Abstract
For several decades scientists have speculated that the key to understanding age-related neurodegenerative disorders may be found in the unusual biology of the prion diseases. Recently, owing largely to the advent of new disease models, this hypothesis has gained experimental momentum. In a remarkable variety of diseases, specific proteins have been found to misfold and aggregate into seeds that structurally corrupt like proteins, causing them to aggregate and form pathogenic assemblies ranging from small oligomers to large masses of amyloid. Proteinaceous seeds can therefore serve as self-propagating agents for the instigation and progression of disease. Alzheimer’s disease and other cerebral proteopathies seem to arise from the de novo misfolding and sustained corruption of endogenous proteins, whereas prion diseases can also be infectious in origin. However, the outcome in all cases is the functional compromise of the nervous system, because the aggregated proteins gain a toxic function and/or lose their normal function. As a unifying pathogenic principle, the prion paradigm suggests broadly relevant therapeutic directions for a large class of currently intractable diseases.
Proteins are essential to cellular metabolism and communication, and they form the framework on which cells and tissues are built. To undertake these roles, most proteins fold into a specific, three-dimensional architecture that is largely determined by their distinctive sequences of amino acids. Others have a degree of structural flexibility that enables them to tailor their shape to the task at hand1,2. For proteins, then, as for the rest of biology, structure governs function. Hence, it is critical for cells to maintain an efficient quality-control system that ensures the proper production, folding and elimination of proteins3,4. When a protein misfolds and evades normal clearance pathways, a pathogenic process can ensue in which the protein aggregates progressively into intracellular and/or extracellular deposits. The consequence is a diverse group of disorders, each of which entails the aggregation of particular proteins in characteristic patterns and locations (Fig. 1)5–8. New insights into the ontogeny of these proteopathies are beginning to emerge from the unusual properties of the prion, arguably one of the most provocative molecules in the annals of medicine.
Figure 1. Commonalities among age-related neurodegenerative diseases.
The deposited proteins adopt an amyloid conformation and show prion-like self-propagation and spreading in experimental settings, consistent with the progressive appearance of the lesions in the human diseases. a, Aβ deposits (senile plaques) in the neocortex of a patient with Alzheimer’s disease. b, Tau inclusion as a neurofibrillary tangle in a neocortical neuron of a patient with Alzheimer’s disease. c, α-Synuclein inclusion (Lewy body) in a neocortical neuron from a patient with Parkinson’s disease/Lewy body dementia. d, TDP-43 inclusion in a motoneuron of the spinal cord from a patient with amyotrophic lateral sclerosis. Scale bars are 50 µm in a and 20 µm in b–d. e–h, Characteristic progression of specific proteinaceous lesions in neurodegenerative diseases over time (t, black arrows), inferred from post-mortem analyses of brains. Aβ deposits and tau inclusions in brains of patients with Alzheimer’s disease (e and f), α-synuclein inclusions in brains of patients with Parkinson’s disease (g), and TDP-43 inclusions in brains of patients with amyotrophic lateral sclerosis (h). Three stages are shown for each disease, with white arrows indicating the putative spread of the lesions (for details see refs 5–8). Panels e and f are reproduced, with permission, from ref. 61.
The prion paradigm
Prions (‘proteinaceous infectious particles’) are unconventional infectious agents consisting of misfolded prion protein (PrP) molecules; in their misshapen state, the molecules aggregate with one another and impose their anomalous structure on benign PrP molecules9–12. Prions thus act as corruptive templates (seeds) that incite a chain-reaction of PrP misfolding and aggregation. As prions grow, fragment and spread, they perturb the function of the nervous system and ultimately cause the death of the affected individual.
The prion diseases in humans include Creutzfeldt–Jakob disease (CJD), Gerstmann–Sträussler–Scheinker disease, fatal insomnia and kuru; in nonhuman species they comprise scrapie, bovine spongiform encephalopathy, chronic wasting disease, transmissible mink encephalopathy and others9–11,13. Prion diseases are remarkable in that they can be genetic, infectious or sporadic in origin. Infectivity involves the transfer of seeds (prions) from one organism to another, whereas the genetic and idiopathic cases seem to develop endogenously, owing to the spontaneous misfolding and nucleation of PrP molecules into a self-propagating seed.
Pathologically, the prionotic brain is marked by spongiform degeneration, loss of neurons, gliosis and the accumulation of aggregated PrP14,15. Despite their similar molecular origins, the prion diseases can vary behaviourally and pathologically within and among species14. The diverse phenotypes are governed in part by the attributes of the affected organism, and in part by the strain-like functional diversity of prions, which in turn is thought to reflect distinct conformations of the misfolded PrP9,16–18. Prions exist in a range of sizes, but the most potent prions are relatively small, soluble assemblies19. Although molecular structure provides a framework for understanding the seeding of prions, there is evidence that prion propagation and prion toxicity are partly distinct20.
The essence of prion disease is a crystallization-like chain reaction by which malformed PrP seeds force naive PrP molecules into a similar pathogenic architecture21. Recent findings now suggest that this ‘prion paradigm’—the seeded corruption of otherwise harmless proteins—also underlies the ontogeny of a widening spectrum of maladies, including common age-related neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease. In many of these disorders, as in prion disease, the aggregating proteins form characteristic lesions generically known as amyloid (Fig. 1).
Prion-like properties of amyloidogenic proteins
‘Amyloid’ refers to multimeric proteinaceous assemblies with distinctive histochemical features in tissues, and a cross-β quaternary structure as determined by X-ray fibre diffraction analysis (Box 1). In each manifestation of amyloidosis a specific protein is involved, and more than 30 different amyloidogenic proteins have been linked to disease22,23. Some types of amyloidosis, such as Alzheimer’s disease and Parkinson’s disease, are localized to the brain, but others affect organs such as the kidney, liver, heart and spleen24.
