Abstract
Recently we reported that middle ear pressure (MEP), middle ear effusion (MEE), and ossicular changes each contribute to the loss of tympanic membrane (TM) mobility in a guinea pig model of acute otitis media (AOM) induced by S. pneumoniae (Guan and Gan, 2013). However, it is not clear how those factors vary along the course of the disease and whether those effects are reproducible in different species. In this study, a chinchilla AOM model was produced by transbullar injection of Haemophilus influenzae. Mobility of the TM at the umbo was measured by laser vibrometry in two treatment groups: 4 days (4D) and 8 days (8D) post inoculation. These time points represent relatively early and later phases of AOM. In each group, the vibration of the umbo was measured at three experimental stages: unopened, pressure-released, and effusion-removed ears. The effects of MEP and MEE and middle ear structural changes were quantified in each group by comparing the TM mobility at one stage with that of the previous stage. Our findings show that the factors affecting TM mobility do change with the disease time course. The MEP was the dominant contributor to reduction of TM mobility in 4D AOM ears, but showed little effect in 8D ears when MEE filled the tympanic cavity. MEE was the primary factor affecting TM mobility loss in 8D ears, but affected the 4D ears only at high frequencies. After the release of MEP and removal of MEE, residual loss of TM mobility was seen mainly at low frequencies in both 4D and 8D ears, and was associated with middle ear structural changes. Our findings establish that the factors contributing to TM mobility loss in the chinchilla ear were similar to those we reported previously for the guinea pig ears with AOM. Outcomes did not appear to differ between the two major bacterial species causing AOM in these animal models.
Keywords: acute otitis media, conductive hearing loss, middle ear pressure, middle ear effusion, ossicular adhesion, umbo vibration, laser vibrometry, Haemophilus influenzae
INTRODUCTION
Acute otitis media (AOM) arises from a rapidly occurring infection of the middle ear and is the most commonly diagnosed disease in young children (Hoberman et al., 2011). The disease is usually caused by bacterial or viral invasion (Gould and Matz, 2010). The principal bacterial pathogens recovered from cases of AOM are nontypeable Haemophilus influenzae (HI) and Streptococus pneumoniae (SP) (Bluestone and Klein, 1983; Bluestone et al., 1992; Giebink, 1999). These two bacterial pathogens have been used to produce AOM models in different species including chinchilla (Bakaletz, 2009; Forbes et al., 2008; Hoa et al., 2009; Reid et al., 2009), mouse (MacArthur et al., 2006; Melhus and Ryan, 2003), rat (Lin et al., 2002; Long et al., 2003), guinea pig (Naguib et al., 1994), and gerbil (Larsson et al., 2003; von Unge et al., 1997). However, middle ear biomechanical changes associated with AOM in the diseased ears of these animal models have rarely been reported.
Recently we utilized a SP type 3 strain to produce a 3-day AOM model in guinea pigs (Guan and Gan, 2013). Data from that study unequivocally showed that changes in middle ear pressure, effusion volume, together with infection-induced ossicular changes each contributed to the loss of middle ear mobility (Guan and Gan, 2013). Because such data are lacking in other animal species used as models of human AOM, it remained unclear whether our findings are species specific (i.e. unique to the guinea pig) or occur generally among the various animal models of AOM. An important additional issue not addressed in our previous study is whether time course-specific changes in the processes altering middle ear mobility occur during the course of AOM. It is known that the level of middle ear pressure (MEP), production of middle ear effusion (MEE), and the degree of inflammation change during the AOM course (Suzuki and Bakaletz 1994; von Unge et al., 1993; von Unge et al., 1997). How these factors contribute to the conductive hearing loss at different phases of middle ear disease is not well understood. The present study was designed to address these issues.
The chinchilla (Chinchilla laniger) is a frequently employed animal model in auditory research. The size of a chinchilla’s tympanic membrane (TM) is close to that of humans (Browning and Granich 1978; Hanamure and Lim 1987; Vrettakos et al., 1988). Nontypeable (acapsular) HI strain 86-028NP, a clinical isolate from a patient with otitis media, frequently has been used in studies investigating host-pathogen interactions during AOM development and resolution in the chinchilla middle ear (Bakaletz et al., 1999; Mason et al., 2003; Morton et al., 2004; Morton et al., 2012; Suzuki and Bakaletz, 1994). Pathologic findings associated with the chinchilla AOM model induced by HI 86-028NP were first characterized by Suzuki and Bakaletz (1994). Otoscopic examination indicated that the middle ear of chinchillas exhibited inflammation as early as one day following transbullar challenge. Thereafter, the degree of inflammation increased and peaked approximately 7–10 days post inoculation (Suzuki and Bakaletz, 1994). This well established and widely used animal model provides an opportunity to investigate the factors decreasing middle ear mobility in ears affected by AOM and their variation at different stages of the disease process. It also allows comparison of AOM outcomes between species and the opportunity to assess whether the bacteria species causing AOM influences the type and severity of the acute biomechanical changes occurring in the middle ear.
To characterize the roles of MEP, MEE, and middle ear structural changes such as ossicular adhesions and soft tissue property changes in sound transmission during the course of middle ear infection, we utilized the chinchilla AOM model initiated by transbullar injection of strain HI 86-028NP. The infected animals were divided into two groups: 4 days (early phase of AOM) and 8 days (later phase of AOM) post inoculation. In each group, the TM vibration at the umbo was measured using laser Doppler vibrometry (LDV) at three experimental stages: the unopened AOM ear with MEP and MEE, upon release of MEP, and after removal of MEE. We then quantified and compared the effects of middle ear pressure, effusion, and structural changes on TM mobility loss at these two phases of AOM.
METHODS
A. Animal preparation
Eighteen chinchillas (Chinchilla lanigera) weighing between 600–780 g were included in this study. The study protocol was approved by the Institutional Animal Care and Use Committee of the University of Oklahoma and met the guideline of the National Institutes of Health. All animals were free from middle ear disease (as evaluated by otoscopic examination) at the beginning of the study.
The animals were divided into control and AOM groups. The control group included eight animals, and the AOM group of 10 animals was subdivided into two groups: the 4 days (4D) group of six animals and the 8 days (8D) group of four animals. AOM was produced by transbullar injection of HI 86-028NP suspension in both ears following the procedure described by Morton et al. (2012). Under general anesthesia [ketamine (10 mg/kg) and xylazine (2 mg/kg)], 0.3 ml bacterial suspension containing 3000 CFU was injected into the superior bulla bilaterally using a 1 cc syringe with a 26 gauge needle. After the challenge dose was administrated, otoscopic examination was performed daily. The animals of control group were untreated.
At the 4th or 8th day post-inoculation, animals were anesthetized as described above. Additional anesthesia was administered as needed to maintain areflexia. The TM was examined microscopically, and then surgery was performed (see description below). In each animal, the experiment was conducted bilaterally (N=16 for control; N=12 for 4D AOM; N=8 for 8D AOM). For both control and AOM groups, the body temperature of the animal was maintained throughout the experiment at approximately 38° C by placing the animal in a prone position on a thermoregulated surgical heating blanket.
