Abstract
The renin-angiotensin-aldosterone system (RAAS) regulates the body's hemodynamic equilibrium, circulating volume, and electrolyte balance, and is a key therapeutic target in hypertension, the world's leading cause of premature mortality. Hypertensive disorders are strongly linked with an overactive RAAS, and RAAS inhibitors, like angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), are routinely used to treat high blood pressure (BP). BP reduction is one of the main goals of current European hypertension guidelines. Oral ACE inhibitors, the oldest category of RAAS inhibitor, were commercially released over 30 years ago in the early 1980s, over a decade before the first ARBs became available. The introduction of ACE inhibitors heralded major changes in the way hypertension and cardiovascular disease were treated. Although the decision of the medical community to replace older ACE inhibitors with more modern ARBs in the 1990s was debatable, it did nevertheless allow scientists to learn more about the angiotensin receptors involved in RAAS stimulation. This and much else of value have been discovered since RAAS inhibitors first became available, but some surprising gaps in our knowledge exist. Until recently, the effect of RAAS inhibition on mortality in hypertension was unknown. This question was recently addressed by a meta-analysis of randomized controlled trials in populations who received contemporary antihypertensive medication. The results of this meta-analysis have helped elucidate the long-term consequences of treatment with RAAS inhibitors on mortality in hypertension. This article will consider the differences between RAAS inhibitors in terms of pharmacological and clinical effects and analyze the impact of the main types of RAAS inhibitor, ACE inhibitors and ARBs, on mortality reduction in hypertensive patients with reference to this latest meta-analysis.
Introduction
The renin-angiotensin-aldosterone system (RAAS) regulates the body's hemodynamic equilibrium, circulating volume, and electrolyte balance, and is a key therapeutic target in hypertension, the world's leading cause of premature mortality.1 Hypertensive disorders are strongly linked with an overactive RAAS,2 and RAAS inhibitors, like angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), are routinely used to treat high blood pressure (BP).3 BP reduction is one of the main goals of current European hypertension guidelines.4
Oral ACE inhibitors, the oldest category of RAAS inhibitor, were commercially released over thirty years ago in the early 1980s, over a decade before the first ARBs became available.5 The introduction of ACE inhibitors heralded major changes in the way hypertension and cardiovascular disease were treated. Although the decision of the medical community to replace older ACE inhibitors with more modern ARBs in the 1990s was debatable, it did nevertheless allow scientists to learn more about the angiotensin receptors involved in RAAS stimulation.
This and much else of value have been discovered since RAAS inhibitors first became available, but some surprising gaps in our knowledge exist. Until recently, the effect of RAAS inhibition on mortality in hypertension was unknown. This question was recently addressed by a meta-analysis of randomized controlled trials in populations who received contemporary antihypertensive medication.6 The results of this meta-analysis have helped elucidate the long-term consequences of treatment with RAAS inhibitors on mortality in hypertension.
This article will consider the differences between RAAS inhibitors in terms of pharmacological and clinical effects and analyze the impact of the main types of RAAS inhibitor, ACE inhibitors and ARBs, on mortality reduction in hypertensive patients with reference to this latest meta-analysis.6
Pharmacological evidence for RAAS inhibition
ACE inhibitors and ARBs inhibit the RAAS in distinct ways. ACE inhibitors prevent the enzyme ACE from converting angiotensin I into angiotensin II (Table 1).7,8 Angiotensin II is a vasoconstrictor that causes a host of deleterious effects, including vascular damage at the endothelial and structural levels.9 Angiotensin II is an important cause of heart, brain, and kidney damage, as well as a modulator of aldosterone, a hormone that increases BP by increasing sodium reabsorption, water retention, and blood volume. Pathological outcomes induced by angiotensin II include myocardial infarction (MI), heart failure, stroke, and renal failure.
Table 1 .
Sites of action and effects of renin-angiotensin-aldosterone system inhibitors on the endothelium.
| ACE inhibitor | ARB | |
| Sites of action | ||
| Impairment of renin formation | – | – |
| Impairment of angiotensin I formation | – | – |
| Impairment of angiotensin II formation | Yes | – |
| AT1 receptor blockade | – | Yes |
| AT2 receptor blockade | – | – |
| AT3 receptor blockade | – | – |
| AT4 receptor blockade | – | – |
| Prevention of bradykinin degradation | Yes | – |
| Positive effects on endothelium | ||
| Reduction in endothelial dysfunction | Yes | Yes |
| Reduction in inflammation | Yes | – |
| Reduction in lipid oxidation | Yes | Yes |
| Reduction in cell adhesion | Yes | Yes |
| Reduction in thrombosis | Yes | Partial |
| Reduction in atherosclerosis | Yes | Yes |
| Decrease in apoptosis | Yes | – |
| Preservation of fibrinolytic balance | Yes | Partial |
| Increase in vasodilation | Yes | – |
| Prevention of vasoconstriction | Yes | Yes |
| Negative effects on endothelium | ||
| Angiotensin II escape | Yes | – |
| Aldosterone escape | Yes | Yes |
| Indirect AT receptor stimulation | – | Partial |
Angiotensin II, which is formed from angiotensin I by angiotensin-converting enzyme (ACE), acts on different angiotensin receptors (ATs) to produce a variety of effects on the heart, vasculature, and kidneys. ACE inhibitors block the formation of angiotensin II and block the degradation of bradykinin. Angiotensin receptor blockers (ARBs) block the AT1 receptor.