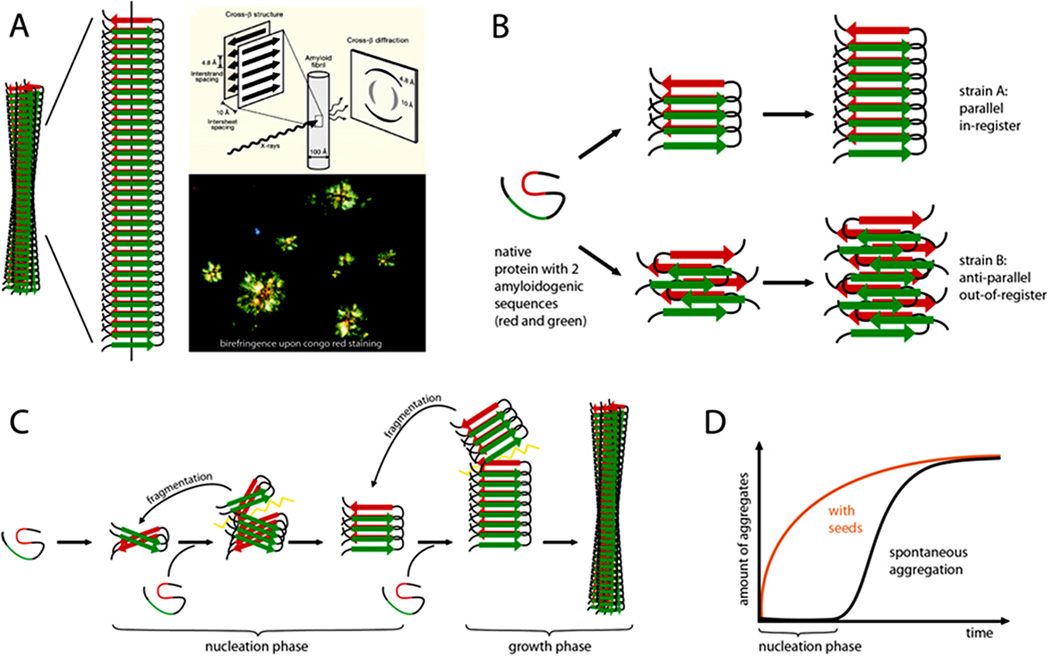
Box 1. The amyloid state of proteins as a framework to explain prion-like protein seeding.
A protein in the amyloid state forms bundles of twisted, unbranched filaments. Each filament is composed of sheets of β-strands (Panel a). These β-sheets run parallel to the filament axis, and the strands are nearly perpendicular to the long axis. This structural arrangement produces a distinctive, cross-β X-ray diffraction pattern that reflects the characteristic spacing between the β-sheets and the β-strands32. Biophysicists classify amyloid based on the X-ray diffraction pattern, whereas pathologists define amyloid as deposits of fibrillar protein in cells or tissues that show reddish/green birefringence under cross-polarized light after staining with the dye Congo red22. Part of panel a is modified, with permission, form ref. 32.
In the most common amyloids, the β-sheets consist of parallel β-strands that are hydrogen-bonded by their backbones. The sheet is ‘in-register’ when identical side chains are on the top of each other. The sheets are bonded to each other via amino acid side chains that are inter-digitated like a zipper. Amyloid ‘steric zippers’ can be formed from identical or different β-strands (homosteric versus heterosteric zippers). An amyloid-forming protein may contribute more than one β-strand segment to the cross-β amyloid backbone (spine)32.
At the molecular level, amyloids can be highly polymorphic; that is, a given β-strand segment is able to form a variety of distinct cross-β amyloid spines and filamentous structures (panel b). Such conformational variants are suggested to be the molecular basis of amyloid ‘strains’, that is, amyloids formed from a particular protein but with different biological activities16,32.
Amyloid formation (panel c) starts with a slow nucleation phase (the aggregation of the protein into a seed) that may go through a series of intermediate states until the initial segment of the amyloid spine is formed21,124. Monomers or oligomeric structures are then bonded to the ends of the initial amyloid seed by conformational conversion. With increasing length, and depending on the conformational stability of the amyloid spine, the growing fibril can eventually break, either spontaneously or actively through cellular processes. In this way, amyloid formation becomes self-propagating through the generation and spread of new amyloid seeds. The kinetics of amyloid fibril formation are a function of the rates of nucleation, growth, and fragmentation21,125. The lag time that precedes protein aggregation in vitro can be greatly shortened by the addition of pre-formed exogenous seeds (panel d).
A protein in the amyloid state (Box 1) is thermodynamically highly stable. Although all proteins have the potential to generate amyloid under the right conditions, protective mechanisms ensure that relatively few do so in living organisms25,26. In some cases, the amyloid state can be biologically useful in nature (‘functional amyloids’)27–31. However, in the amyloidoses the aggregation of proteins disrupts function either because the aggregates directly harm cells, tissues and organs (a gain of function), and/or because molecules sequestered within aggregates are unable to execute their required tasks24,32. Amyloidogenesis also involves the formation of intermediate assemblies known as oligomers and protofibrils, which can themselves be highly toxic33.
The molecular structure responsible for the toxicity of amyloid and amyloid intermediates is not fully understood (Box 1). Contemporary work suggests that a deviation from the energetically favourable and stable parallel and in-register β-sheet amyloid state is linked to toxicity34,35. Consistent with this concept, assemblies consisting of out-of-register β-sheets have been identified as toxic amyloid entities36. There is also evidence that the process of amyloid formation itself can be harmful to cells37. Like the prion protein9,17,28, other amyloidogenic proteins also can adopt conformationally distinct amyloid ‘strains’ (Box 1) with different biological activities and toxicities32,38,39.