B. Experimental protocol
To expose the entrance of the ear canal, the pinna and the skin covering the ear canal were removed surgically. The TM was examined under a microscope to identify signs of AOM. Then the middle ear pressure and energy absorbance were measured by using a wideband tympanometer (Model AT235h, Interacoustic, MN).
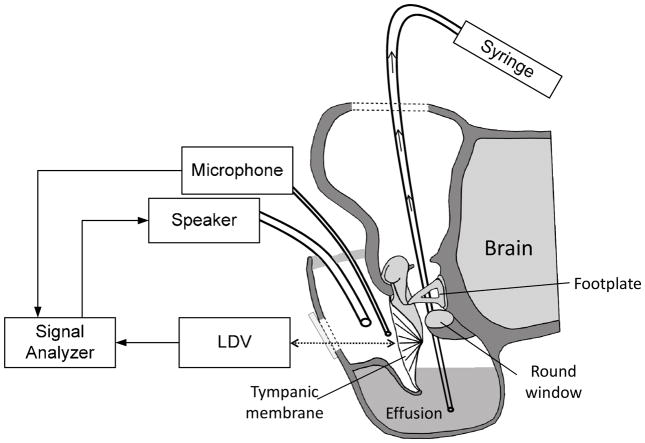
Upon the completion of tympanometry, a 3 mm diameter hole was drilled in the lateral wall of the ear canal to expose the umbo. Then a laser reflective tape (0.2 × 0.2 mm2, < 0.01 mg, 3M, St. Paul, MN) was passed through the hole and placed on the center of the lateral surface of the TM (umbo) to establish the laser target for measuring TM vibration (Fig. 1).
Figure 1.
Schematic diagram of the experimental setup with laser vibrometry at the umbo in the tympanic membrane in chinchillas and the methods for aspiration of the middle ear effusion.
The TM vibration measurement in AOM ears was performed in three experimental: OM-1, undisturbed bulla had intrinsic middle ear pressure and effusion; OM-2, the pressure was released from the middle ear; and OM-3, the effusion was drained from the middle ear. A sound delivery tube and a probe microphone were placed in the ear canal and the movement of the TM at umbo was measured. At each experimental stage, the TM measurement was conducted bilaterally using LDV.
After completion of the vibration measurement in stage OM-1, the skin of the superior temporal bone was partly removed to expose the middle ear bony wall on top of the temporal bone. A hole of 1 mm diameter was drilled into the roof of the middle ear to release the middle ear pressure. After sealing the hole with dental cement (PD-135, Pac-Dent, CA), the OM-2 evaluation was performed.
Upon the completion of LDV measurements for stage OM-2, the hole on top of the temporal bone was opened and enlarged to 3–4 mm in diameter using a drill (Fig. 1). Under microscopic visualization a silicone tube was inserted to the bottom of the middle ear cavity through this hole. The middle ear effusion was then aspirated manually from the cavity with a 1 ml syringe. The aspiration process was repeated as necessary until no additional fluid could be drained from the tympanic cavity. The total effusion volume obtained from each ear was then recorded.
Adhesions were frequently found on the malleus head and between the manubrium and the cochlear promontory when the top cavity was opened for stage OM-3. These ossicular adhesions were not disturbed during the aspiration of the effusion. The opening on top of the temporal bone was then covered by a thin glass sheet, and sealed with dental cement. Then, LDV was performed for stage OM-3. After measurements were completed at all 3 OM stages, the bulla was harvested. Then another hole (diameter of ~4 mm) was opened on the posterior area from the medial side to allow a microscopic examination of the middle ear. The ossicles were examined from the superior view to assess the malleus-incus complex and from the posterior-medial view to evaluate the manubrium and the area adjacent to the round window.
Control ears were prepared and analyzed in the same manner as described above for ears with AOM. To exclude the effect of middle ear pressure in anesthetized animals (Guinan and Peake 1967), a hole of 1 mm diameter was drilled on the top of the middle ear cavity to release any pre-existing pressure. A reflective tape was placed on the umbo and the small opening on the roof of the temporal bone was sealed by dental cement. TM vibration was then measured by LDV as described in the next section. Note that the pressure was not measured in the control ears since any unbalanced pressure had been released in the preparation.
C. Laser Doppler vibrometry measurement
Figure 1 shows a schematic of the experimental setup for measuring TM vibration at the umbo with LDV. The methods of stimulus generation and umbo vibration measurement closely resembled those used in our previous guinea pig study (Guan and Gan, 2013). Briefly, pure tones at 80 dB SPL were presented into the ear canal sequentially from 100 Hz to 10 kHz for 50 cycles at each tone through a sound delivery tube. A probe microphone (Model ER-7C, Etymotic Research, IL) was inserted in the ear canal and the tip of the probe was placed approximately 2 mm from the umbo to monitor the input SPL. After the sound delivery tube and the probe microphone were placed, the entrance of the ear canal was sealed with dental cement. As shown in Fig. 1, the opening in the lateral surface of the ear canal wall was covered by a transparent glass sheet, and the gap between the glass sheet and the bony wall was sealed with dental cement. Vibrations of the TM were measured by the laser vibrometer (Polytec CLV 2534, Tustin, CA). The direction of the laser beam was approximately normal to the lateral surface of the umbo. The peak-to-peak displacements (dp-p in unit of μm) of the TM at the umbo were calculated from the voltage output of the laser vibrometer velocity decoder by dp-p= 2Avoltp/πf, where Avolt is the amplitude of vibrometer output (velocity) in Volts and f is the frequency of pure tone in kHz. To obtain the TM displacement phase, the measured velocity phase was shifted by −90° at all tested frequencies.
Surgical preparation of each animal (inclusion of both ears) required 15–20 minutes. Tympanometry was completed within 2 minutes, and the LDV measurement at each experimental stage usually took about 10 minutes. The TM vibration data for the unopened bulla (OM-1) were collected about 30 minutes after the anesthesia. For other OM stages, the TM vibration data were obtained within 5 minutes after re-sealing the opening on the bulla to avoid any pressure variations that might occur with a longer interval.
RESULTS
A. Microscopic observation and MEP of AOM ears
After removing the pinna, the TM was examined microscopically to identify signs of AOM. The thin, translucent TM typical of normal (uninfected control) chinchillas is shown in Fig. 2A. The changes typical for the TM of an AOM ear at 4D are shown in Fig. 2B. After this short time post infection, the TM appeared opaque and a middle ear effusion (yellow in color) was evident. However, at this low magnification such TMs exhibited no observable structural changes compared to control TMs. As shown in Fig. 2B, an air-fluid interface behind the TM was usually visible and associated with AOM in 4D ears. The volume of MEE in 4D AOM ears ranged from 0.3–0.6 ml and had a mean value of 0.42 ml (± 0.11 ml standard deviation, SD) when the MEE was aspirated for experimental stage OM-2. In two of the ears exhibiting AOM at 4D, the volume of effusion was sufficiently large (~0.6 ml) to fill the middle ear over the umbo. The effusion level in the other ears from the 4D group filled the middle ear cavity to a level below the umbo.