ACE inhibition impairs angiotensin II production, resulting in a number of positive cardiovascular benefits. Attenuation of angiotensin II reduces levels of proinflammatory markers and prevents atherogenesis. It also inhibits fibrosis and reduces endothelial dysfunction.9 Decreases in the concentrations of plasminogen activator inhibitor-1 and tissue factor, caused by the reduction of angiotensin II levels, inhibit thrombosis.8 For these positive inhibitory effects to occur, it is important that local ACE is inhibited.
The advantages of angiotensin II reduction by ACE inhibition are substantial, but may be compromised in the long term because of “escape” effects related to angiotensin II and aldosterone.10 Disrupted negative feedback mechanisms cause renin and angiotensin I concentrations to rise, eventually leading to angiotensin II escape when non-ACE enzymes, such as chymase, convert angiotensin I to angiotensin II.11 Similarly, aldosterone escape occurs after long-term ACE inhibitor therapy, due to progressive elevation of aldosterone levels. In addition, angiotensin II can be produced by enzymes other than ACE.
Given this scenario, one might expect ACE inhibitors to lose all their efficacy over the long term, but this is not the case, thanks to a complementary mechanism of action related to ACE inhibition. By inhibiting ACE, ACE inhibitors also increase concentrations of the vasodilatory peptide bradykinin, which is broken down into inactive peptides by ACE. Bradykinin causes the release of the vasodilator nitric oxide and other relaxing factors, such as prostaglandins, prostacyclin, and endothelium-derived hyperpolarizing factor.12 Physiologically, bradykinin can be regarded as having opposite effects to those of angiotensin II, in that it reduces BP, protects the heart, and improves arterial function.13 Apoptosis is also inhibited by bradykinin.9 These bradykinin-mediated effects help counter the “escape” effects and maintain the efficacy of ACE inhibition in the long term.
The mode of action of ARBs also limits the deleterious effects of angiotensin II. ARBs prevent the binding of angiotensin II to AT1 receptors (Table 1).7,8 Vasoconstriction, sympathetic stimulation, oxidative stress, release of inflammatory factors, and aldosterone release are all effectively reduced by this selective AT1 receptor blockade. Compared with ACE inhibition, selective AT1 receptor blockade has certain distinct advantages, like the absence of angiotensin II escape and pronounced inhibition of deleterious effects regulated via AT1 receptor stimulation and blockade of all angiotensin II, independently from the site of production. Pure AT1 receptor blockade may, however, be a mixed blessing; angiotensin II formation and concentration increase in response to blockade, and free angiotensin II binds to free angiotensin receptors (AT2, AT3, and AT4). AT2 receptor activation causes plaque to become unstable and thrombuses to form.14 Activation of these receptors also induces hypertrophy, inflammation, and apoptosis, but also positive effects like vasodilation and diminished proliferation. The AT2 receptor is also responsible for regulating aldosterone escape in ARBs.15 Not much is known about the effect of AT3 receptor stimulation, while AT4 receptor stimulation is thought to promote thrombosis.7
In brief, ACE inhibitors prevent the enzyme ACE from converting angiotensin I into angiotensin II and also prevent the breakdown of bradykinin, resulting in beneficial cardiovascular protection. Selective blockade of AT1 receptors by ARBs also prevents a wide range of negative cardiovascular effects, but this selectivity may also be responsible for unintentional clinical effects, both positive and negative. These different modes of RAAS inhibition may explain some of the clinical differences between ACE inhibitors and ARBs.
Clinical evidence for RAAS inhibition
At first view, ACE inhibitors and ARBs may appear clinically similar: the two are used to treat cardiovascular risk factors,16 and they both reduce BP, stroke, and symptoms of heart failure.8 A longer look, however, reveals the existence of substantial clinical differences between the two classes of RAAS inhibitor, in particular with regards to cardiovascular risk reduction.
The relationship between cardiovascular risk reduction and BP reduction is not clear-cut; trials that have compared ACE inhibitors versus ARBs, like ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) and DETAIL (Diabetics Exposed to Telmisartan And enalaprIL), show that large decreases in BP do not automatically decrease the risk of cardiovascular outcomes and mortality.17,18 The results of these two prospective trials indicate there is no difference in outcome between ACE inhibitors and ARBs in patients with high cardiovascular risk (ONTARGET)17 or patients with diabetic nephropathy (DETAIL).18 ARBs, it could be argued, should have reduced cardiovascular risk more, as mean BP was reduced more with ARBs in both trials. Another element that should have favored ARBs was the fact that the ACE inhibitors used in these respective trials, ramipril and enalapril, have shorter durations of action than telmisartan, the ARB used in both trials, and were administered in the morning, which meant patients in the ACE inhibitor arm were theoretically at greater risk of cardiovascular events following early morning surges in BP.