In vivo conditions that promote amyloid formation are: increased concentration of the culpable protein; mutations that destabilize the native form and allow the amyloid-prone segments of a protein to interact with each other; exposure and/or de novo generation of amyloid-prone segments through cleavage of the native protein or aberrant translation; thermodynamically destabilizing conditions such as conducive pH or temperature; and/or a deterioration of cellular protein quality control (proteostasis), as occurs with advancing age3,32. However, a highly effective way to stimulate amyloid formation is by seeding naive amyloidogenic protein molecules with β-sheet-rich aggregates of a cognate protein21 (Box 1). Studies in experimental animals provide compelling evidence that some systemic amyloidoses can be induced in this way40–44. Moreover, recent research indicates that amyloidogenic proteins linked to various neurodegenerative diseases also exhibit prion-like seeded self-propagation in vivo and in vitro.
The prion-like properties of amyloid-β
The amyloid-β protein (Aβ) is a normal cleavage product of the Aβ precursor protein (APP)45. The physiological role of Aβ remains indeterminate, but it is now apparent that Aβ aggregation is a key player in Alzheimer’s disease, the most common cause of dementia46–48. The self-propagation of aggregates of Aβ was predicted decades ago from in vitro studies21 and from inoculation experiments with nonhuman primates49. However, it has only recently been established that Aβ can be induced to deposit in the living brain by a prion-like mechanism, as a result of experiments using genetically modified rodent models.
Rodent Aβ does not readily form amyloid in vivo; however, transgenic animal models expressing human APP generate Aβ plaques and cerebral Aβ-amyloid angiopathy (CAA) at predictable ages50. The intracerebral infusion of dilute Aβ-amyloid-rich brain extracts from Alzheimer’s disease patients or from aged APP-transgenic mice stimulates the premature formation of plaques and CAA in these models51,52. The pathogenic process is also inducible in transgenic rodents that express human-sequence Aβ but do not otherwise develop Aβ deposits within their normal lifespans53,54 (Box 2). The induction of Aβ deposition is dependent both on the concentration of Aβ seeds in the extract and on the production of human-sequence Aβ by the host brain52. Control brain extracts lacking aggregated Aβ do not induce Aβ deposition, and the denaturation of proteins or the selective removal of Aβ completely negates the ability of the extracts to seed Aβ aggregation52. Seeding of Aβ deposition also is abrogated by active or passive immunization of the mice against Aβ52.
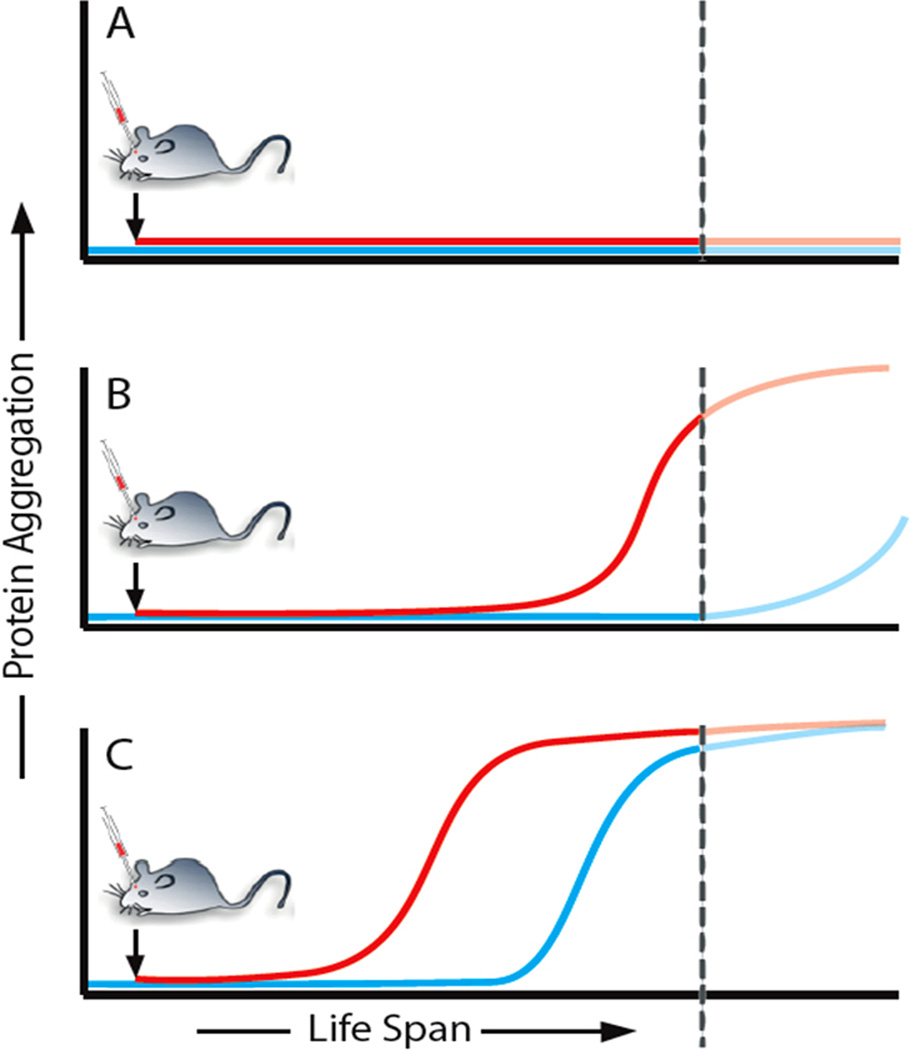
Box 2. Hypothetical model of seeded cerebral amyloid induction in mice.
An endogenous protein (panel a, blue) is not amyloidogenic under physiological conditions. In this case, the application of a seed (panel a, red) will not induce protein aggregation. A protein is amyloidogenic but does not aggregate during the lifespan of the mouse (which ends at the dotted line), either because endogenously formed seeds are removed by an effective proteostasis network, or because seed formation is inefficient and therefore unlikely to occur during the mouse’s lifetime (panel b, blue). However, with an appropriate seed (single inoculation), the onset of protein aggregation is advanced and occurs before the mouse reaches the end of its lifespan (panel b, red). A protein is highly amyloidogenic and typically aggregates with ageing of the mouse (panel c, blue). The addition of an appropriate seed advances the onset of protein aggregation (panel c, red).