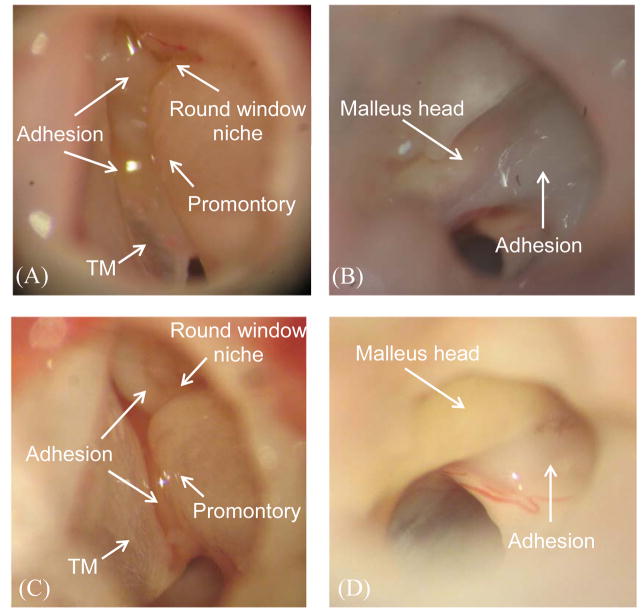
Figure 2.
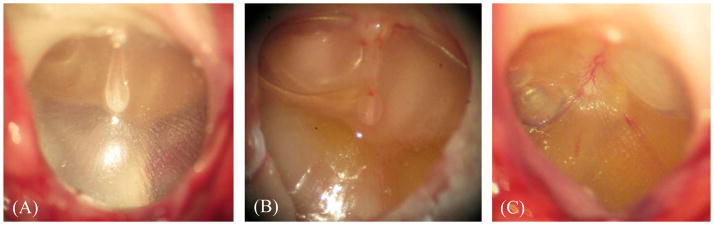
Microscopic photographs of (A) control eardrum, (B) 4 days AOM eardrum, and (C) 8 days AOM eardrum.
A view of the TM in an 8D AOM ear is shown in Fig. 2C. TMs from this time period were hyperemic and opaque. A yellow-colored MEE was observed in each ear. In these ears, the effusion almost filled the entire middle ear space. MEE volume ranged from 0.7–0.9 ml with an average value of 0.82 ml (± 0.09 ml, SD). The observed mean fluid volume was significantly greater than that found in AOM ears after infection for four days (student t-test, p < 0.05). MEEs from both 4D and 8D ears were clearly purulent. The appearance of MEEs obtained at these two times following bacterial challenge could not be distinguished from one another by the unaided eye or under low power light microscopy.
Next we evaluated the occurrence of changes in ossicular appearance and structure at 4D and 8D post challenge (Fig. 3). Prior to these assessments, MEEs were removed to better visualize the changes which occurred. Adhesions were formed between the TM and the cochlear promontory as well as around the round window niche by Day 4 (Fig. 3A). The manubrium, stapes, and long process of the incus were covered with adhesions. As shown in Fig. 3B, adhesions were also observed on the malleus head.
Figure 3.
Microscopic photographs of (A) middle ear cavity and ossicles in 4 days AOM ear, (B) malleus head in 4 days AOM ear, (C) middle ear cavity and ossicles in 8 days AOM ear, and (D) malleus head in 8 days AOM ear.
The typical appearance of the ossicles in ears of chinchillas experiencing AOM for 8 days is shown in Figs. 3C and 3D. Modest adhesions were observed in the round window niche and between the manubrium and promontory (Fig. 3C). Figure 3D illustrates the adhesions formed on the malleus head. Adhesions also were observed between the ossicles and the adjacent middle ear walls in both 4D and 8D AOM animals. These AOM-associated ossicular changes in the chinchilla closely resemble those previously described in the guinea pig (Guan and Gan, 2013) and the gerbil AOM models (von Unge et al., 1997). Adhesions between the manubrium and cochlear promontory in 4D ears appeared to be thicker than those observed in 8D ears. In contrast, adhesions around the malleus head in 4D ears did not appear to differ from those occurring at 8D. In Fig. 3D, we also observed dilated capillaries in the mucosa near the malleus head. The changes of capillaries suggest a possible thickening of the mucosa layer.
The MEP in stage OM-1 was measured by wideband tympanometry before the measurement of TM vibration. The MEP of all 4D AOM ears was negative and had a mean value of −176 ± 54 daPa. In the 8D AOM group, four ears exhibited a flat tympanogram, and the MEP was could not be identified. We believe that this observation probably reflects large amount of effusion present in the middle ear cavity (Jerger 1970; Paradise et al., 1976). The MEP of the remaining 8D ears with AOM were all negative (mean value −145 ± 65 daPa).
B. TM mobility change in 4D AOM ears
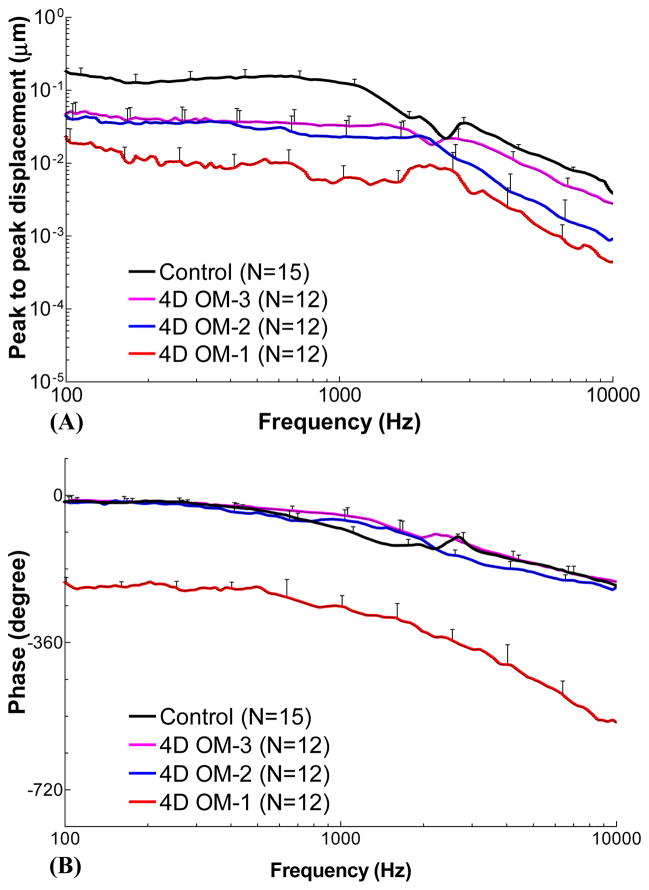
Figure 4 shows the individual and mean curves for TM displacement at the umbo in response to 80 dB SPL pure tones over frequencies of 0.1–10 kHz measured in twelve 4D AOM and 15 control ears. In each AOM ear, TM vibration was recorded at three experimental stages: OM-1, OM-2, and OM-3. The upper panels display the frequency response curves of the peak-to-peak displacement magnitude. The displacement phase curves are shown in the lower panels.