As regards ARB trials versus placebo, no reductions in cardiovascular mortality have been observed despite mean systolic BP reductions of 3.2 mm Hg in SCOPE (Study on COgnition and Prognosis in the Elderly), 4 mm Hg in TRANSCEND (Telmisartan Randomized AssessmeNt Study in aCE iNtolerant subjects with cardiovascular Disease), and 3.8 mm Hg in PRoFESS (PReventiOn regimen For Effectively avoiding Second Strokes).19–21 ARB meta-analyses have also concluded that BP reduction with ARBs does not reduce the risk of MI.22–24
Conversely, minor falls in BP with ACE inhibitors may lead to substantial reductions in cardiovascular risk. In a meta-analysis of 146 838 patients with hypertension,25 decreases in BP with ACE inhibitor therapy were small, but led to a supplementary 9% relative risk reduction (95% confidence interval [CI], 3% to 14%) in coronary heart disease, independent of BP. In fact, the same meta-analysis also revealed that with ARBs, there was a supplementary 8% increase in the relative risk of coronary heart disease (95% CI, − 17% to 39%), independent of BP, and that this interclass difference was significant (p = 0.002).25
A meta-analysis of MI in 55 050 ARB patients painted a similar picture, this time with regards to MI.14 The rate of MI in this meta-analysis was deemed to be excessive in nine trials and significant in two (one versus active comparator and one versus placebo). With ARBs, there was no effect on all-cause mortality (odds ratio, 1.01; 95% CI, 0.96 to 1.06; p = 0.80), but the risk of MI rose significantly by 8% (95% CI, 1% to 16%; p = 0.03). On the other hand, ACE inhibitors were able to significantly reduce all-cause mortality, cardiovascular death, and MI by 9% (95% CI, 0.86 to 0.95; p < 0.001), 12% (95% CI, 0.82 to 0.95; p < 0.001), and 14% (95% CI, 0.82 to 0.90; p < 0.001), regardless of comparator.14 Recent evidence also confirms that ARBs do not reduce mortality; a meta-analysis of 37 ARB trials in 147 020 patients in 2011 showed that ARBs did not reduce the relative risk of all-cause mortality (relative risk [RR], 1.00; 95% CI, 0.97-1.02; p = 0.75) or cardiovascular mortality (RR, 0.99; 95% CI, 0.94-1.04; p = 0.73) compared with controls.26
Class-specific effects like diminution of oxidative stress and endothelial dysfunction, and inhibition and stabilization of atherosclerotic plaque arguably account for some of the differences between ACE inhibitors and ARBs in terms of mortality reduction in hypertension.9,25 Mortality reduction in hypertension is contingent on more than simple BP reduction. Furthermore, abundant evidence already exists showing that there are differences between ACE inhibitors and ARBs in terms of mortality reduction.
Mortality reduction with RAAS inhibitors in contemporary trials of hypertension: a meta-analytic approach
The most recent meta-analysis of mortality reduction with RAAS inhibition in hypertension, published in the European Heart Journal,6 again confirmed a difference between ACE inhibitors and ARBs in terms of mortality reduction in hypertension. For this meta-analysis, English publications of contemporary (2000 to 2011) ACE inhibitor and ARB trials in hypertension were identified.6 Twenty trials were included on the basis of a sufficient number of patients having hypertension (>66%) and an acceptable incidence of all-cause death (n>10). Data for all-cause mortality were available for all 20 trials,19–21,27–43 while data for cardiovascular mortality were available for 16 of the 20 trials.19–21,27–33,35,39–43
Overall, there were 76 615 patients from ACE inhibitor trials and 82 383 patients from ARB trials in the meta-analysis. Approximately half the 158 998 patients were randomized to active treatment (n = 71 401) and half to control (n = 87 597). Fifty-eight percent of patients were male, and most patients were hypertensive (91%). Mean age was 67 years (range 59 to 84 years) and mean baseline systolic BP was 153 mm Hg (range 135 to 182 mm Hg).6
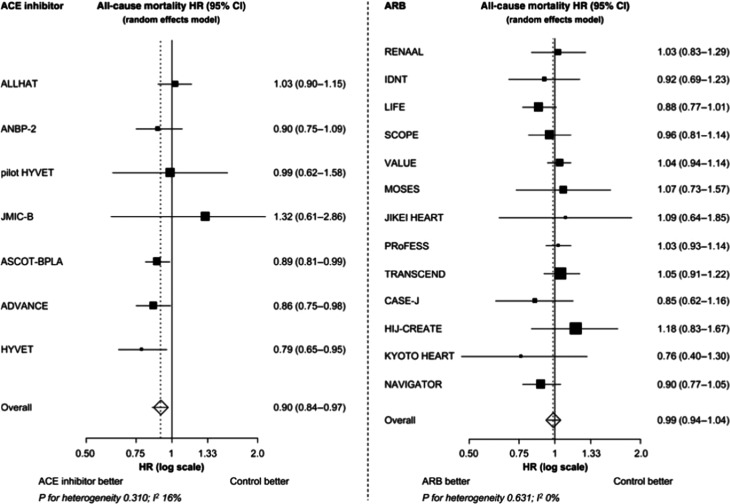
The relative risk of all-cause mortality fell significantly by 5% (hazard ratio [HR], 0.95; 95% CI, 0.91 to 1.00; p = 0.032) with RAAS inhibitors.6 ACE inhibitors were responsible for much of this mortality reduction, with the relative risk of all-cause mortality falling significantly by 10% (HR, 0.90; 95% CI, 0.84 to 0.97; p = 0.004) with ACE inhibitors (Figure 1). In contrast, there was no significant relative risk reduction in all-cause mortality with ARBs (HR, 0.99; 95% CI, 0.94 to 1.04; p = 0.683). There was also a significant difference in treatment effect between ACE inhibitors and ARBs (p = 0.036).