Like prions, Aβ seeds range in size from small, soluble, protease-sensitive aggregates to large, insoluble, protease-resistant fibrils55. Aβ lesions in APP transgenic mice are inducible by injections of pure, synthetic human Aβ fibrils56, although (similar to recombinant prion protein57), the potency of synthetic Aβ seeds is less than that of Aβ aggregates formed within the living brain. The reasons for the relative ineffectiveness of synthetic Aβ seeds are not yet apparent; it may be that additional co-factors or chaperones must participate in their production (as has been shown for recombinant prions58), or that Aβ (like PrP) can fold into polymorphic and polyfunctional strains (Box 1)32,38,52,59. Indeed, strain-like variations in the morphology of Aβ lesions can be seeded in APP-transgenic mice by the injection of Aβ-laden brain extracts from different sources60.
Injection of Aβ-rich brain extract into one brain region eventually triggers deposits in axonally coupled regions61. Over time, widespread areas of the brain are involved, including neocortical and subcortical regions62, similar to areas affected in Alzheimer’s disease (Fig. 1). In addition, Aβ deposition is inducible in the brain by the injection of extract into the peritoneal cavity63. Taken together, these studies reinforce the hypothesis that the aggregation of Aβ can be seeded in susceptible hosts by a mechanism that closely resembles the molecular templating of prions.
The prion-like properties of tau and α-synuclein
Accumulating experimental data indicate that the seeding principle also applies to other pathogenic proteins, many of which form amyloid-like inclusions within cells. Tau is a cytoplasmic protein that normally helps to stabilize microtubules, but it becomes hyperphosphorylated and prone to aggregation (Fig. 1) in a variety of neurodegenerative conditions, including Alzheimer’s disease, frontotemporal lobar degeneration, progressive supranuclear palsy, corticobasal degeneration, chronic traumatic encephalopathy and others64,65. The intracerebral injection of brain extracts containing aggregated tau induces tauopathy in tau-transgenic host mice, and the induced tau lesions propagate systematically from the injection site to axonally connected areas, consistent with neuronal uptake, transport and release of tau seeds66. Unlike Aβ lesions, tauopathy can be seeded by exogenous tau aggregates in non-transgenic (wild-type) mice67,68, indicating that endogenous murine tau itself is amyloidogenic, even though wild-type mice do not develop tauopathy spontaneously (Box 2, panel b). Tau aggregation is also inducible by recombinant tau fibrils in cultured cells69 and in tau-transgenic mice70. As in the case of Aβ and prions19,55, tau seeds are of many sizes, and small, soluble assemblies are effective seeds67. Intracerebral injections of brain extracts from various human tauopathies have shown that tau lesions in mice can be induced to resemble those in the corresponding human diseases68. These results exemplify the conformation-dependent templated propagation of tau multimers, and evoke the hypothesis that specific tau conformers give rise to clinically distinct tauopathies, reminiscent of prion strains.
In α-synucleinopathies such as Parkinson’s disease and dementia with Lewy bodies, misfolded α-synuclein assembles into intracellular fibrillar inclusions called Lewy bodies (Fig. 1) and Lewy neurites71. Clues to the induction of these lesions in vivo emerged from the surprising discovery that some foetal dopaminergic neurons transplanted into the brains of Parkinson’s disease patients contain α-synuclein-positive Lewy bodies 11 to 16 years after transplant surgery72,73. Studies in vitro74–76 and in experimental animals74,75,77–79 suggest that the inductive agent probably consists of α-synuclein seeds formed within the host brain that transfer to the grafted neurons to induce α-synuclein aggregation. Consistent with such a transfer, intracerebral injection of brain extracts containing aggregated α-synuclein into young, α-synuclein-transgenic mice stimulates the formation of α-synuclein lesions in the host78,79; the lesions then emerge in anatomically linked regions of the brain79, suggesting a parallel with the apparent spread of α-synuclein deposits in the human brain (Fig. 1). Eventually, the mice develop progressive neurodegeneration, signs of Parkinson’s-disease-like motor dysfunction, and premature death78,79. Importantly, intracerebral injections of synthetic (human or mouse) α-synuclein fibrils (as well as autopsy-derived brain extracts from a case of Lewy body disease) also induce Lewy-body-like pathology and neuronal degeneration in non-transgenic (wild-type) host mice80,81. Finally, synthetic α-synuclein fibrils are able to induce tauopathy, possibly by a cross-seeding mechanism82. When synthetic fibrils with distinct proteinase-K cleavage sites are generated, these variant α-synuclein fibrils differ in their ability to stimulate tauopathy82. Differential proteinase-K cleavage of α-synuclein aggregates is also observed in the brains of Parkinson’s patients, indicative of alternative α-synuclein conformations82.
The growing family of prion-like proteins
Amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) are two representatives of a cluster of related brain disorders83,84. Genetic factors play a conspicuous role in the ontogeny of these diseases, which can result from mutations involving genes coding for tau, superoxide dismutase-1 (SOD1), TAR DNA-binding protein 43 (TDP-43), fused in sarcoma (FUS), C9orf72, heterogeneous nuclear ribonucleoproteins (hnRNPs) and others83–86. A commonality among these proteins is their susceptibility to aggregation, particularly in the context of ribonucleoprotein granules that form in cells as a result of stress. Several of these proteins include aggregation-prone stretches of amino acids known as ‘prion-like domains’, which occur in a significant subgroup of RNA-binding proteins87. Under stressful conditions, the proteins form an amyloid-like cross-β sheet conformation that enables them to recruit and structurally convert like molecules, in which state their function is blocked88. In some instances, the aggregating proteins trap and thereby disable other proteins as well89. Importantly, ribonucleoprotein granules can be disassembled when conditions are more favourable for the cell88. However, the amyloid-like cross-β sheet conformations can also convert into stable amyloid conformations either naturally or by disease-linked mutations in the prion domains, as shown for hnRNPs86 and probably also for TDP-43 (ref. 88). Indeed, TDP-43 inclusions in sporadic ALS appear in a sequential pattern consistent with the hypothesis of seeded propagation (Fig. 1). Although it has not been shown in vivo, a prion-like cell-to-cell propagation of TDP-43 and SOD1 aggregates has been demonstrated in cell cultures90–92, supporting the hypothesis that the prion paradigm may also apply to diseases in the ALS–FTLD spectrum93.