Figure 4.
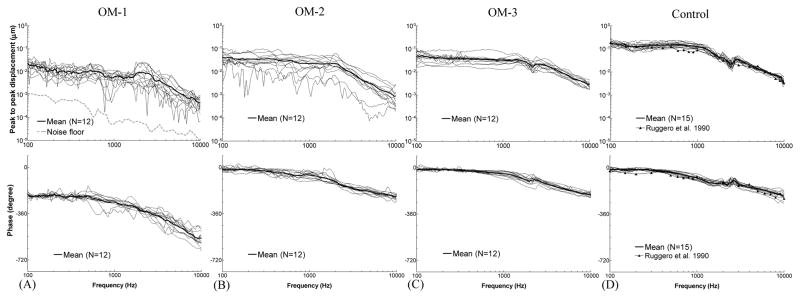
Peak to peak displacement magnitude (upper panel) and phase angle (lower panel) of the TM at umbo in response to 80 dB SPL sound input at the ear canal in 4 days (A) OM-1, (B) OM-2, (C) OM-3, and (D) control ears. The solid lines represent the mean curves; the dotted lines represent the individual curves; the black line with triangles represents the TM displacement of normal chinchilla ears reported by Ruggero et al. (1990).
TM displacement at the umbo measured in OM-1 with mean and individual curves is shown in Fig. 4A. At stage OM-1, the middle ear was unopened, and TM movement was affected by the MEP, MEE, and structural changes in the middle ear. The mean displacement magnitude gradually decreased from 0.023 μm to 0.009 μm over 0.1–2 kHz, and a continued reduction to 0.44 nm at 10 kHz was observed. The mean phase of OM-1 was flat for frequencies between 0.1–0.5 kHz with a value of −212° to −230°, and decreased at higher frequencies. The noise level for LDV measurement is plotted in the upper panel of Fig. 4A. It shows that the TM displacement magnitude in OM-1 was at least 10 dB greater than the noise level for most tested frequencies. Considering that TM mobility in OM-1 should be the lowest when compared to the other OM stages in this study, the LDV measurement is expected to be reliable.
At stage OM-2 (Fig. 4B), the mean TM displacement curve was flat at 0.1–2 kHz with a value of 0.022–0.045 μm and decreased after 2 kHz as frequency increased when the MEP was released. The mean phase decreased slowly from −14° to −230° over 0.1–10 kHz. We noted that individual TM displacement values exhibited relatively large variations compared to the curves obtained in stage OM-1. The two lowest displacement curves were obtained from the ears with 0.6 ml of MEE, the largest amount observed in the 4D ears.
The magnitude and phase of TM displacement after the MEE was removed (stage OM-3) are shown in Fig. 4C. The variation of the individual displacement curves in OM-3 was smaller than that in OM-1 and OM-2. The mean displacement was flat between 0.1–1.5 kHz with a value of 0.034–0.052 μm, and decreased continually as frequency increased. The mean phase angle of OM-3 gradually decreased from −11° to −210° over 0.1–10 kHz. It is important to note that the purulent adhesions still remained on the ossicles at this stage, a finding different from control ears.
The TM displacement magnitude and phase determined from 15 control ears without AOM are displayed in Fig. 4D (16 control ears were initially involved in this study, but one TM was damaged during preparation and was excluded from our study for this reason). Mean TM displacement was flat between 0.1–1.2 kHz with a value of 0.11–0.18 μm, then decreased to 0.004 μm as the frequency increased to 10 kHz. The mean phase curve slowly decreased from −11° to −210° over the tested frequencies. Published data from Ruggero et al. (1990) describing pressure-released normal chinchilla ears were included in Fig. 4D for comparison with our newly obtained data. The displacement magnitude and phase curves obtained in our current study closely resemble those based on Ruggero’s data.
The mean TM displacement curves at control and 3 OM stages in Fig. 4 are extracted and displayed with SD bars in Fig. 5. The statistical results (p-values) for Fig. 5A displacement data are listed in Table 1. Repeated-Measures ANOVA and Turkey post-hoc tests were used to compare the three OM stages since the displacements were measured from the same population. An unpaired t-test was used to compare OM-3 to uninfected control ears because these two middle ear conditions were from different populations. Statistical analysis was performed using Prism software (Graphpad, La Jolla, CA). The p-values of post-hoc test were reported as inequalities in the software.
Figure 5.
Mean peak to peak displacement magnitude (A) and phase angle (B) of the TM at umbo with SD in response to 80 dB SPL sound input at the ear canal in 4 days OM-1 (red line), OM-2 (blue line), OM-3 (purple line), and control ears (black line).
Table 1.
List of P values derived from: (1) One way Repeated-Measures ANOVA test and Turkey post-hoc test on the TM displacement magnitude data between three 4D OM stages; (2) Unpaired Student t-test on the TM magnitude data between OM-3 and control.
| Frequency (kHz) | ANOVA P value | Turkey P value OM-1 vs OM-3 | Turkey P value OM-1 vs OM-2 | Turkey P value OM-2 vs OM-3 | Unpaired t-test P value OM-3 vs Control |
|---|---|---|---|---|---|
| 0.25 | 0.0014 | < 0.01 | < 0.05 | > 0.05 | 0.0000 |
| 0.5 | 0.0016 | < 0.01 | < 0.05 | > 0.05 | 0.0000 |
| 0.75 | 0.0009 | < 0.001 | < 0.05 | > 0.05 | 0.0000 |
| 1 | 0.0001 | < 0.001 | < 0.05 | > 0.05 | 0.0001 |
| 2 | 0.0110 | < 0.05 | < 0.05 | > 0.05 | 0.0000 |
| 4 | 0.0001 | < 0.001 | > 0.05 | < 0.001 | 0.0000 |
| 6 | 0.0001 | < 0.001 | > 0.05 | < 0.001 | 0.0000 |
| 8 | 0.0001 | < 0.001 | > 0.05 | < 0.001 | 0.0000 |
| 10 | 0.0001 | < 0.001 | > 0.05 | < 0.001 | 0.0160 |
Note: The P values with grey background represent significant difference between groups (P < 0.05). The P values of Turkey post-hoc test are shown as inequalities since no exact value is reported by the software.
