Abstract
Introduction
Carboxyamido-triazole (CAI) is a synthetic inhibitor of non-voltage-gated calcium channels that reversibly inhibits angiogenesis, tumor cell proliferation, and metastatic potential. This study examined the efficacy, safety and pharmacokinetics of oral CAI in the treatment of patients with newly diagnosed glioblastoma multiforme (GBM) in an open-label, single arm non-randomized phase 2 trial.
Methods
Eligible patients with histologically confirmed GBM started CAI therapy on the first day of radiation (6000 cGy in 30 fractions) and continued until progression, unless side effects became intolerable. The primary outcome was survival compared to historical controls within the NABTT CNS Consortium database. Secondary outcomes included toxicity and pharmacokinetic parameters.
Results
Fifty-five patients were enrolled with a median Karnofsky performance status of 90 and age of 56 years. Forty-six (84%) of these patients had debulking surgeries and 52 have died. The median survival was 10.3 months (95% confidence interval (CI), 8.5–12.8) compared to 12.1 months (95% CI, 10.3–13.3) in the NABTT reference group (p=0.97). Significant toxicities included 2 incidents of reversible vision loss. The mean CAI plasma concentration for patients taking enzyme inducing antiepileptic drugs (EIAED) was 1.35 ±1.22 compared to 4.06 ± 1.50 (p<0.001) for subjects not taking these agents. Overall survival and grade ≥ 3 toxicities were comparable by EIAED status.
Conclusions
This study demonstrated that 1) CAI can be administered safely with concomitant cranial irradiation, 2) the pharmacokinetics of CAI are significantly affected by co-administration of EIAED, and 3) the survival of patients with newly diagnosed GBM was not improved with this novel agent, despite achieving adequate drug levels.
Keywords: CAI, Phase II, angiogenesis, GBM
Introduction
Carboxyamido-triazole (CAI)2 is an agent initially identified in a tumor migration assay as an inhibitor of receptor-mediated calcium influx [1]. It appears to function by inhibiting a G-protein common to the signal transduction machinery of receptor-mediated events [2,3]. CAI inhibits tumor cell migration and angiogenesis in vitro [4] and arrests growth and metastasis of transplanted human melanoma and ovarian cancer xenografts [3]. Recent studies with inhibitors of angiogenesis in animal models have suggested efficacy, with synergy demonstrated with concurrent therapy with external beam radiation [5]. Some studies have indicated that this synergistic activity may require only brief exposure to angio-inhibitory agents, limited to the duration of delivery of radiation therapy (RT) [6].
Two phase 1 clinical trials with CAI in systemic cancer have been published in conjunction with pharmacokinetics [7,8]. In the study by Kohn and colleagues, chronic oral daily dosing of CAI in 49 patients resulted in stable serum concentrations in the range known to inhibit calcium signaling with no evidence of drug accumulation. Toxicity was typically mild. Although no complete or partial responses were seen, 49% of patients had stabilization of their disease. CAI pharmacokinetics were described by a two-compartment open linear model with no evidence of saturable elimination. Grade 1 and 2 gastrointestinal side effects were seen in up to 50% of patients. Dose limiting toxicities, consisting of reversible grade 2/3 cerebellar ataxia in one patient and confusion in one patient, were observed in patients treated at 350 mg/m2/day. Given the critical importance of tumor invasion and angiogenesis in gliomas and the inevitable malignant progression seen despite conventional therapy, a phase 2 trial of CAI was undertaken in this population.