Finally, it is worth noting that diseases involving prion-like seeding mechanisms are likely to expand beyond the classical amyloidoses and neurodegenerative disorders. The tumour suppressor protein p53 acts mainly in the nucleus to prevent cells from dividing uncontrollably, and in many human cancers p53 function is diminished94. Interestingly, p53 can aggregate into amyloid-like assemblies that seed the further aggregation and mislocalization of the protein; in this ectopic state, it is thought that p53 loses its ability to suppress cell proliferation, thus promoting malignant growth95,96.
The spectre of infectivity
A defining feature of prions is their infectivity9; that is, their capacity to cause disease when transferred from an affected individual to a naive recipient. The ease with which prion disease is transmissible from animal to animal under ordinary circumstances varies; natural infection by prions is facile in some nonhuman prionoses, such as chronic wasting disease in cervids, and scrapie in sheep97,98. However, in contemporary humans exogenous transmission of prion disease is rare, totalling approximately 2,700 documented cases of kuru among the Fore people of Papua New Guinea between 1957 and 2004 (ref. 99) and approximately 700 known cases of CJD as of 2012 (ref. 100). Most instances of human transmission have involved unusual routes of exposure such as ritual cannibalism (for kuru), treatment with contaminated growth hormone extracted from human pituitaries, or the use of contaminated dura mater in neurosurgical procedures. With the cessation of cannibalism among the Fore in the 1950s, recognition of the prion as the disease agent, and implementation of preventive measures, the transmission of prion disease to humans has virtually ceased99,100.
We do not yet know why some proteopathies can move from animal to animal whereas others do not61,101. To become infectious under natural conditions, pathogenic seeds must exit the body and travel intact to another organism, where they resume replication. During this journey, the seeds must resist destruction and overcome a number of biological and physical barriers. Prions thus owe their infectivity, at least in part, to their durability, their replication over multiple serial passages from one host to another, and to attributes of the host that either promote or restrain transmission9–11. The precise structure of the infectious PrP assembly has not yet been solved, although most data point to a β-sheet-rich amyloid-like conformation34. It is conceivable that the infectivity of prions results from a particular molecular architecture of PrP (Box 1). A comparison of prions with other self-propagating protein assemblies, including systemic amyloids with transmissible properties40,43, could furnish clues to the features that determine whether or not a proteopathic agent is infectious under everyday circumstances.
Therapeutic implications
Proteins that aggregate in disparate neurodegenerative disorders share with prions the molecular properties of nucleation, templating, growth, multiplication and spread. Each of these phenomena presents potential therapeutic targets. For example, reducing the production or stimulating the removal of amyloidogenic proteins will lower the concentration of the protein and thereby impede nucleation and growth32. Another strategy is to stabilize the native conformation of an amyloidogenic protein, an approach that has been pioneered to treat transthyretin amyloidosis102. Stabilizing existing aggregates also could have therapeutic value, by hindering their fragmentation and thus the multiplication of seeds. Another option may be to exploit structural elements of the cross-β sheet to prevent the corruptive templating of cognate proteins. This strategy is particularly promising when the atomic structure of the seed is known, and interfering agents can therefore be designed to bind distinct amyloid conformations103.
The progressive march of symptoms in neurodegenerative diseases has been proposed to involve the systematic advance of a pathogen along neuronal pathways5–8, 61, 71, 104, 105. Imaging studies confirm that the differential vulnerability of brain regions to neurodegenerative changes is correlated with the strength of neuronal connections among the affected areas106–109. These patterns could reflect the trafficking of proteopathic seeds among interconnected brain regions. The discharge of Aβ110,111 and tau112 into the extracellular space is regulated by neuronal activity. Accordingly, the progression of disease may be abrogated either by arresting seeds as they travel between cells113, or by targeting the cellular processes of release, uptake and transport77,92,114–121.
Finally, a hallmark of chronic diseases is a long silent phase of pathogenesis preceding the onset of symptoms122. In Alzheimer’s disease, the pathological cascade is thought to begin 10 to 20 years or more before the first clinical symptoms appear47,48. Thus, in addition to being therapeutic targets, small, soluble proteinaceous seeds in bodily fluids could serve as early biomarkers for pre-symptomatic disease.
Perspectives
The theoretical and practical implications of the prion paradigm hinge on the resolution of a number of issues. Perhaps the most pressing need is a precise description of the molecular structure of the amyloidogenic seeds. The physicochemical and cellular conditions that promote the formation, growth and proliferation of seeds in vivo also are still ambiguous. Another question is whether the clinical and pathological diversity of neurodegenerative diseases reflects the strain-like structural diversity of the aggregates in vivo. In addition, different proteopathies often coincide in the ageing brain, and it remains uncertain whether these diseases result from independent pathologic processes, a coincidental response to a common instigator, or from the ‘cross-seeding’ of one type of aggregated protein by another. At present, it seems unlikely that non-prion neurodegenerative diseases are infectious under ordinary circumstances61,101, but further epidemiological studies are warranted, particularly with regard to uncommon routes of exposure61.