Significant changes in TM displacement occurred among the three OM stages (Table 1, column 2). As shown in Fig. 5A and Table 1, the TM displacement of OM-3 was significantly greater than that of OM-1 over frequencies of 0.1–10 kHz (column 3 of Table 1). After pressure in the middle ear was released (OM-2), the TM displacement increased significantly at low frequencies (0.1–2 kHz, see column 4 of Table 1). As the effusion was drained from the cavity (OM-3), TM displacement was significantly increased compared to OM-2 at 4–10 kHz (column 5 of Table 1). When the OM-3 values were compared with those of control ears, there was a significant difference at all tested frequencies (column 6 of Table 1). It should be noted that at frequencies > 2 kHz, the TM displacement of OM-3 was slightly, but significantly, lower than that of controls (Fig. 5A).
Figure 5B shows the mean ± SD of the TM displacement phase curves measured in control ears and three experimental stages of 4D AOM ears. The mean phase in undisturbed AOM ears (OM-1) was much lower than that in control and other stages by 190° (roughly a half-cycle) over 0.1–10 kHz. After middle ear pressure was released (OM-2), the TM phase generally overlapped with the control at frequencies below 800 Hz and was greater at 0.8–2 kHz and lower at f > 2 kHz compared with the control curve. After the effusion was removed (OM-3), the TM phase was almost the same as that of controls at all tested frequencies except 0.6–2.4 kHz, where the phase was greater than control.
C. TM mobility change in 8D AOM ears
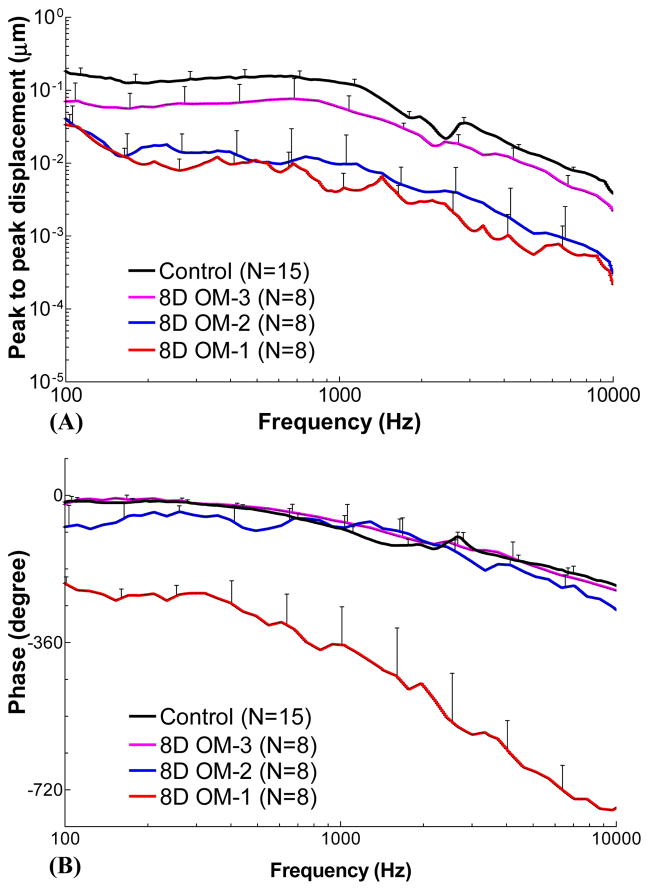
Figure 6 shows the individual and mean curves of the TM displacement at the umbo in response to 80 dB SPL pure tones over frequencies of 0.1–10 kHz measured from eight 8D AOM ears at the three experimental stages. The control curves shown in Fig. 6D are the same as those in Fig. 4D for comparison purposes. The upper panels display the frequency response curves of the displacement magnitude and the lower panels show the displacement phase curves.
Figure 6.
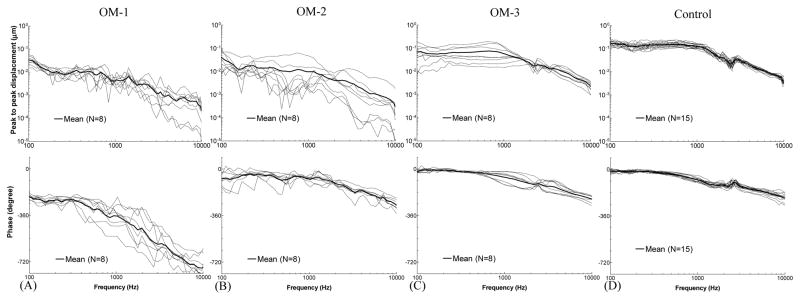
Peak to peak displacement magnitude (upper panel) and phase angle (lower panel) of the TM at umbo in response to 80 dB SPL sound input at the ear canal in 8 days (A) OM-1, (B) OM-2, (C) OM-3, and (D) control ears. The solid lines represent the mean curves; the dotted lines represent the individual curves.
The TM displacement curves measured from unopened ears (OM-1) are shown in Fig. 6A. The mean displacement continually decreased from 0.03 μm to 0.22 nm over 0.1–10 kHz. The mean phase curve was flat at 100–300 Hz with a value of −215° to −240°, and decreased at higher frequencies.
Figure 6B shows the TM displacement curves after the MEP was released (OM-2). The individual variation of displacement magnitude at OM-2 was large, similar to the 4D OM-2 ears. This variation may relate to the different levels of middle ear effusion and the degree of middle ear ossicular and soft tissue changes between individual ears. The mean displacement decreased from 0.04 μm to 0.3 nm over 0.1–10 kHz. The mean phase was −77° at 100 Hz, increased to −40° at 200 Hz, then gradually decreased to −280° at 10 kHz.
Figure 6C displays the TM displacement curves at stage OM-3, in which the effusion was removed but the adhesions on the ossicles remained unaltered. Inter-individual variation in TM displacement was smaller than that in OM-2 and occurred mainly at frequencies below 2 kHz. The individual difference in 8D ears was greater than that in 4D ears (Fig. 4C) at stage OM-3. The mean displacement curve of OM-3 in 8D was flat with a value of 0.06–0.08 μm at 0.1–1 kHz, and decreased to 2 nm at 10 kHz. The mean phase slowly decreased from −10° to −230° over 0.1–10 kHz.
The mean TM displacement curves from control ears and three OM stages in Fig. 6 were collected and displayed in Fig. 7. Figure 7A shows that the TM vibration slightly increased upon the release of pressure in 8D ears and the increase was smaller than that observed in 4D ears (Fig. 5A). The difference of TM displacement between OM-2 and controls in 8D ears (blue line versus black line) was larger than the difference between OM-2 and controls in 4D ears (blue line versus black line) shown in Fig. 5A. After removal of the effusion, the TM displacement increased substantially and the difference between OM-3 and control in 8D ears (pink line versus black line) was smaller than that in 4D ears. This observation indicates that MEE is a primary factor contributing to the loss of TM mobility in 8D ears.
Figure 7.
Mean peak to peak displacement magnitude (A) and phase angle (B) of the TM at umbo with SD in response to 80 dB SPL sound input at the ear canal in 8 days OM-1 (red line), OM-2 (blue line), OM-3 (purple line), and control ears (black line).