Methods
Eligibility Criteria
Patients had histologically confirmed supratentorial grade 4 astrocytoma (GBM) untreated except for biopsy or surgery, and corticosteroids. They must have recovered from the immediate post-operative period, and if taking steroids, be maintained on a stable dose for at least five days. Other eligibility criteria included: age ≥ 18 years, Karnofsky performance status (KPS) ≥ 60, estimated life expectancy > 2 months, absolute neutrophil count > 1500/mm3, platelet count > 100,000 mm3, hemoglobin > 9.0 g/dl, creatinine < 1.7 mg/dl, bilirubin < 1.2 mg/dl, transaminases < 2 times above the upper limit of normal. Exclusion criteria included intercurrent illness that might interfere with protocol treatment, and concurrent malignancy unless disease-free > 5 years. Women of child bearing potential were required to have a negative serum beta human chorionic gonadotropin pregnancy test, and agreed not to breast feed. Males and females were required to use a standard contraception regimen. Each patient signed informed consent before participating in the study. This study was approved by the National Cancer Institute’s Clinical Trials Evaluation Program and the Institutional Review Board of each participating institution.
Treatment Plan
Treatment with micronized CAI, 250 mg po daily, was started on the first day of conventional fractionated external beam radiation therapy (6000 cGy in 30 fractions) and continued daily until progression unless side effects became intolerable. The induction cycle was 10 weeks and followed by maintenance cycles of 4 weeks each. No additional standard or experimental chemotherapy was permitted to be given with the radiation and CAI.
Pharmacology
During the first week of therapy, serum CAI samples were obtained prior to administration and then daily for the first 4 days. A single sample was then obtained once every 7 ± 1 days for 9 weeks. Thereafter, a sample was collected once every 56 ± 1 days until the patient was removed from the study. Blood specimens (7 mL) were collected in heparinized tubes and promptly centrifuged. Plasma CAI concentrations were determined using a previously published method [9]. Briefly, internal standard was added to 1 mL thawed patient samples and standards. The drug and internal standard were extracted using Bond Elute SPE columns. The eluant was evaporated to dryness and reconstituted in mobile phase and transferred to glass injector vials. The reconstituted sample was injected into a mobile phase consisting of acetonitrile with 0.01M ammonium acetate: water with 0.01M ammonium acetate (pH=6.92) (50:50) at a flow rate of 1.0 ml/min. One Waters Nova-Pak® C18 (3.9 × 300 mm) column (Waters Corp., Milford, MA ) and a Waters Nova-Pak® C-18 pre-column was used to separate the compounds of interest. Detection was accomplished using diode-array detector (CAI 264 nm and Harmine at 323 nm). Mean CAI plasma concentration was obtained by averaging the observed CAI plasma concentrations from week 2 through the end of the induction therapy. After the pharmacokinetic results were known, a post hoc analysis of survival by enzyme inducing anticonvulsants (EIAED) status was conducted for patients in this trial.
Statistical Considerations
The primary endpoint of this study was survival. The primary efficacy analysis included all accrued patients, and analyses were intention-to-treat. Overall survival time was calculated as time from histological diagnosis until death from any cause. Event times were censored if patient was alive at time of last follow-up. The failure rate was calculated as the number of deaths divided by the total exposure (follow-up time). Survival distributions were estimated using the product limit method [10], and compared using the log-rank test [11].
In addition, patient characteristics and survival of the patients treated on this trial were compared to patients treated on similar trials of therapies administered concurrently with RT for newly diagnosed GBM by the New Approaches to Brain Tumor Therapy (NABTT) CNS Consortium. To control for the effects of prognostic factors on survival, adjusted risk ratios were calculated using the proportional hazards regression model [12]. These prognostic factors included age, KPS, and extent of surgical resection coded as craniotomy for resection or biopsy.
The sample size of 54 was chosen to provide sufficient events over two years of study to have 78% power to find a 30% reduction in the hazard rate compared to the NABTT historical database to be statistically significant with a one-sided 0.10 alpha-level test. No interim analyses were planned or conducted.
Secondary endpoints included toxicity and correlations of pharmacokinetic parameters with toxicity and drug activity. Toxicities are reported as frequencies and percents.
Differences in patient characteristics between groups were tested for statistical significance using chi-square and Student t tests. Confidence intervals (CI) were calculated using standard methods. SAS software versions 8 and 9 (SAS Institute, Cary, NC) were used to perform analyses. All reported P-values are two-sided.