After decades of controversy, the prion now is widely accepted as an unorthodox, but formidable, agent of disease. On the margins of the prion debate has long been the notion that prion-like processes might drive the misfolding and aggregation of proteins involved in other diseases9,123. Recent experimental work supports this hypothesis. By establishing seeded protein aggregation as a cardinal pathogenic principle, the prion paradigm stands to consolidate and focus treatment strategies for a broad spectrum of diseases.
Acknowledgements
We thank D. Eisenberg, H. LeVine, A. Aguzzi, J. Collinge, R. Rosen, Y. Eisele, A. Mehta, M. Gearing, J. Manson, M. Neumann, and the members of our laboratories for critical discussions and comments. The help of H. Braak with Fig. 1, and the help of S. Eberle with the manuscript and figures is gratefully acknowledged. This work was supported by grants from the Competence Network on Degenerative Dementias (BMBF-01GI0705), ALZKULT (BMBF-031A198A), NGFN2 (BMBF-01GS08131), and anonymous foundations (to M.J.), and by National Institutes of Health grants R21AG040589, P51RR165, P51OD11132, and the CART Foundation (to L.C.W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions M.J. and L.C.W. contributed to the writing of the review
The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
References
- 1.Uversky VN, Dunker AK. The case for intrinsically disordered proteins playing contributory roles in molecular recognition without a stable 3D structure. F1000 Biol. Rep. 2013;5:1. doi: 10.3410/B5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malinovska L, Kroschwald S, Alberti S. Protein disorder, prion propensities, and self-organizing macromolecular collectives. Biochim. Biophys. Acta. 2013;1834:918–931. doi: 10.1016/j.bbapap.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 4.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 5.Thal DR, Rub U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 8.Brettschneider J, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013 doi: 10.1002/ana.23937. http://dx.doi.org/10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prusiner SB. Prions. Proc. Natl Acad. Sci. USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu. Rev. Neurosci. 2001;24:519–550. doi: 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- 11.Aguzzi A, Calella AM. Prions: protein aggregation and infectious diseases. Physiol. Rev. 2009;89:1105–1152. doi: 10.1152/physrev.00006.2009. [DOI] [PubMed] [Google Scholar]
- 12.Caughey B, Baron GS, Chesebro B, Jeffrey M. Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions. Annu. Rev. Biochem. 2009;78:177–204. doi: 10.1146/annurev.biochem.78.082907.145410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Head MW, Ironside JW. Review: Creutzfeldt–Jakob disease: prion protein type, disease phenotype and agent strain. Neuropathol. Appl. Neurobiol. 2012;38:296–310. doi: 10.1111/j.1365-2990.2012.01265.x. [DOI] [PubMed] [Google Scholar]
- 14.DeArmond SJ, Prusiner SB. Prion protein transgenes and the neuropathology in prion diseases. Brain Pathol. 1995;5:77–89. doi: 10.1111/j.1750-3639.1995.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 15.Wadsworth JD, Collinge J. Molecular pathology of human prion disease. Acta Neuropathol. 2011;121:69–77. doi: 10.1007/s00401-010-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M, Collins SR, Toyama BH, Weissman JS. The physical basis of how prion conformations determine strain phenotypes. Nature. 2006;442:585–589. doi: 10.1038/nature04922. [DOI] [PubMed] [Google Scholar]
- 17.Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–936. doi: 10.1126/science.1138718. [DOI] [PubMed] [Google Scholar]
- 18.Colby DW, Prusiner SB. Prions. Cold Spring Harb. Perspect. Biol. 2011;3:a006833. doi: 10.1101/cshperspect.a006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silveira JR, et al. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandberg MK, Al-Doujaily H, Sharps B, Clarke AR, Collinge J. Prion propagation and toxicityin vivooccur in two distinct mechanistic phases. Nature. 2011;470:540–542. doi: 10.1038/nature09768. [DOI] [PubMed] [Google Scholar]
- 21.Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 22.Sipe JD, et al. Amyloid fibril protein nomenclature: 2012 recommendations from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2012;19:167–170. doi: 10.3109/13506129.2012.734345. [DOI] [PubMed] [Google Scholar]
- 23.Buxbaum JN, Linke RP. A molecular history of the amyloidoses. J. Mol. Biol. 2012;421:142–159. doi: 10.1016/j.jmb.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Blancas-Mejía LM, Ramirez-Alvarado M. Systemic amyloidoses. Annu. Rev. Biochem. 2013 doi: 10.1146/annurev-biochem-072611-130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem. Sci. 1999;24:329–332. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 26.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 27.Maji SK, et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwald J, Riek R. Biology of amyloid: structure, function, and regulation. Structure. 2010;18:1244–1260. doi: 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Hou F, et al. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanco LP, Evans ML, Smith DR, Badtke MP, Chapman MR. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 2012;20:66–73. doi: 10.1016/j.tim.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wickner RB, et al. Amyloids and yeast prion biology. Biochemistry. 2013;52:1514–1527. doi: 10.1021/bi301686a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harbor Perspect. Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tycko R, Wickner RB. Molecular structures of amyloid and prion fibrils: consensus versus controversy. Acc. Chem. Res. 2013;46:1487–1496. doi: 10.1021/ar300282r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C, et al. Out-of-register β-sheets suggest a pathway to toxic amyloid aggregates. Proc. Natl Acad. Sci. USA. 2012;109:20913–20918. doi: 10.1073/pnas.1218792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laganowsky A, et al. Atomic view of a toxic amyloid small oligomer. Science. 2012;335:1228–1231. doi: 10.1126/science.1213151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jan A, et al. Aβ42 neurotoxicity is mediated by ongoing nucleated polymerization process rather than by discrete Aβ42 species. J. Biol. Chem. 2011;286:8585–8596. doi: 10.1074/jbc.M110.172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petkova AT, et al. Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 39.Toyama BH, Weissman JS. Amyloid structure: conformational diversity and consequences. Annu. Rev. Biochem. 2011;80:557–585. doi: 10.1146/annurev-biochem-090908-120656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westermark GT, Westermark P. Prion-like aggregates: infectious agents in human disease. Trends Mol. Med. 2010;16:501–507. doi: 10.1016/j.molmed.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Yan J, et al. Cross-seeding and cross-competition in mouse apolipoprotein A-II amyloid fibrils and protein A amyloid fibrils. Am. J. Pathol. 2007;171:172–180. doi: 10.2353/ajpath.2007.060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xing Y, et al. Transmission of mouse senile amyloidosis. Lab. Invest. 2001;81:493–499. doi: 10.1038/labinvest.3780257. [DOI] [PubMed] [Google Scholar]