The statistical analyses for the data in Fig. 7A are tabulated in Table 2 using the same methods as those for 4D ears. Comparing Table 2 with Table 1, we observed that the TM displacement in 8D ears did not change significantly at all tested frequencies after pressure was released (OM-1 versus OM-2). In contrast, releasing MEP significantly increased the TM mobility in 4D ears at low frequencies. After effusion was removed, the TM mobility in 8D ears increased significantly over the entire frequencies (OM-2 versus OM-3), but in 4D ears significant changes were observed only at high frequencies. In both 4D and 8D ears, the TM mobility at OM-3 was significantly different from controls.
Table 2.
List of P values derived from: (1) One way Repeated-Measures ANOVA test and Turkey post-hoc test on the TM displacement magnitude data between three 8D OM stages; (2) Unpaired Student t-test on the TM magnitude data between OM-3 and control.
| Frequency (kHz) | ANOVA P value | Turkey P value OM-1 vs OM-3 | Turkey P value OM-1 vs OM-2 | Turkey P value OM-2 vs OM-3 | Unpaired t-test P value OM-3 vs Control |
|---|---|---|---|---|---|
| 0.25 | 0.0019 | < 0.01 | > 0.05 | < 0.01 | 0.0031 |
| 0.5 | 0.0055 | < 0.05 | > 0.05 | < 0.05 | 0.0043 |
| 0.75 | 0.0106 | < 0.05 | > 0.05 | < 0.05 | 0.0210 |
| 1 | 0.0014 | < 0.01 | > 0.05 | < 0.01 | 0.0014 |
| 2 | 0.0001 | < 0.001 | > 0.05 | < 0.001 | 0.0001 |
| 4 | 0.0001 | < 0.001 | > 0.05 | < 0.001 | 0.0012 |
| 6 | 0.0001 | < 0.001 | > 0.05 | < 0.001 | 0.0042 |
| 8 | 0.0001 | < 0.001 | > 0.05 | < 0.001 | 0.0002 |
| 10 | 0.0001 | < 0.001 | > 0.05 | < 0.001 | 0.0037 |
Note: The P values with grey background represent significant difference between groups (P < 0.05). The P values of Turkey post-hoc test are shown as inequalities since no exact value is reported by the software.
For a quantitative comparison of TM mobility changes in two infection periods, Table 3 summarizes the mean values for TM displacement magnitude in 4D, 8D, and control ears. Six frequencies were selected to represent low, middle, and high frequency ranges.
Table 3.
List of mean peak-to-peak TM displacement magnitude of control, 4D, and 8D ears at the three experimental stages (μm).
| Frequency (kHz) | 4D | 8D | Control | ||||
|---|---|---|---|---|---|---|---|
| OM-1 | OM-2 | OM-3 | OM-1 | OM-2 | OM-3 | ||
| 0.25 | 0.0105 | 0.0350 | 0.0421 | 0.0081 | 0.0152 | 0.0651 | 0.1338 |
| 0.5 | 0.0102 | 0.0294 | 0.0370 | 0.0108 | 0.0105 | 0.0711 | 0.1530 |
| 1 | 0.0058 | 0.0227 | 0.0331 | 0.0042 | 0.0097 | 0.0587 | 0.1269 |
| 4 | 0.0027 | 0.0045 | 0.0128 | 0.0010 | 0.0019 | 0.0126 | 0.0198 |
| 8 | 0.0007 | 0.0013 | 0.0040 | 0.0005 | 0.0007 | 0.0040 | 0.0072 |
| 10 | 0.0004 | 0.0009 | 0.0028 | 0.0002 | 0.0003 | 0.0022 | 0.0039 |
The TM phase data measured in control and three OM stages in 8D ears are shown in Fig. 7B. The mean phase in unopened AOM ears (OM-1) was much lower than that in control and other OM stages by 190°, and these differences increased with increasing frequency. After release of MEP, the mean phase curve in stage OM-2 was greater than control at 0.8–2 kHz but lower at other frequencies. After the removal of MEP and MEE, the phase of OM-3 stage generally overlapped with the control curve but was slightly greater than control at 0.9–2 kHz. The large phase lag of the umbo movement observed in stage OM-1 of 8D ears is similar to that of 4D ears (Fig. 5B).
DISCUSSION
A. Factors affecting TM mobility loss in AOM ears
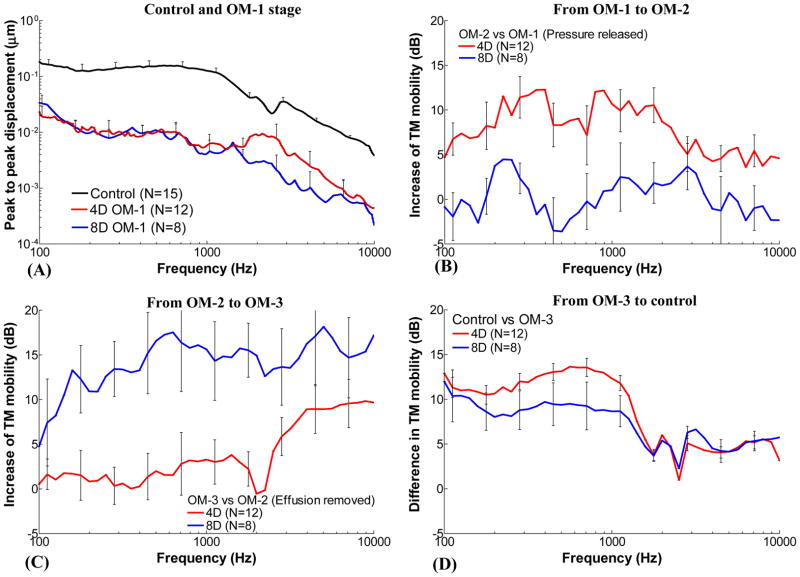
In this study, the TM mobility of chinchilla AOM ears was investigated at 4 days and 8 days post transbullar inoculation with H. influenzae. These time points represent relatively early and later stages of AOM. Comparing the TM displacement curves obtained in the unopened 4D and 8D ears (stage OM-1) with that of control ears (Fig. 8A), the TM mobility at 8D did not differ significantly from that of infected ears at 4D at f < 1.5 kHz (also see columns of “OM-1” under 4D and 8D in Table 3). However, the results in Tables 1 and 2 suggest that the middle ear pressure (OM-1 versus OM-2), effusion (OM-2 versus OM-3), and ossicular structure change (OM-3 versus Control) at early infection contributed to the loss of TM mobility in a different manner from those at the later infection period. To quantify the effects of three factors (MEP, MEE, and structural change) on the loss of TM mobility at two infection periods, the results in Figs. 5A and 7A were calculated as the increase of TM mobility (displacement in dB) at an OM stage with respect to the previous stage as shown in Figs. 8B–D. The effects of MEP, MEE, and middle ear structural changes on TM mobility over frequencies from 100 Hz to 10 kHz are shown in these figures and described in following sections.