Results
Between March and October of 2000, 55 patients with histologically confirmed newly diagnosed GBM were accrued to this trial at 8 NABTT CNS Consortium institutions. Baseline demographic and clinical characteristics of the study patients and the reference group are reported in Table 1. The reference group consisted of 164 patients enrolled in four other NABTT trials of therapies concurrent with RT that have been found to be non-efficacious for patients with newly diagnosed GBM. The trials in the reference group had very similar eligibility criteria and accrual took place at many of the same sites as the present study [13,14,15,16].
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | CAI (n=55) | NABTT Reference (n=164) |
|---|---|---|
| Sex, % male | 60 | 68 |
| Race, % white | 95 | 96 |
| Median age (range), years | 59.0 (31.0 – 79.4) | 54.9 (28.4 – 78.8) |
| Median Karnofsky performance status (range) | 90 (60–100) | 90 (60–100) |
| Surgery type, % resection | 87 | 82 |
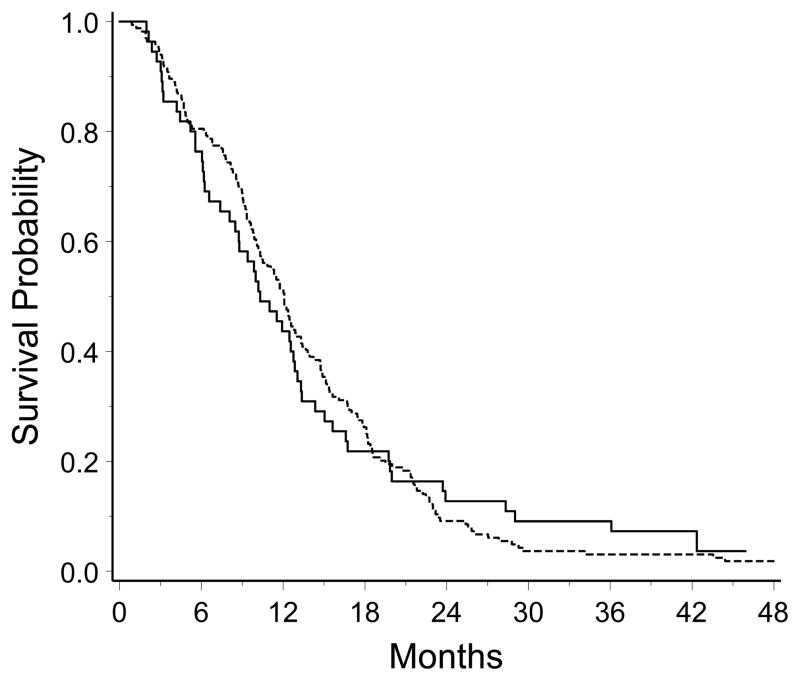
Fifty-two of the 55 patients accrued to this study have died. The three surviving patients have been followed for more than 38 months. The 12-month survival rate was 44% (95% CI, 30% to 58%). Median survival was 10.3 months (95% CI, 8.5 to 12.8). In the reference group, 163 of the 164 patients have died. Follow-up time for the surviving patient is 60 months. The hazard rates for the CAI patients and the reference group are in Table 2. Median survival for the reference group was 12.1 months (95% CI, 10.3 to 13.3). Kaplan-Meier survival curves for the CAI patients and the NABTT reference group are depicted in Figure 1. There was no difference in overall survival for the patients treated with CAI compared to the reference group (log-rank, P = 0.97; estimated hazard ratio = 0.99). Adjusting for age, KPS and extent of surgical resection, which are known prognosticators of survival in this patient population, the hazard ratio for patients in the CAI study compared to those in NABTT trials of other agents administered concurrently with RT was similar to the unadjusted risk ratio (estimated hazard ratio = 1.03, P = 0.87)
Table II.
Event rates and 95% confidence intervals
| Cohort | N | Deaths | Person-years of follow-up | Event Ratea | 95% CI of Event Rate |
|---|---|---|---|---|---|
| CAI | 55 | 52 | 61.1 | 0.85 | 0.65–1.12 |
| NABTT reference | 164 | 163 | 189.1 | 0.86 | 0.74–1.01 |
Deaths per person-year.