- 43.Zhang B, et al. Fecal transmission of AA amyloidosis in the cheetah contributes to high incidence of disease. Proc. Natl Acad. Sci. USA. 2008;105:7263–7268. doi: 10.1073/pnas.0800367105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korenaga T, et al. Transmission of amyloidosis in offspring of mice with AApoAII amyloidosis. Am. J. Pathol. 2006;168:898–906. doi: 10.2353/ajpath.2006.050350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol. Rev. 2010;90:465–494. doi: 10.1152/physrev.00023.2009. [DOI] [PubMed] [Google Scholar]
- 46.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci. Transl. Med. 2011;3:77s71. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bateman RJ, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N. Engl. J. Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villemagne VL, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 49.Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Evidence for the experimental transmission of cerebral β-amyloidosis to primates. Int. J. Exp. Pathol. 1993;74:441–454. [PMC free article] [PubMed] [Google Scholar]
- 50.Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nature Med. 2010;16:1210–1214. doi: 10.1038/nm.2224. [DOI] [PubMed] [Google Scholar]
- 51.Kane MD, et al. Evidence for seeding of β-amyloid by intracerebral infusion of Alzheimer brain extracts in β-amyloid precursor protein-transgenic mice. J. Neurosci. 2000;20:3606–3611. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer-Luehmann M, et al. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 53.Morales R, Duran-Aniotz C, Castilla J, Estrada LD, Soto C. De novoinduction of amyloid-β depositionin vivo. . Mol. Psychiatry. 2012;17:1347–1353. doi: 10.1038/mp.2011.120. [DOI] [PubMed] [Google Scholar]
- 54.Rosen RF, et al. Exogenous seeding of cerebral β-amyloid deposition in βAPP-transgenic rats. J. Neurochem. 2012;120:660–666. doi: 10.1111/j.1471-4159.2011.07551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langer F, et al. Soluble Aβ seeds are potent inducers of cerebral β-amyloid deposition. J. Neurosci. 2011;31:14488–14495. doi: 10.1523/JNEUROSCI.3088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stöhr J, et al. Purified and synthetic Alzheimer's amyloid beta (Aβ) prions. Proc. Natl Acad. Sci. USA. 2012;109:11025–11030. doi: 10.1073/pnas.1206555109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Legname G, et al. Synthetic mammalian prions. Science. 2004;305:673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 58.Wang F, Wang X, Yuan CG, Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levine H, III, Walker LC. Molecular polymorphism of Aβ in Alzheimer's disease. Neurobiol. Aging. 2010;31:542–548. doi: 10.1016/j.neurobiolaging.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heilbronner G, et al. Seeded strain-like transmission of beta-amyloid morphotypes in APP transgenic mice. 2013 Sep 3; doi: 10.1038/embor.2013.137. http://dx.doi.org/10.1038/embor.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamaguchi T, et al. The presence of Aβ seeds, and not age per se, is critical to the initiation of Aβ deposition in the brain. Acta Neuropathol. 2012;123:31–37. doi: 10.1007/s00401-011-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eisele YS, et al. Peripherally applied Aβ-containing inoculates induce cerebral β-amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 65.Goldstein LE, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Science Transl. Med. 2012;4:134ra160. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clavaguera F, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nature Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lasagna-Reeves CA, et al. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Scientific Rep. 2012;2:700. doi: 10.1038/srep00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clavaguera F, et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl Acad. Sci. USA. 2013;110:9535–9540. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo JL, Lee VM. Neurofibrillary tangle-like tau pathology induced by synthetic tau fibrils in primary neurons over-expressing mutant tau. FEBS Lett. 2013;587:717–723. doi: 10.1016/j.febslet.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iba M, et al. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J. Neurosci. 2013;33:1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nature Rev. Neurology. 2013;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 72.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nature Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 73.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nature Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 74.Hansen C, et al. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kordower JH, et al. Transfer of host-derived α synuclein to grafted dopaminergic neurons in rat. Neurobiol. Dis. 2011;43:552–557. doi: 10.1016/j.nbd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Volpicelli-Daley LA, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl Acad. Sci. USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mougenot AL, et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol. Aging. 2012;33:2225–2228. doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 79.Luk KC, et al. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 2012;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luk KC, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Masuda-Suzukake M, et al. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136:1178–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo JL, et al. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013;154:103–117. doi: 10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Langenhove T, van der Zee J, Van Broeckhoven C. The molecular basis of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum. Ann. Med. 2012;44:817–828. doi: 10.3109/07853890.2012.665471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nature Rev. Neurol. 2012;8:423–434. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cruts M, Gijselinck I, Van Langenhove T, van der Zee J, Van Broeckhoven C. Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci. 2013;36:450–459. doi: 10.1016/j.tins.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 86.Kim HJ, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495:467–473. