Introduction
The two adult types of bone marrow (BM) can be distinguished: the red marrow consisting of hematopoietic tissue, including Hematopoietic Stem Cells (HSCs) capable of producing around 500 billion blood cells per day; and the yellow marrow mainly made up of fat cells. BM contains two types of stem cells: hemopoietic (which can produce blood cells) and stromal (which can produce fat, cartilage and bone).
Mesenchymal Stem Cells (MSCs), first described in 1970 in BM,1 emerged as an extremely promising therapeutic cell based agent for tissue regeneration and were broadly characterized in that sense.2,3In vitro studies demonstrated that MSCs present attractive characteristics like immune-regulatory effects; capacity to stimulate neovascularization, endogenous stem cell proliferation and differentiation, capacity to modify the micro-environment, prevent scarification, fibrosis and aptitude to differentiate into various mesoderm derived tissue. Notably, myocytes and cardiomyocyte differentiation ability was shown in multiple studies.4–9 All together these properties drove enthusiasm for MSCs and particularly BM derived MSCs (BM-MSCs) use in cardiac regeneration. MSCs represent 0.001 to 0.01% of BM nucleated cells and, it is now broadly accepted that MSCs cultures represent a mix of various cells with various degrees of “stemness”.10,11
The unforeseen discovery that HSCs isolated from BM present the ability to repair infarcted myocardium12 also prompted extensive research in this direction. Though, HSCs only represent ∼0.01% of BM mononucleated cells, and there expansion in vitro remains elusive,13 therefore, clinical trials to date relied on whole BM use, impairing the clear identification of which cellular actor drives the observe effect.
Therefore, very short time after the publication of the first experimental study of the use of Bone Marrow Cells (BMCs) for the treatment of post Myocardial Infarction (MI) heart failure (HF) in a small animal model,14 clinical trials of this form of therapy started.15 This was followed by an extremely large number of trials with mixed results,16–20 and even the alarming establishment of commercial clinics in different countries.
All together, these findings lead in the last year to witness the publication of several trials in the field using either whole BMCs or BM-MSCs. The results of the most recently publish results are reviewed here with the hope of clarifying some of the major issues in the field. In addition, an article expressing concerns regarding some of the early trials is described.
Time
The Timing in Myocardial Infarction Evaluation “TIME” trial is a multicenter 2 by 2 randomized, placebo controlled trial performed as part of the Cardiovascular Cell Therapy Research Network (CCTRN) sponsored by the National Heart Lung and Blood Institute (NHLBI). The objectives of the trial was to determine the effect of timing of intracoronary injection of 1.5 × 108 autologous BM derived cells on recovery of Left Ventricle (LV) function after successful primary Percutaneous Coronary Intervention (PCI) for anterior ST-Elevation Myocardial Infarction (STEMI).21 Overall, 120 patients were recruited. The inclusion criteria included patients with an ejection fraction equal to or less than 0.45 after PCI. Cells were injected 3 or 7 days after PCI. The primary end points were global and regional LV function at 6 months after treatment.
This is a very well designed trial representing real life conditions encountered in many centers. In spite of the relatively small number of patients dealt with in each center, the trial is likely to influence practice by dampening some of the prevailing enthusiasm for infusing unmodified BM mononuclear cells into the coronaries at 3 or 7 days after MI. Indeed, no significant influence in either treatment group versus placebo on both end points was observed.
Swiss-ami
The four-month results of this trial were published in Circulation earlier this year.22 Like TIME, SWiss multicenter Intracoronary Stem cells Study in Acute Myocardial Infarction (SWISS-AMI) trial was designed to examine the effect of timing of intracoronary injection of 10 mL of mononuclear BM derived cells at different interval, 3–4 days or 5–7 weeks after successful primary PCI.23 The authors randomized 200 patients to controls, early and late cell infusion. The primary end point was left ventricular function determined by cardiac MRI, at different time points. The current publication relates to the 4 month results. At this point, there was no difference in the primary end point between the three groups. Although the period of follow up was very short, the findings confirm those of the TIME trial.