Figure 1.
Kaplan-Meier survival curves for the 55 patients in the CAI study (solid line) and the 164 patients in the NABTT reference group (broken line). The curve for the reference group is truncated at 48 months, though 3 patients had longer survival.
CAI was generally well tolerated. Patients were treated for a median time of 2 months (23 days to 46 months). The percent of patients experiencing toxicities are listed in Table 3. Twenty-nine percent of patients (16/55) experienced grade 3 or greater toxicity considered to be at least possibly related to CAI treatment. There were two instances of transient visual loss (one near complete), which were reversible upon drug withdrawal. Following re-introduction of drug one patient had recurrence of visual loss, but it subsequently improved at reduced dose. Other major toxicities were single instances of febrile neutropenia, hemorrhage (fatal), hepatotoxicity and hyperuricemia. The most frequent grade 3 or 4 toxicity reported was fatigue.
Table III.
Common (> 1 occurrence) treatment-related toxicity ≥ grade 3
| Adverse Event | N (%) |
|---|---|
| Dyspnea | 2 (4) |
| Fatigue | 5 (9) |
| Anemia | 2 (4) |
| Reversible Vision Loss | 2 (4) |
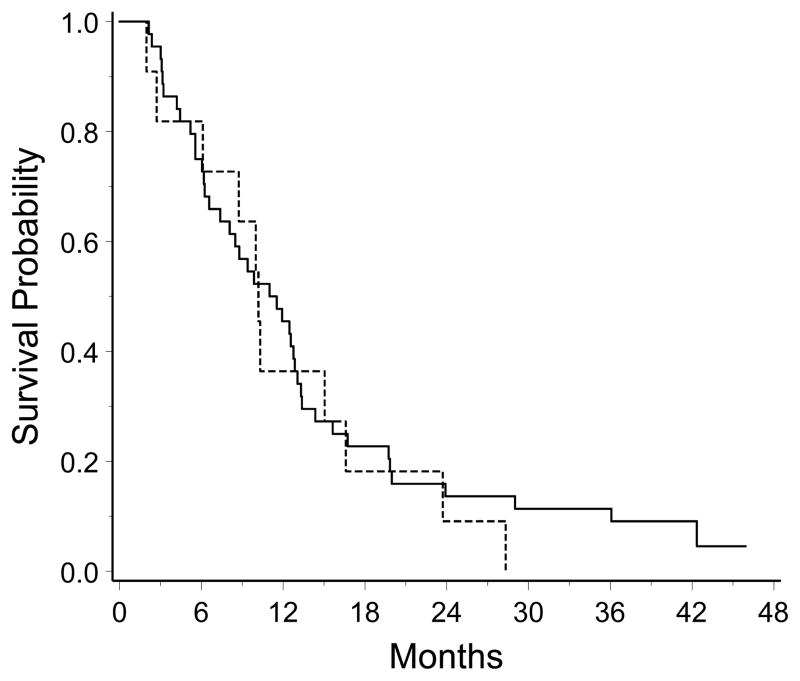
Plasma samples were obtained from 50 subjects enrolled in this study. The CAI plasma concentration from EIAED+ patients was 1.35 ± 1.22 (mean ± standard deviation) compared with 4.06 ± 1.50 (p<0.001) for EIAED− patients. Because the plasma concentrations differed, we compared survival by EIAED status. Three of the 44 patients taking EIAED are alive while none of the 11 patients not taking EIAED have survived. The median survival for the EIAED+ group was 11.3 (95% CI, 7.4 to 13.0) months, and was not statistically different than the EIAED− group median survival of 10.2 (95% CI, 6.1 to 16.6) months (Figure 2; log-rank, P = 0.62).
Figure 2.
Kaplan-Meier survival curves for the patients in this study stratified by use of enzyme inducing anti-convulsants (EIAED+, n=44, solid line; EIAED−, n=11, broken line).