Figure 1. .
The effect of treatment on all-cause mortality in ACE inhibitor and ARB hypertension trials. The effect of treatment on all-cause mortality was significant with ACE inhibitors (p = 0.004), but not with ARBs (p = 0.683). Copied from reference.6Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CI, confidence interval; HR, hazard ratio.
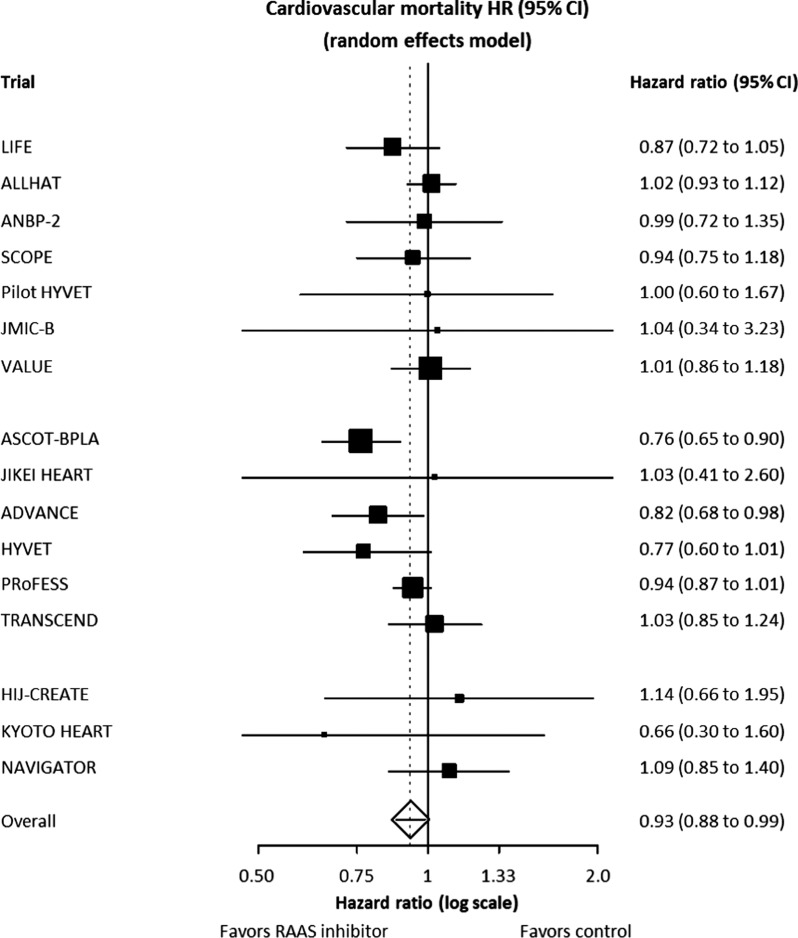
With regard to cardiovascular mortality, RAAS inhibition was shown to significantly reduce the relative risk of cardiovascular mortality by 7% (HR, 0.93; 95% CI, 0.88 to 0.99; p = 0.018) (Figure 2).6 Analysis of 73 100 patients from nine ARB trials that reported cardiovascular mortality data showed that ARBs were not responsible for this reduction (HR, 0.96; 95% CI, 0.90 to 1.01; p = 0.143). Again, mortality reduction was dominated by the effect of ACE inhibitors, with a trend towards a relative risk reduction in cardiovascular mortality of 12% (HR, 0.88; 95% CI, 0.77 to 1.00; p = 0.051) in 76 615 patients from 7 ACE inhibitor trials.
Figure 2. .
Random effects model comparison of cardiovascular mortality reduction in ACE inhibitor and ARB trials. Modified from reference.6Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; RAAS, renin-angiotensin-aldosterone system.