Cellwave
This trial was designed to test the possible beneficial effect of application of shock waves to the heart 24 h before intracoronary infusion of BM derived cells in patients with chronic post infarction HF.24 The authors randomized patients to placebo (n = 20), high dose (n = 40) and low doses (n = 42) shock waves. The primary endpoint was to assess a change in global left ventricular ejection fraction (LVEF), measured at 4 months after treatment. They observed a modest improvement in LVEF from 1% in the placebo group to 3.5% in the shockwave + BM-MSCs group. Regional wall thickening also improved modestly from 0.5% in the placebo group to 3.6% in the shockwave + BM-MSCs group. This trial highlights the need for additional modalities to improve the results of these procedures.
Poseidon
This early trial of PercutaneOus StEm cell Injection Delivery effects On Neomyogenesis (POSEIDON) examines the use of autologous versus allogenic BM-MSCs injected into the myocardium through the trans endocardial route in patients with post myocardial infarction HF.25 The authors randomized patients (5 in each group) to autologous versus allogenic MSCs, in the absence of a control group.26 The endpoints were a combination of clinical and hemodynamic parameters with emphasis on safety profile at one month. The results showed that both types of cells were safe and produced comparable improvements in endpoints, with little evidence of an immune reaction to the allogenic cells. Another interesting finding in this trial was the benefit of injection of a smaller number of cells (20 million) was superior to larger numbers (100–200 million). This proof of principle trial suggest that the use of “off the shelf” allogenic MSCs might be feasible in the future, and strengthens the need for processing BM derived cells before injection.
C-cure
The Cardiopoietic stem Cell therapy in heart failURE (C-CURE), is a multi-center randomized placebo controlled trial of feasibility, safety and efficacy of BM derived cardiopoietic mesenchymal stem cells use in post myocardial infarction chronic HF. BM was harvested and isolated mesenchymal stem cells were exposed to a cardiogenic “cocktail”. The cells were injected through the endocardium using a Nog catheter (Medtronic). The end point was a composite score incorporating several hemodynamic and clinical parameters.27
Overall, 21 patients received the cell therapy and 15 acted as controls. Strict quality control and testing for beginning myocardial differentiation was performed. The ejection fraction improved by 7% and the left ventricular end-systolic volume was reduced by 16 mL in the treatment group as compared to no change in the placebo. Similarly, there was improvement in the composite score in the treatment group. This trial is a model of what can be done in this field. Longer term results of this trial and similar trials are awaited with great interest.
Discussion
Although the use of unprocessed BM derived cells for regenerative therapy is extremely attractive because of availability and the short time required for its preparation, the collective evidence from the trials discussed above and previous clinical trials (elegantly reviewed in a meta-analysis of 33 trials)28 strongly suggests that we must move on.
We are awaiting with great interest the results of additional ongoing clinical trials such as: the Transendocardial Autologous Cells (hMSC or hBMC) in ischemic Heart Failure Trial (TAC-HFT),29 PROspective randomized study of MEsenchymal stem cell THErapy in patients undergoing cardiac Surgery (PROMETEUS), POSEIDON-DCM (studying MSCs therapy for the treatment of idiopathic dilated cardiomyopathy) and the larger scaled phase II intracoronary reinfusion of Bone marrow-derived mononuclear cells (BM-MNC) on all-cause mortality the in Acute Myocardial Infarction (BAMI) trial.
