00000542-199105000-00012. [DOI] [PubMed] [Google Scholar]
- [128].Sessler DI, Schroeder M. Heat loss in humans covered with cotton hospital blankets. Anesth Analg. 1993;77:73–77. doi: 10.1213/00000539-199307000-00014. [DOI] [PubMed] [Google Scholar]
- [129].Sessler DI, Moayeri A, Stoen R, Glosten B, Hynson J, McGuire J. Thermoregulatory vasoconstriction decreases cutaneous heat loss. Anesthesiology. 1990;73:656–660. doi: 10.1097/00000542-199010000-00011. [DOI] [PubMed] [Google Scholar]
- [130].Morris RH, Kumar A. The effect of warming blankets on maintenance of body temperature of the anesthetized, paralyzed adult patient. Anesthesiology. 1972;36:408–411. doi: 10.1097/00000542-197204000-00020. [DOI] [PubMed] [Google Scholar]
- [131].Sessler DI, Moayeri A. Skin-surface warming: heat flux and central temperature. Anesthesiology. 1990;73:218–224. [PubMed] [Google Scholar]
- [132].Borms SF, Engelen SL, Himpe DG, Suy MR, Theunissen WJ. Bair hugger forced-air warming maintains normothermia more effectively than thermo-lite insulation. J Clin Anesth. 1994;6:303–307. doi: 10.1016/0952-8180(94)90077-9. [DOI] [PubMed] [Google Scholar]
- [133].Negishi C, Hasegawa K, Mukai S, Nakagawa F, Ozaki M, Sessler DI. Resistive-heating and forced-air warming are comparably effective. Anesth Analg. 2003;96(6):1683–1687. doi: 10.1213/01.ANE.0000062770.73862.B7. [DOI] [PubMed] [Google Scholar]
- [134].Rosenberg H, Davis M, James D, Pollock N, Stowell K. Malignant hyperthermia. Orphanet J Rare Dis. 2007;2:21. doi: 10.1186/1750-1172-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Tautz TJ, Urwyler A, Antognini JF, Riou B. Case scenario: Increased end-tidal carbon dioxide: a diagnostic dilemma. Anesthesiology. 2010;112:440–446. doi: 10.1097/ALN.0b013e3181ca7c38. [DOI] [PubMed] [Google Scholar]
- [136].Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- [137].Glahn KPE, Ellis FR, Halsall PJ, Müller CR, Snoeck MMJ, Urwyler A, Wappler F. Recognizing and managing a malignant hyperthermia crisis: guidelines from the European Malignant Hyperthermia Group. Br J Anaesth. 2010;105:417–420. doi: 10.1093/bja/aeq243. [DOI] [PubMed] [Google Scholar]
- [138].Inan S, Wei H. The cytoprotective effects of dantrolene: a ryanodine receptor antagonist. Anesth Analg. 2010;111:1400–1410. doi: 10.1213/ANE.0b013e3181f7181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Girard T, Ginz H, Urwyler A. MaligneHyperthermie. Schweiz Med Forum. 2004:1192–1197. [Google Scholar]
- [140].Lichtman A, Oribabor C. Malignant hyperthermia following systemic rewarming after hypothermic cardiopulmonary bypass. Anesth Analg. 2006;102:372–375. doi: 10.1213/01.ane.0000189596.70694.36. [DOI] [PubMed] [Google Scholar]
- [141].Siddik-Sayyid SM, Moussa AR, Baraka AS. Can we prevent malignant hyperthermia after hypothermic cardiopulmonary bypass in a malignant hyperthermia-susceptible patient? Anesth Analg. 2006;102:372–375. doi: 10.1213/01.ane.0000249792.37338.cc. [DOI] [PubMed] [Google Scholar]