As the findings are based on data from nearly 160 000 randomized controlled trial subjects,6 the meta-analysis can be considered fundamentally robust in terms of data quality and numbers analyzed.
Mortality reduction in hypertension with RAAS inhibitors: are they all the same?
As the results of the meta-analysis show, ARBs have no effect on either all-cause or cardiovascular mortality, so our attention should quite naturally first turn toward ACE inhibitors in the search of explanations about successful mortality reduction in hypertension.6 When the results of ACE inhibitor trials of the meta-analysis were examined in greater depth, it was found that there was a significant reduction in the relative risk of all-cause mortality in only three of the seven ACE inhibitor trials: ASCOT-BPLA (Anglo-Scandinavian Cardiac Outcomes Trial Blood Pressure–Lowering Arm), ADVANCE (Action in Diabetes and Vascular disease: PreterAx and DiamicroN MR Controlled Evaluation), and HYVET (HYpertension in the Very Elderly Trial) (Figure 1).31–33
The relative risk of all-cause mortality was reduced in these three trials by 11% (p = 0.025), 14% (p = 0.025), and 21% (p = 0.02), respectively. Perindopril was used in the active treatment arms of all three trials. The best that can be said for ARBs is a trend toward a 12% reduction in the relative risk of all-cause mortality (p = 0.077) reported in LIFE (Losartan Intervention For Endpoint reduction in hypertension),39 which compared a losartan-based regimen versus an atenolol-based regimen.
The relative risk of cardiovascular mortality was reduced significantly in only two of 16 trials, and these were both ACE inhibitor trials: ASCOT-BPLA and ADVANCE (Figure 2).31,32 In ASCOT-BPLA, the relative risk of cardiovascular mortality was reduced by 24% (p = 0.001), while in ADVANCE it fell by 18% (p = 0.027). In the other perindopril-based trial, HYVET,33 there was a trend towards a 23% reduction (p = 0.06).
From the above, it appears in this meta-analysis that perindopril-based trials accounted for a substantial part of the all-cause and cardiovascular mortality reduction with RAAS inhibitors in hypertension. The results with perindopril are probably due to a combination of effects. Perindopril acts on all the main parameters of BP,31,44–46 and its efficacy has been established in a wide range of hypertensive patients.47,48 Examination of its characteristics shows that perindopril is lipophilic and has a long duration of antihypertensive action (trough:peak ratio, 75% to 100%).49,50 Maximum inhibition is seen approximately 8 h after administration, although levels stay elevated (>70%) 24 h after administration,51 an effect confirmed in clinical practice.48
With regards to the efficacy of perindopril in hypertension, this has been confirmed in a wide range of hypertensive patients, including the young and old, men and women, and patients of various ethnicities.48 In a three-month study of clinical hypertension, mean sitting BP decreased significantly with perindopril, from 157/95 mm Hg at baseline to 139/84 mm Hg at study end (p < 0.001). Furthermore, perindopril was found to be well tolerated and safe in high-risk patients, in addition to all other hypertensive subgroups.47 The use of full-dose perindopril was recently investigated and found to be an efficient therapeutic approach in a range of hypertensive patients.52
In addition to reducing BP, perindopril has been shown to have a beneficial effect on endothelium, an important regulator of physiological homoeostasis.9 The endothelium, a continuous layer of cells lining blood vessels with a surface area of over 800 m2, has a lifespan of one to three months. When the natural life cycle of the endothelium is disrupted and the rate of apoptosis exceeds that of regeneration, the continuity of the endothelial layer is compromised. This situation favors the development and progression of atherosclerosis. In a stable coronary population, perindopril reduced endothelial apoptosis by 31% (p < 0.05 versus placebo),53 as well as normalizing fibrinolytic balance. Perindopril decreased levels of angiotensin II by 27% and increased those of bradykinin by 17% after one year (p < 0.05 vs baseline).