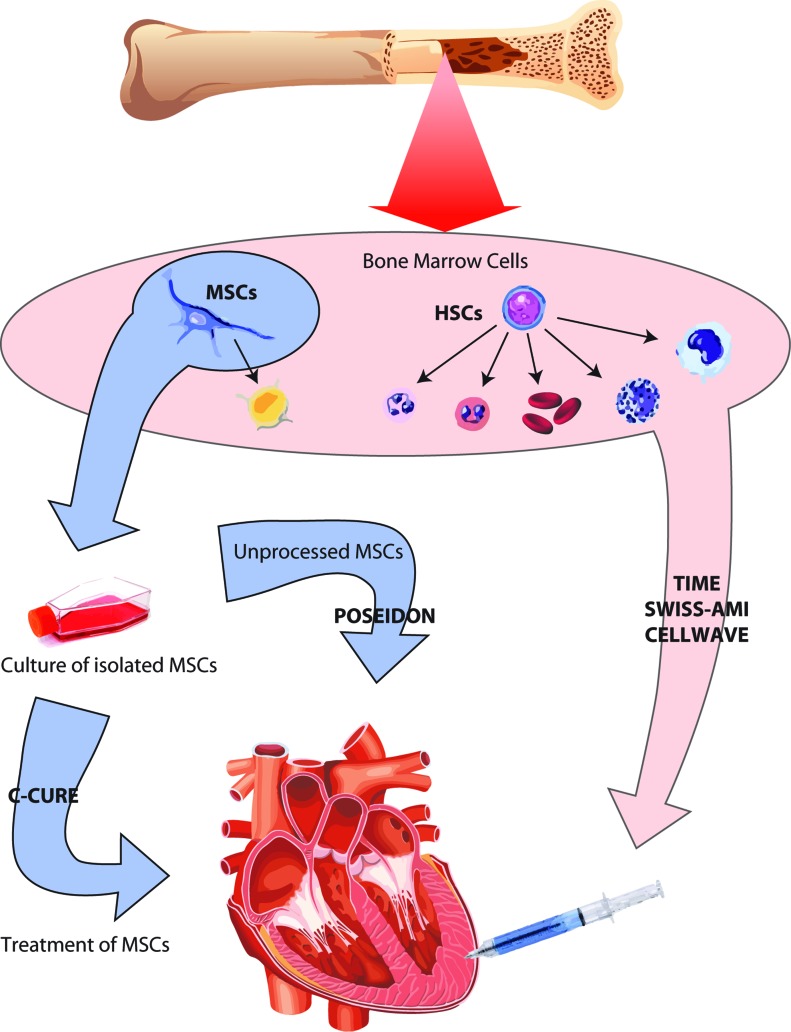
Figure 1. .
Bone marrow cells used in the different trials. Flow chart of bone marrow cells used and treatments: TIME, SWISS-AMI and CELLWAVE used unprocessed bone marrow cells including HSCs and MSCs. POSEIDON and C-CURE used isolated and purified MSCs from bone marrow but while in POSEIDON the MSCs used were unprocessed, in C-CURE the isolated MSCs were treated in culture with a “cardiogenic cocktail” prior injection.
Though, the overall results of the large number of clinical trials already performed suggest that even if proven safe, and when using allogenous MSCs, modest improvement of patient condition sustained on long term is observed.Procedures designed to enhance the performance of these cells seems therefore to be warranted. Four main areas of research could be actively pursued. As far as HSCs are concerned, new solutions have been developed recently for HSCs in vitro amplifications and should be further tested for heart failure treatment.30
As for MSCs, pre-treatment of the cells and there reprogramming towards cardiac fate prior to injection seems be the direction of choice based on the better results observed with C-CURE. New modifications via genetic alteration or Wnt and TGF-β pathway targeting could be further persued. BM-MSCs might also not be the candidate of choice for heart therapy, and alternative source of MSCs could be tested. Indeed MSCs can be isolated from a broad array of tissues (not exhaustively: adipose tissues, cord blood, placenta etc.) that present acute in vitro and preclinical capacity of differentiation into cardiomyocytes.31–34 Nevertheless, the same restricted improvement might also be seen with these MSCs. Evidence of pre-clinical and clinical data suggest that most of the beneficial effect of BM-MSCs treatment rely on their paracrine effects more than direct cardiomyocyte differentiation. In that case, and considering the safety of their injection and there immuno regulatory effect, MSCs use could be rethought as a complement to other cell based therapies (like cardiac stem cells). Indeed, as stressed in the previous issue of the Journal,35 alternative methods using cardiac derived stem cells, exploiting the self-renewal capacity of the differentiated myocardial cell, or cell reprogramming must be actively pursued.