Figure 8.
(A) Mean TM displacement of control, 4D and 8D AOM ears at OM-1 from Fig. 5A and Fig. 7A. (B) Mean increase (with SD) of TM displacement at umbo after release of middle ear pressure in 4 days and 8 days AOM ears (OM-2 vs OM-1). (C) Mean increase of TM displacement after removal of middle ear effusion in 4 days and 8 days AOM ears (OM-3 vs OM-2). (D) Mean difference of TM displacement between control and OM-3 of 4 days and 8 days AOM ears (Control vs OM-3). Red lines represent the TM displacement increase in 4 days group. Blue lines represent the TM displacement increase in 8 days group.
A1. Effect of MEP on TM mobility along the course of AOM
Figure 8B displays the increase of TM displacement caused by release of MEP after 4 days or 8 days of infection. In 4D ears (red line), an average 5–12 dB increase at frequencies below 3 kHz and a smaller increase (about 5 dB) at frequencies above 3 kHz were observed after the release of MEP. However, in 8D ears the TM displacement increase due to pressure release was under 4 dB or even negative at some frequencies (blue line).
Pressure in the middle ear increases the stiffness of the TM and thus decreases the TM movement as reported by Dai et al. (2008). Lee and Rosowski (2002) reported the reduction of the umbo mobility with controlled MEP in gerbil ears. Release of the MEP would reduce the tension in the TM and increase the TM mobility. In our present study, the increase of TM displacement upon releasing MEP in 4D is larger than that in 8D ears. The effect of MEP on TM mobility may relate to the residual air space in the middle ear in these two inflammatory phases. In 4D ears the air-fluid interface was at or below the umbo and about half of the tympanic cavity was air-filled. In contrast, the effusion almost filled the entire space behind the TM (i.e., tympanic cavity) in 8D ears and only the superior cavity of the chinchilla middle ear remained air filled. The air space behind the TM is critical to its mobility and Ravicz et al. (2004) reported that even a small amount of air would be sufficient to facilitate umbo’s motion. However, there was little air space behind the TM in 8D ears compared with 4D ears. After the air pressure was released in 8D ears, the increase of TM motion was very limited because the tympanic cavity was almost filled by fluid as observed just before the aspiration of MEE. In 4D ears the TM displacement significantly increased upon the release of MEP due to sufficient air space behind the TM. Therefore, the MEP was a dominant factor on TM mobility loss in the early infection of AOM ears. In the extended (8D) AOM infection, MEP had little effect on TM movement because MEE filled the tympanic cavity.
MEP in 4D and 8D ears substantially changed the phase of the umbo movement. In gerbil ears it has been reported that a small negative MEP stiffened the middle ear and led to a flat extension into the high frequencies in the phase curve (Lee and Rosowski 2001; Rosowski and Lee 2002). However, when the negative MEP became large, e.g. −250 to −300 daPa in gerbil ears, a half-cycle phase lag in the umbo vibration was observed (Fig. 7 in Lee and Rosowski 2001; Fig. 5 in Rosowski and Lee 2002). In the streptococcal AOM model recently reported by Guan and Gan (2013) in guinea pigs, one ear at stage OM-1 showed the half-cycle phase change. The large phase lag observed in the present study is probably related to large negative MEP or tissue infection in AOM. The mechanisms behind these observations are not clear and need further study.
A2. Effect of MEE on TM mobility along the course of AOM
As can be seen in Fig. 8C, TM mobility increased by an average of 10–18 dB over 140 Hz to 10 kHz after the effusion was removed in 8D ears (blue line). In 4D ears, removal of the effusion resulted in an average of 5–10 dB increase of TM movement at frequencies greater than 3 kHz but had not much effect at low frequencies (red line). There was a huge difference of the MEE’s effect on TM mobility loss between 8D and 4D ears over the frequency range. The principal reason for this TM mobility change during the disease course is the increase of MEE amount in the middle ear (on average from 0.42 to 0.84 ml) and the decrease of middle ear air space from 4D to 8D infection. The MEE in 4D ears neither covered the entire TM nor filled the tympanic cavity as shown in Fig. 2. There might be a possible viscosity change in MEE from 4D to 8D, but we did not measure the viscosity of the MEE in the present study.
Middle ear fluid decreases TM mobility by two mechanisms: stiffening of the middle ear by reducing air space, and increasing effective mass of the TM (Ravicz et al. 2004). The loss of middle ear air space and the increase of TM mass together reduce the umbo’s mobility at low and high frequencies, respectively. An increase in MEE contacting the TM would lead to an increase of TM mass and more reduction of displacement at high frequencies as determined in human temporal bones by Ravicz et al. (2004) and Gan et al. (2006). The increase of TM displacement at high frequencies after the removal of the MEE in 8D ears was greater than that in 4D ears. This new observation in our present study is in agreement with the findings of the Ravicz et al. and Gan et al. studies.
The TM mobility at low frequencies was decreased by reduction of the air space in the middle ear cavity and this effect became notable only when the middle ear was almost filled with fluid (Ravicz et al., 2004; Gan et al., 2006; Guan and Gan, 2011). In the present study, the volume of air space in the tympanic cavity of chinchilla ears is 0.9–1.0 ml. In 4D ears, the MEE occupied less than half of the tympanic cavity (Fig. 2) and the restoration of air space had little effect on TM movement at low frequencies. In 8D ears, the effusion occupied almost the entire middle ear air space, which not only increased the mass of the TM but also substantially decreased the middle ear air volume. Therefore, removal of the effusion significantly increased TM mobility over all tested frequencies in 8D ears. Thus, the MEE was the primary contributor to decreased TM mobility in the late stage of OM.
Thornton et al. (2013) reported changes of the umbo velocity and cochlear microphonic threshold in chinchillas when the middle ear was filled with different volumes of silicone oil. Their results demonstrated that a reduction of the umbo mobility and an elevation of cochlear microphonic threshold both increased as middle ear fluid volume increased from 0.5 ml to 1.25 ml (Fig. 2A and Fig. 5A in their paper). Their study also demonstrated that the decrease of TM mobility at umbo was related to frequency. A small decrease in umbo velocity at low frequencies was observed when a small amount of fluid was instilled into the middle ear. As more fluid was added, the reduction of umbo velocity extended to high frequencies and a large value of reduction was observed (Fig. 5A in their paper). The effect of MEE on TM movement reported in the present study is in general agreement with the findings of Thornton et al.