In this study,53 levels of von Willebrand factor, a marker of endothelial damage, were significantly reduced after one year in patients treated with perindopril compared with those on placebo (p < 0.001). Interestingly, perindopril also appears to promote endothelial regeneration by increasing the rate of production of endothelial progenitor cells in bone marrow.54
Perindopril has also been shown to modulate neovascularization, regress atherosclerosis, and reduce arterial stiffness (a marker of vascular remodeling).55 Arterial stiffness was shown to diminish in adults with mild-to-moderate essential hypertension who took perindopril.56
Conclusion
With their predominant role in clinical practice, the superiority of ARBs versus ACE inhibitors should be clearly demonstrable, not only in terms of side effect reduction but also efficacy. This is not the case. The latest meta-analysis, once again, highlights differences in mortality reduction—the primary aim of antihypertensive therapy—with different classes of RAAS inhibitor in hypertension.4 These differences between ACE inhibitors and ARBs are so marked that they have already led to calls for changes in the way RAAS inhibitors are used in clinical practice and for the preferential use of ACE inhibitors ahead of ARBs in hypertension, except in cases of ACE inhibitor intolerance.57
Medicine today should be practiced according to evidence. In the case of mortality reduction in hypertension, by denying patients the use of drugs with proven benefits—ACE inhibitors—in favor of those with no evidence of benefits—ARBs—we are denying patients access to effective treatment and thereby harming them indirectly. In the latest meta-analysis, there was a substantial amount of heterogeneity between ACE inhibitors; treatment with perindopril, in particular, was associated with significant reductions in all-cause and cardiovascular mortality.6 More generally, once-a-day administration and an ability to modulate cardiovascular risk factors, both characteristics of perindopril, are deemed important by European hypertension guidelines.4 Given what we know today about the effects of ACE inhibitors and ARBs on mortality in hypertension, perhaps now is the moment to reconsider how we prescribe these agents.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13:9–20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong ND, Dede J, Chow VH, Wong KS, Franklin SS. Global cardiovascular risk associated with hypertension and extent of treatment and control according to risk group. Am J Hypertens. 2012;25:561–567. doi: 10.1038/ajh.2012.2. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Kjeldsen SE, Erdine S, Narkiewicz K, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Cifkova R, Dominiczak A, van Zwieten P, Viigimaa M, Waeber B, Williams B, Zamorano JL. Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 5.Sica DA. The evolution of renin-angiotensin blockade: angiotensin-converting enzyme inhibitors as the starting point. Curr Hypertens Rep. 2010;12:67–73. doi: 10.1007/s11906-010-0091-9. [DOI] [PubMed] [Google Scholar]
- 6.van Vark LC, Bertrand M, Akkerhuis KM, Brugts JJ, Fox K, Mourad JJ, Boersma E. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension - a meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone-system inhibitors involving 158,998 patients. Eur Heart J. 2012;33:2088–2097. doi: 10.1093/eurheartj/ehs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unger T, Stoppelhaar M. Rationale for double renin-angiotensin-aldosterone system blockade. Am J Cardiol. 2007;100:25J–31J. doi: 10.1016/j.amjcard.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Probstfield JL, O'Brien KD. Progression of cardiovascular damage: the role of renin-angiotensin system blockade. Am J Cardiol. 2010;105:10A–20A. doi: 10.1016/j.amjcard.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari R, Fox K. Insight into the mode of action of ACE inhibition in coronary artery disease: The ultimate ‘EUROPA’ story. Drugs. 2009;69:265–277. doi: 10.2165/00003495-200969030-00003. [DOI] [PubMed] [Google Scholar]
- 10.Athyros VG, Mikhailidis DP, Kakafika AI, Tziomalos K, Karagiannis A. Angiotensin II reactivation and aldosterone escape phenomena in renin-angiotensin-aldosterone system blockade: is oral renin inhibition the solution? Expert Opin Pharmacother. 2007;8:529–535. doi: 10.1517/14656566.8.5.529. [DOI] [PubMed] [Google Scholar]
- 11.Hollenberg NK, Fisher ND. Renal circulation and blockade of the renin-angiotensin system. Is angiotensin-converting enzyme inhibition the last word? Hypertension. 1995;26:602–609. doi: 10.1161/01.hyp.26.4.602. [DOI] [PubMed] [Google Scholar]
- 12.Comini L, Bachetti T, Cargnoni A, Bastianon D, Gitti GL, Ceconi C, Ferrari R. Therapeutic modulation of the nitric oxide pathway: are all ACE inhibitors equivalent? Pharmacol Res. 2007;56:42–48. doi: 10.1016/j.phrs.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Ceconi C, Francolini G, Olivares A, Comini L, Bachetti T, Ferrari R. Angiotensin-converting enzyme (ACE) inhibitors have different selectivity for bradykinin binding sites of human somatic ACE. Eur J Pharmacol. 2007;577:1–6. doi: 10.1016/j.ejphar.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 14.Strauss MH, Hall AS. Angiotensin receptor blockers may increase risk of myocardial infarction: unraveling the ARB-MI paradox. Circulation. 2006;114:838–854. doi: 10.1161/CIRCULATIONAHA.105.594986. [DOI] [PubMed] [Google Scholar]