References
- 1.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 2.Hao L, Sun H, Wang J, Wang T, Wang M, Zou Z. Mesenchymal stromal cells for cell therapy: besides supporting hematopoiesis. Int J Hematol. 2012;95(1):34–46. doi: 10.1007/s12185-011-0991-8. [DOI] [PubMed] [Google Scholar]
- 3.Raynaud CM, Rafii A. The necessity of a systematic approach for the use of MSCs in the clinical setting. Stem Cells Int. 2013;2013:892340. doi: 10.1155/2013/892340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18(12):1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Yu X, Lin Q, Deng C, Shan Z, Yang M, Lin S. Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment. J Mol Cell Cardiol. 2007;42(2):295–303. doi: 10.1016/j.yjmcc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, Zhou H, Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229(7):623–631. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 7.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103(5):697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu M, Wani M, Dai YS, Wang J, Yan M, Ayub A, Ashraf M. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110(17):2658–2665. doi: 10.1161/01.CIR.0000145609.20435.36. [DOI] [PubMed] [Google Scholar]
- 9.Pijnappels DA, Schalij MJ, Ramkisoensing AA, van Tuyn J, de Vries AA, van der Laarse A, Ypey DL, Atsma DE. Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circ Res. 2008;103(2):167–176. doi: 10.1161/CIRCRESAHA.108.176131. [DOI] [PubMed] [Google Scholar]
- 10.Lee CC, Christensen JE, Yoder MC, Tarantal AF. Clonal analysis and hierarchy of human bone marrow mesenchymal stem and progenitor cells. Exp Hematol. 2010;38(1):46–54. doi: 10.1016/j.exphem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113(Pt 7):1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- 12.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 13.Butler JM, Gars EJ, James DJ, Nolan DJ, Scandura JM, Rafii S. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood. 2012;120(6):1344–1347. doi: 10.1182/blood-2011-12-398115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orlic D. Adult bone marrow stem cells regenerate myocardium in ischemic heart disease. Ann N Y Acad Sci. 2003;996:152–157. doi: 10.1111/j.1749-6632.2003.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94(1):92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108(7):792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Liu Z, Tian N, Zhang J, Yei F, Duan B, Zhu Z, Lin S, Kwan TW. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol. 2006;18(11):552–556. [PubMed] [Google Scholar]
- 18.Katritsis DG, Sotiropoulou PA, Karvouni E, Karabinos I, Korovesis S, Perez SA, Voridis EM, Papamichail M. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv. 2005;65(3):321–329. doi: 10.1002/ccd.20406. [DOI] [PubMed] [Google Scholar]
- 19.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assmus B, Rolf A, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Tillmanns H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Tonn T, Dimmeler S, Dill T, Zeiher AM, Schächinger V, REPAIR-AMI Investigators Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3(1):89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 21.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, Perin EC, Chambers J, Baran KW, Raveendran G, Lambert C, Lerman A, Simon DI, Vaughan DE, Lai D, Gee AP, Taylor DA, Cogle CR, Thomas JD, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Kappenman C, Westbrook L, Piller LB, Simpson LM, Baraniuk S, Loghin C, Aguilar D, Richman S, Zierold C, Spoon DB, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moyé LA, Simari RD, Cardiovascular Cell Therapy Research Network (CCTRN) Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308(22):2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sürder D, Manka R, Lo Cicero V, Moccetti T, Rufibach K, Soncin S, Turchetto L, Radrizzani M, Astori G, Schwitter J, Erne P, Zuber M, Auf der Maur C, Jamshidi P, Gaemperli O, Windecker S, Moschovitis A, Wahl A, Bühler I, Wyss C, Kozerke S, Landmesser U, Lüscher TF, Corti R. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation. 2013;127(19):1968–1979. doi: 10.1161/CIRCULATIONAHA.112.001035. [DOI] [PubMed] [Google Scholar]