Discussion
Although agents of this category are purported to synergize with therapeutic radiation in vitro and in vivo animal models, the median survival of the 55 patients with newly diagnosed GBM in this trial was not improved by administering CAI during and following radiation therapy. The reasons for this remain unclear. It is likely that the drug reached the therapeutic target as the tumor vasculature is not hidden behind the blood brain barrier. Another possibility is that an insufficient dose was given as we observed few dose limiting toxicities in this study. Methods for the optimization of anti-angiogenic dosing have not been well established, especially when toxicities may not be dose-related and, therefore, somewhat unpredictable. Unfortunately, no surrogate endpoints have yet to be described that adequately correlate with response or survival from these novel agents. It is also possible that CAI is inactive against this particular tumor histology.
This study is the first to document that CAI is subject to Cyp3a induction by anticonvulsants. Similar findings have been described with many other cytotoxic and non-cytotoxic agents in this patient population where potent hepatic enzyme inducing anticonvulsants are frequently co-administered to patients. Examples include 9-aminocamptothecin [17], phenylbutyrate [18], and other signal transduction agents, including Gefitinib ([19]. This finding re-emphasizes the importance of assessing the pharmacology of novel agents in patients taking p450 inducers to ensure that therapeutic levels are achieved prior to initiating efficacy trials.
In summary, this study design permitted evaluation of an innovative therapeutic agent in a population of treatment-naïve patients with GBM. In comparison with a control population aggregated from other studies of similar design, no significant efficacy was found. In addition, patients on EIAED were found to have lower serum steady-state levels of CAI compared to patients not taking EIAED. Finally, efficacy was not observed in patients achieving serum CAI concentrations comparable to that seen in patients treated with other malignancies. These data suggest that this agent should not be pursued further in patients with GBM.
Acknowledgments
Supported by NIH grant #CA062475
Abbreviations used are as follows
- CAI
Carboxyamido-triazole
- CI
Confidence interval
- EIAED
enzyme inducing anticonvulsants
- GBM
glioblastoma multiforme
- KPS
Karnofsky performance status
- NABTT
New Approaches to Brain Tumor Therapy
- RT
radiation therapy
References
- 1.Kohn EC, Liotta LA. L651582: A novel antiproliferative and antimetastasis agent. J Natl Cancer Inst. 1990;82:54–60. doi: 10.1093/jnci/82.1.54. [DOI] [PubMed] [Google Scholar]
- 2.Felder CC, Ma AL, Liotta LA, Kohn EC. The antiproliferative and antimetastatic compound L651582 inhibits muscarinic acetylcholine receptor-stimulated calcium influx and arachidonic acid release. J Pharmacol Exp Ther. 1991;257:967–971. [PubMed] [Google Scholar]
- 3.Kohn EC, Sandeen MA, Liotta LA. In vivo efficacy of a novel inhibitor of selected signal transduction pathways including calcium, arachidonate, and inositol phophates. Cancer Res. 1992;52:3208–3212. [PubMed] [Google Scholar]
- 4.Jacobs W, Mikkelsen T, Smith R, Nelson K, Rosenblum ML, Kohn EC. Inhibitory effects of CAI in glioblastoma growth and invasion. J Neurooncol. 1997;32:93–101. doi: 10.1023/a:1005777711567. [DOI] [PubMed] [Google Scholar]
- 5.Mauceri HJ, Hanna NN, Beckett MA, Gorski DH, Staba MJ, Stellato KA, Bigelow K, Heimann R, Gately S, Dhanabal M, Soff GA, Sukhatme VP, Kufe DW, Weichselbaum RR. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:287–291. doi: 10.1038/28412. [DOI] [PubMed] [Google Scholar]