- 15.Wolf G, Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney Int. 2005;67:799–812. doi: 10.1111/j.1523-1755.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 16.Chrysant SG, Chrysant GS, Chrysant C, Shiraz M. The treatment of cardiovascular disease continuum: focus on prevention and RAS blockade. Curr Clin Pharmacol. 2010;5:89–95. doi: 10.2174/157488410791110742. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 18.Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, Mustonen J. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–1961. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 19.Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A, SCOPE Study Group The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlöf B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, Vander Maelen C, Voigt T, Weber M, Yoon BW, PRoFESS Study Group Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 22.McDonald MA, Simpson SH, Ezekowitz JA, Gyenes G, Tsuyuki RT. Angiotensin receptor blockers and risk of myocardial infarction: systematic review. BMJ. 2005;331:873. doi: 10.1136/bmj.38595.518542.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verdecchia P, Angeli F, Gattobigio R, Reboldi GP. Do angiotensin II receptor blockers increase the risk of myocardial infarction? Eur Heart J. 2005;26:2381–2386. doi: 10.1093/eurheartj/ehi445. [DOI] [PubMed] [Google Scholar]
- 24.Volpe M, Mancia G, Trimarco B, Angiotensin receptor blockers and myocardial infarction: deeds and misdeeds. J Hypertens. 2005;23:2113–2118. doi: 10.1097/01.hjh.0000194114.12228.16. [DOI] [PubMed] [Google Scholar]
- 25.Turnbull F, Neal B, Pfeffer M, Kostis J, Algert C, Woodward M, Chalmers J, Zanchetti A, MacMahon S Blood Pressure Lowering Treatment Trialists' Collaboration. Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens. 2007;25:951–958. doi: 10.1097/HJH.0b013e3280bad9b4. [DOI] [PubMed] [Google Scholar]
- 26.Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147 020 patients from randomised trials. BMJ. 2011;342:d2234. doi: 10.1136/bmj.d2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wing LM, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Marley JE, Morgan TO, West MJ, Second Australian National Blood Pressure Study Group A comparison of outcomes with angiotensin-converting–enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583–592. doi: 10.1056/NEJMoa021716. [DOI] [PubMed] [Google Scholar]
- 28.Yui Y, Sumiyoshi T, Kodama K, Hirayama A, Nonogi H, Kanmatsuse K, Origasa H, Iimura O, Ishii M, Saruta T, Arakawa K, Hosoda S, Kawai C, Japan Multicenter Investigation for Cardiovascular Diseases-B Study Group Comparison of nifedipine retard with angiotensin converting enzyme inhibitors in Japanese hypertensive patients with coronary artery disease: the Japan Multicenter Investigation for Cardiovascular Diseases-B (JMIC-B) randomized trial. Hypertens Res. 2004;27:181–191. doi: 10.1291/hypres.27.181. [DOI] [PubMed] [Google Scholar]
- 29.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 30.Bulpitt CJ, Beckett NS, Cooke J, Dumitrascu DL, Gil-Extremera B, Nachev C, Nunes M, Peters R, Staessen JA, Thijs L, Hypertension in the Very Elderly Trial Working Group Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens. 2003;21:2409–2417. doi: 10.1097/00004872-200312000-00030. [DOI] [PubMed] [Google Scholar]
- 31.Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J, ASCOT Investigators Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 32.Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 33.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ, HYVET Study Group Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 34.Ogihara T, Fujimoto A, Nakao K, Saruta T, CASE-J Trial Group ARB candesartan and CCB amlodipine in hypertensive patients: the CASE-J trial. Expert Rev Cardiovasc Ther. 2008;6:1195–1201. doi: 10.1586/14779072.6.9.1195. [DOI] [PubMed] [Google Scholar]
- 35.Kasanuki H, Hagiwara N, Hosoda S, Sumiyoshi T, Honda T, Haze K, Nagashima M, Yamaguchi J, Origasa H, Urashima M, Ogawa H, HIJ-CREATE Investigators Angiotensin II receptor blocker-based vs. non-angiotensin II receptor blocker-based therapy in patients with angiographically documented coronary artery disease and hypertension: the heart institute of Japan candesartan randomized trial for evaluation in coronary artery disease (HIJ-CREATE) Eur Heart J. 2009;30:1203–1212. doi: 10.1093/eurheartj/ehp101. [DOI] [PubMed] [Google Scholar]
- 36.Schrader J, Lüders S, Kulschewski A, Hammersen F, Plate K, Berger J, Zidek W, Dominiak P, Diener HC, MOSES Study Group Morbidity and Mortality After Stroke, Eprosartan Compared with Nitrendipine for Secondary Prevention: principal results of a prospective randomized controlled study (MOSES) Stroke. 2005;36:1218–1226. doi: 10.1161/01.STR.0000166048.35740.a9. [DOI] [PubMed] [Google Scholar]