- 23.Sürder D, Schwitter J, Moccetti T, Astori G, Rufibach K, Plein S, Lo Cicero V, Soncin S, Windecker S, Moschovitis A, Wahl A, Erne P, Jamshidi P, Auf der Maur C, Manka R, Soldati G, Bühler I, Wyss C, Landmesser U, Lüscher TF, Corti R. Cell-based therapy for myocardial repair in patients with acute myocardial infarction: rationale and study design of the SWiss multicenter Intracoronary Stem cells Study in Acute Myocardial Infarction (SWISS-AMI) Am Heart J. 2010;160(1):58–64. doi: 10.1016/j.ahj.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 24.Assmus B, Walter DH, Seeger FH, Leistner DM, Steiner J, Ziegler I, Lutz A, Khaled W, Klotsche J, Tonn T, Dimmeler S, Zeiher AM. Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. JAMA. 2013;309(15):1622–1631. doi: 10.1001/jama.2013.3527. [DOI] [PubMed] [Google Scholar]
- 25.Hare JM. Translational development of mesenchymal stem cell therapy for cardiovascular diseases. Tex Heart Inst J. 2009;36(2):145–147. [PMC free article] [PubMed] [Google Scholar]
- 26.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61(23):2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 28.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;2:Cd006536. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 29.Trachtenberg B, Velazquez DL, Williams AR, McNiece I, Fishman J, Nguyen K, Rouy D, Altman P, Schwarz R, Mendizabal A, Oskouei B, Byrnes J, Soto V, Tracy M, Zambrano JP, Heldman AW, Hare JM. Rationale and design of the Transendocardial Injection of Autologous Human Cells (bone marrow or mesenchymal) in Chronic Ischemic Left Ventricular Dysfunction and Heart Failure Secondary to Myocardial Infarction (TAC-HFT) trial: a randomized, double-blind, placebo-controlled study of safety and efficacy. Am Heart J. 2011;161(3):487–493. doi: 10.1016/j.ahj.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Raynaud CM, Butler JM, Halabi NM, Ahmad FS, Ahmed B, Rafii S, Rafii A. Endothelial cells provide a niche for placental hematopoietic stem/progenitor cell expansion through broad transcriptomic modification. Stem Cell Research. 2013;11(3):1074–1090. doi: 10.1016/j.scr.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 31.van Dijk A, Niessen HW, Zandieh Doulabi B, Visser FC, van Milligen FJ. Differentiation of human adipose-derived stem cells towards cardiomyocytes is facilitated by laminin. Cell Tissue Res. 2008;334(3):457–467. doi: 10.1007/s00441-008-0713-6. [DOI] [PubMed] [Google Scholar]
- 32.Gaustad KG, Boquest AC, Anderson BE, Gerdes AM, Collas P. Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochem Biophys Res Commun. 2004;314(2):420–427. doi: 10.1016/j.bbrc.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 33.Schimrosczyk K, Song YH, Vykoukal J, Vykoukal D, Bai X, Krohn A, Freyberg S, Alt EU. Liposome-mediated transfection with extract from neonatal rat cardiomyocytes induces transdifferentiation of human adipose-derived stem cells into cardiomyocytes. Scand J Clin Lab Invest. 2008;68(6):464–472. doi: 10.1080/00365510701836907. [DOI] [PubMed] [Google Scholar]
- 34.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28(21):2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 35.Yacoub MH, Terrovitis J. CADUCEUS, SCIPIO, ALCADIA: cell therapy trials using cardiac-derived cells for patients with post myocardial infarction LV dysfunction, still evolving. GCSP. 2013;2013(1):3. doi: 10.5339/gcsp.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]