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.King OD, Gitler AD, Shorter J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 2012;1462:61–80. doi: 10.1016/j.brainres.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li YR, King OD, Shorter J, Gitler AD. Stress granules as crucibles of ALS pathogenesis. J. Cell Biol. 2013;201:361–372. doi: 10.1083/jcb.201302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Olzscha H, et al. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell. 2011;144:67–78. doi: 10.1016/j.cell.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 90.Furukawa Y, Kaneko K, Watanabe S, Yamanaka K, Nukina N. A seeding reaction recapitulates intracellular formation of Sarkosyl-insoluble transactivation response element (TAR) DNA-binding protein-43 inclusions. J. Biol. Chem. 2011;286:18664–18672. doi: 10.1074/jbc.M111.231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Münch C, O'Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc. Natl Acad. Sci. USA. 2011;108:3548–3553. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grad LI, et al. Intermolecular transmission of superoxide dismutase 1 misfolding in living cells. Proc. Natl Acad. Sci. USA. 2011;108:16398–16403. doi: 10.1073/pnas.1102645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Polymenidou M, Cleveland DW. The seeds of neurodegeneration: prion-like spreading in ALS. Cell. 2011;147:498–508. doi: 10.1016/j.cell.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 95.Xu J, et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nature Chem. Biol. 2011;7:285–295. doi: 10.1038/nchembio.546. [DOI] [PubMed] [Google Scholar]
- 96.Ano Bom AP, et al. Mutant p53 aggregates into prion-like amyloid oligomers and fibrils: implications for cancer. J. Biol. Chem. 2012;287:28152–28162. doi: 10.1074/jbc.M112.340638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sigurdson CJ, Aguzzi A. Chronic wasting disease. Biochim. Biophys. Acta. 2007;1772:610–618. doi: 10.1016/j.bbadis.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoinville LJ. A review of the epidemiology of scrapie in sheep. Rev. Sci. Tech. 1996;15:827–852. doi: 10.20506/rst.15.3.959. [DOI] [PubMed] [Google Scholar]
- 99.Collinge J, et al. Kuru in the 21st century—an acquired human prion disease with very long incubation periods. Lancet. 2006;367:2068–2074. doi: 10.1016/S0140-6736(06)68930-7. [DOI] [PubMed] [Google Scholar]
- 100.Brown P, et al. Iatrogenic Creutzfeldt–Jakob disease, final assessment. Emerg. Infect. Dis. 2012;18:901–907. doi: 10.3201/eid1806.120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Irwin DJ, et al. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol. 2013;70:462–468. doi: 10.1001/jamaneurol.2013.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnson SM, Connelly S, Fearns C, Powers ET, Kelly JW. The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug. J. Mol. Biol. 2012;421:185–203. doi: 10.1016/j.jmb.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sievers SA, et al. Structure-based design of non-natural amino-acid inhibitors of amyloid fibril formation. Nature. 2011;475:96–100. doi: 10.1038/nature10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saper CB, Wainer BH, German DC. Axonal and transneuronal transport in the transmission of neurological disease: potential role in system degenerations, including Alzheimer's disease. Neuroscience. 1987;23:389–398. doi: 10.1016/0306-4522(87)90063-7. [DOI] [PubMed] [Google Scholar]
- 105.Ravits JM, La Spada AR. ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology. 2009;73:805–811. doi: 10.1212/WNL.0b013e3181b6bbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eidelberg D, Surmeier DJ. Brain networks in Huntington disease. J. Clin. Invest. 2011;121:484–492. doi: 10.1172/JCI45646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–1227. doi: 10.1016/j.neuron.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raj A, Kuceyeski A, Weiner M. A network diffusion model of disease progression in dementia. Neuron. 2012;73:1204–1215. doi: 10.1016/j.neuron.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gardner RC, et al. Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann. Neurol. 2013;73:603–616. doi: 10.1002/ana.23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bero AW, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nature Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dolev I, et al. Spike bursts increase amyloid-β 40/42 ratio by inducing a presenilin-1 conformational change. Nature Neurosci. 2013;16:587–595. doi: 10.1038/nn.3376. [DOI] [PubMed] [Google Scholar]
- 112.Pooler AM, Phillips EC, Lau DH, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013;14:389–394. doi: 10.1038/embor.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bae EJ, et al. Antibody-aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J. Neurosci. 2012;32:13454–13469. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Polymenidou M, Cleveland DW. Prion-like spread of protein aggregates in neurodegeneration. J. Exp. Med. 2012;209:889–893. doi: 10.1084/jem.20120741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Holmes BB, Diamond MI. Cellular mechanisms of protein aggregate propagation. Curr. Opin. Neurol. 2012;25:721–726. doi: 10.1097/WCO.0b013e32835a3ee0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu JW, et al. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J. Biol. Chem. 2013;288:1856–1870. doi: 10.1074/jbc.M112.394528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:1856–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 118.Nath S, et al. Spreading of neurodegenerative pathology via neuron-to-neuron transmission of β-amyloid. J. Neurosci. 2012;32:8767–8777. doi: 10.1523/JNEUROSCI.0615-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem. 2012;287:19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Freundt EC, et al. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Ann. Neurol. 2012;72:517–524. doi: 10.1002/ana.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ren PH, et al. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nature Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Selkoe DJ. Resolving controversies on the path to Alzheimer's therapeutics. Nature Med. 2011;17:1060–1065. doi: 10.1038/nm.2460. [DOI] [PubMed] [Google Scholar]
- 123.Gajdusek DC. Spontaneous generation of infectious nucleating amyloids in the transmissible and nontransmissible cerebral amyloidoses. Mol. Neurobiol. 1994;8:1–13. doi: 10.1007/BF02778003. [DOI] [PubMed] [Google Scholar]
- 124.Lee J, Culyba EK, Powers ET, Kelly JW. Amyloid-β forms fibrils by nucleated conformational conversion of oligomers. Nature Chem. Biol. 2011;7:602–609. doi: 10.1038/nchembio.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Knowles TP, Buehler MJ. Nanomechanics of functional and pathological amyloid materials. Nature Nanotechnol. 2011;6:469–479. doi: 10.1038/nnano.2011.102. [DOI] [PubMed] [Google Scholar]