- 37.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Group Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 38.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 39.Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, LIFE Study Group Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 40.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A, VALUE trial group Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 41.Mochizuki S, Dahlöf B, Shimizu M, Ikewaki K, Yoshikawa M, Taniguchi I, Ohta M, Yamada T, Ogawa K, Kanae K, Kawai M, Seki S, Okazaki F, Taniguchi M, Yoshida S, Tajima N, Jikei Heart Study group Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2007;369:1431–1439. doi: 10.1016/S0140-6736(07)60669-2. [DOI] [PubMed] [Google Scholar]
- 42.Sawada T, Yamada H, Dahlöf B, Matsubara H. Effects of valsartan on morbidity and mortality in uncontrolled hypertensive patients with high cardiovascular risks: KYOTO HEART Study. Eur Heart J. 2009;30:2461–2469. doi: 10.1093/eurheartj/ehp363. [DOI] [PubMed] [Google Scholar]
- 43.McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamás G, Tognoni G, Tuomilehto J, Villamil AS, Vozár J, Califf RM, NAVIGATOR Study Group Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1477–1490. doi: 10.1056/NEJMoa1001121. [DOI] [PubMed] [Google Scholar]
- 44.Manisty CH, Zambanini A, Parker KH, Davies JE, Francis DP, Mayet J, McG Thom SA, Hughes AD, Anglo-Scandinavian Cardiac Outcome Trial Investigators Differences in the magnitude of wave reflection account for differential effects of amlodipine- versus atenolol-based regimens on central blood pressure: an Anglo-Scandinavian Cardiac Outcome Trial substudy. Hypertension. 2009;54:724–730. doi: 10.1161/HYPERTENSIONAHA.108.125740. [DOI] [PubMed] [Google Scholar]
- 45.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M, CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 46.Dolan E, Stanton AV, Thom S, Caulfield M, Atkins N, McInnes G, Collier D, Dicker P, O'Brien E, ASCOT Investigators Ambulatory blood pressure monitoring predicts cardiovascular events in treated hypertensive patients–an Anglo-Scandinavian cardiac outcomes trial substudy. J Hypertens. 2009;27:876–885. doi: 10.1097/HJH.0b013e328322cd62. [DOI] [PubMed] [Google Scholar]
- 47.Guo W, Turlapaty P, Shen Y, Dong V, Batchelor A, Barlow D, Lagast H. Clinical experience with perindopril in patients nonresponsive to previous antihypertensive therapy: a large US community trial. Am J Ther. 2004;11:199–205. doi: 10.1097/00045391-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Julius S, Cohn JN, Neutel J, Weber M, Turlapaty P, Shen Y, Dong V, Batchelor A, Lagast H. Antihypertensive utility of perindopril in a large, general practice-based clinical trial. J Clin Hypertens (Greenwich) 2004;6:10–17. doi: 10.1111/j.1524-6175.2004.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Physicians' Desk Reference (58th ed.) Thompson Healthcare (2004) ISBN: 9781563634710
- 50.Ferrari R. Angiotensin-converting enzyme inhibition in cardiovascular disease: evidence with perindopril. Expert Rev Cardiovasc Ther. 2005;3:15–29. doi: 10.1586/14779072.3.1.15. [DOI] [PubMed] [Google Scholar]
- 51.Louis WJ, Conway EL, Krum H, Workman B, Drummer OH, Lam W, Phillips P, Howes LG, Jackson B. Comparison of the pharmacokinetics and pharmadocynamics of perindopril, cilazapril and enalapril. Clin Exp Pharmacol Physiol Suppl. 1992;19:55–60. doi: 10.1111/j.1440-1681.1992.tb02811.x. [DOI] [PubMed] [Google Scholar]
- 52.Tsoukas G, Anand S, Yang K. Dose-dependent antihypertensive efficacy and tolerability of perindopril in a large, observational, 12-week, general practice-based study. Am J Cardiovasc Drugs. 2011;11:45–55. doi: 10.2165/11587000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 53.Ceconi C, Fox KM, Remme WJ, Simoons ML, Bertrand M, Parrinello G, Kluft C, Blann A, Cokkinos D, Ferrari R, EUROPA Investigators; PERTINENT Investigators and the Statistical Committee ACE inhibition with perindopril and endothelial dysfunction. Results of a substudy of the EUROPA study: PERTINENT. Cardiovasc Res. 2007;73:237–246. doi: 10.1016/j.cardiores.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 54.Cangiano E, Marchesini J, Campo G, Francolini G, Fortini C, Carrà G, Miccoli M, Ceconi C, Tavazzi L, Ferrari R. ACE inhibition modulation of endothelial apoptosis and renewal via endothelial progenitor cells in patients with acute coronary syndromes. Am J Cardiovasc Drugs. 2011;11:189–198. doi: 10.2165/11589400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Yazawa H, Miyachi M, Furukawa M, Takahashi K, Takatsu M, Tsuboi K, Ohtake M, Murase T, Hattori T, Kato Y, Murohara T, Nagata K. Angiotensin-converting enzyme inhibition promotes coronary angiogenesis in the failing heart of Dahl salt-sensitive hypertensive rats. J Card Fail. 2011;17:1041–1050. doi: 10.1016/j.cardfail.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Asmar R, Topouchian J, Pannier B, Benetos A, Safar M. Scientific, Quality Control, Coordination and Investigation Committees of the Complior Study. Pulse wave velocity as endpoint in large-scale intervention trial. The Complior study. Scientific, Quality Control, Coordination and Investigation Committees of the Complior Study. J Hypertens. 2001;19:813–818. doi: 10.1097/00004872-200104000-00019. [DOI] [PubMed] [Google Scholar]
- 57.Ruschitzka F, Taddei S. Angiotensin-converting enzyme inhibitors: first-line agents in cardiovascular protection? Eur Heart J. 2012;33:1996–1998. doi: 10.1093/eurheartj/ehs108. [DOI] [PubMed] [Google Scholar]