- 6.Gorski DH, Mauceri HJ, Salloum RM, Gately S, Hellman S, Beckett MA, Sukhatme VP, Soff GA, Kufe DW, Weichselbaum RR. Potentiation of the antitumor effect of ionizing radiation by brief concomitant exposures to angiostatin. Cancer Res. 1998;58:5686–5689. [PubMed] [Google Scholar]
- 7.Bauer KS, Figg WD, Hamilton JM, Jones EC, Premkumar A, Steinberg SM, Dyer V, Linehan WM, Pluda JM, Reed E. A pharmacokinetically guided Phase II study of carboxyamido-triazole in androgen-independent prostate cancer. Clin Cancer Res. 1999;5:2324–2329. [PubMed] [Google Scholar]
- 8.Kohn EC, Figg WD, Sarosy GA, Bauer KS, Davis PA, Soltis MJ, Thompkins A, Liotta LA, Reed E. Phase I trial of micronized formulation carboxyamidotriazole in patients with refractory solid tumors: pharmacokinetics, clinical outcome, and comparison of formulations. J Clin Oncol. 1997;15:1985–1993. doi: 10.1200/JCO.1997.15.5.1985. [DOI] [PubMed] [Google Scholar]
- 9.Simmons BR, Bauer KS, McCall NA, Kohn E, William D. An optimized method for the quantitation of carboxyamido-triazole (CAI) in human plasma with solid phase extraction and reverse phase HPLC. Anal Lett. 1997;30:2009–2021. [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 11.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley and Sons; 1980. Estimation of the survivor function; pp. 16–19. [Google Scholar]
- 12.Cox DR. Regression models and life tables (with discussion) JR Stat Soc B. 1972;34:187–220. [Google Scholar]
- 13.Brem S, Grossman SA, Carson K, New P, Phuphanich S, Alavi JB, Mikkelsen T, Fisher JD The New Approaches to Brain Tumor Therapy CNS Consortium. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro-Oncology. 2005;7:246–253. doi: 10.1215/S1152851704000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinberg L, Grossman SA, Piantadosi S, Pearlman J, Engelhard H, Lesser G, Ruffer J, Gerber M For the New Approaches to Brain Tumor Therapy Central Nervous System Consortium. (Phase I trial to determine the safety, pharmacodynamics and pharmacokinetics of RSR13, a novel radioenhancer, in newly diagnosed glioblastoma multiforme. J Clin Oncol. 1999;17:2593–2603. doi: 10.1200/JCO.1999.17.8.2593. [DOI] [PubMed] [Google Scholar]
- 15.Kleinberg L, Grossman SA, Carson K, Lesser G, O’Neill A, Pearlman J, Phillips P, Herman T, Gerber M. Survival of patients with newly diagnosed glioblastoma multiforme treated with RSR13 and radiotherapy: results of a phase II New Approaches to Brain Tumor Therapy CNS Consortium safety and efficacy study. J Clin Oncol. 2002;20:3149–3155. doi: 10.1200/JCO.2002.01.096. [DOI] [PubMed] [Google Scholar]
- 16.Laterra JJ, Grossman SA, Carson KA, Lesser GJ, Hochberg FH, Gilbert M. Suramin and radiotherapy in newly diagnosed glioblastoma: phase 2 NABTT CNS Consortium study. Neuro-Oncology. 2004;6:15–20. doi: 10.1215/S1152851703000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossman SA, Hochberg F, Fisher J, Chen TL, Kim L, Gregory R, Grochow LB, Piantadosi S. Increased 9-aminocamptothecin dose requirements in patients on anticonvulsants. NABTT CNS Consortium. The New Approaches to Brain Tumor Therapy. Cancer Chemother Pharmacol. 1998;42:118–126. doi: 10.1007/s002800050794. [DOI] [PubMed] [Google Scholar]
- 18.Phuphanich S, Baker SD, Grossman SA, Carson KA, Gilbert MR, Fisher JD, Carducci MA. Oral sodium phenylbutyrate in patients with recurrent malignant gliomas: a dose escalation and pharmacologic study. Neuro-Oncology. 2005;7:177–182. doi: 10.1215/S1152851704000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKillop D, McCormick AD, Millar A, Miles GS, Phillips PJ, Hutchison M. Cytochrome P450-dependent metabolism of gefitinib. Xenobiotica. 2005;35:39–50. doi: 10.1080/00498250400026464. [DOI] [PubMed] [Google Scholar]