It should be noted that the volume of MEE and the area of TM covered by the effusion in our study was different from the study by Thornton et al. (2013). From our observation, the entire TM was covered by MEE with 0.8–0.9 ml in the infected ears. Thornton et al. (2013) used 1.25 ml of silicone oil and the TM was 100% covered by the fluid, simulating a MEE in a normal ear. In our AOM model, we found a small amount of pus remained in the small crevices of the middle ear cavity during the post experiment examination. This purulent material could not be aspirated with the MEE in the OM-3 stage. In addition, the middle ear mucosa was thickened in our infected chinchilla ears. It is possible that the space for the fluid in the more severely infected middle ear was smaller than that in the healthy ear, and thus required a smaller fluid volume to cover the TM in our infected ears.
A3. Effect of middle ear structural changes on TM mobility along the course of AOM
As shown in Fig. 8D, the TM mobility of control ears was higher than that of OM-3 (MEP released and MEE removed) in both 4D and 8D ears. The residual reduction of TM mobility after release of pressure and removal of effusion suggested that infection-induced middle ear structural changes, including the ossicular adhesions and the possible micro-structural changes of middle ear soft tissues, contributed to the TM mobility loss. The residual TM mobility loss was frequency dependent as displayed in Fig. 8D. The middle ear structural changes in early and late stages of AOM resulted in about 5 dB loss of TM mobility at f > 1 kHz. The reduction of TM mobility was enhanced to 8 dB for 8D ears and 13 dB for 4D ears at f < 1 kHz. In addition, the phase at OM-3 in both 4D and 8D ears was slightly higher than control at frequencies around 1 kHz (pink line vs black line in Figs. 5B and 7B). These changes show that the middle ear was stiffer than controls in both 4D and 8D situations at stage of OM-3. Similar results were observed in our previous study of guinea pig AOM ears (Guan and Gan, 2013). The AOM-induced structural changes in chinchillas may increase middle ear stiffness and caused TM mobility loss mainly at low frequencies, similar to the AOM ear of guinea pigs.
Ossicular adhesions have been observed in AOM models of gerbils (von Unge et al., 1997), rats (Caye-Thomasen et al., 1996; Caye-Thomasen and Tos, 2000), and guinea pigs (Guan and Gan, 2013). In the gerbil AOM model, von Unge et al. (1997) reported that TM stiffness was increased in ears with ossicular adhesions compared with normal ears. In this study it is possible that the adhesions in AOM ears (Fig. 3) could have fixed the manubrium, malleus head, incus, and stapes and increase the overall stiffness of the middle ear.
Rosowski et al. (2008) reported the umbo vibration in patients with malleus and stapes fixation and their results indicated that significant loss of umbo mobility occurred at f < 3 kHz in both stapes-fixed and malleus-fixed groups (Fig. 9 in their paper). In a study of human temporal bones by Dai et al. (2007), fixation of the malleus caused a reduction of 15 dB at the umbo or stapes at low frequencies. Nakajima et al. (2005A; 2005B) reported the vibration patterns of the umbo and stapes when adhesives were applied to the ossicles in human temporal bones and their results suggested that ossicular fixation increased the stiffness of the middle ear, reduced the umbo’s mobility mainly at f < 1.5 kHz, and increased the phase near 1 kHz (see Figs. 5–7 in Nakajima et al. 2005A).
The residual TM mobility loss observed in the chinchilla AOM ears seems to be consistent with those published data measured from ears with ossicular fixation. If we assume that the adhesions fixed the ossicles in 4D and 8D ears, TM mobility at the umbo would be decreased. In addition, the mass of the adhesions might also affect the TM mobility at high frequencies. Therefore, ossicular adhesions are likely to contribute to the residual mobility loss of the TM in our model. Figure 8D also suggests that the effect of ossicular changes in 4D ears was greater than that in 8D ears at f < 1 kHz, which may relate to the fact that the adhesions in 4D ears were generally thicker or denser compared with 8D ears as observed in Fig. 3. A possible explanation is that the inflammatory process has changed by the 8th day of AOM.
Infection in the middle ear also commonly causes structural changes of middle ear soft tissues such as the TM (Larsson et al., 2003; von Unge et al., 1993; von Unge et al., 1997) and round window membrane (Gan et al., 2013). The mechanical properties of the TM in diseased ears are different from those of normal ears as reported by Luo et al. (2009); this also could affect middle ear vibration. Gan et al. (2013) reported soft tissue property changes of the round window membrane in a guinea pig AOM model; this might alter the cochlear load or cochlear input impedance to the middle ear. Pathological changes of the ossicular joints and stapedial annular ligament may also occur during middle ear infection. Therefore, in addition to the ossicular adhesions, the mechanical property changes of the TM, round window membrane, and other middle ear soft tissues may also contribute to the difference in TM mobility between OM-3 and uninfected control ears. However, it is difficult to differentiate the effects between ossicular adhesions and middle ear tissue structural changes in live animals. Future studies are needed to quantify whether and how such soft tissue changes contribute to the middle ear mobility change during the course of AOM.
B. Comparison of chinchilla and guinea pig AOM models
In our previous study of the guinea pig AOM model (Guan and Gan 2013), AOM was created by injection of Streptococcus pneumoniae into the middle ear and the change in TM mobility was measured after 3 days of inoculation. In the current study, Haemophilus influenzae was used to induce the middle ear infection and produce this AOM model in chinchillas. SP and HI are two leading bacterial pathogens commonly used for creating AOM in animals. To our knowledge, the middle ear biomechanics during AOM was reported only in SP-induced models (von Unge et al., 1997; Larsson et al., 2003; Guan and Gan, 2013). The current study is the first to evaluate the middle ear mechanics in a HI-induced AOM model.
Comparing the loss in TM mobility at the early phase of the disease in these two species with different bacterial pathogens, we found that the factors causing TM mobility loss at the early phase of the disease (3 days for guinea pig and 4 days for chinchilla) were similar for these two species although the bacterial pathogens used for creating AOM were different. In both AOM models, negative pressure was generated in the middle ear and resulted in about 10 dB reduction in TM (umbo) vibration at f < 2 kHz. At higher frequencies, the effect of MEP on TM motion was not significant. Purulent effusion was found in both chinchilla and guinea pig models. The effusion filled about half of the tympanic cavity in guinea pigs and slightly less than half of the tympanic cavity in chinchillas. MEE led to the loss of TM mobility mainly at frequencies greater than 2 kHz at early phase of infection in both models (9–15 dB for guinea pig and 5–10 dB for chinchilla). In both species, ossicular adhesions were present and commonly located between the manubrium and cochlear promontory and around the round window niche. After the MEP and MEE were removed from the middle ear, TM mobility at the umbo was lower than in control ears, mainly at low frequencies (< 2 kHz) for both species, which corresponds to the effect of ossicular adhesions and other middle ear structural changes on middle ear mechanics. Therefore, the middle ear pressure, effusion, and middle ear structural changes are the main factors leading to TM mobility loss in guinea pig and chinchilla AOM models in the early phase of the disease.
The chinchilla AOM model reported in this study showed a slow development of middle ear infection compared with other AOM models such as the AOM model in guinea pigs reported in our previous study (Guan and Gan 2013). The type of bacteria and infective dosage in the middle ear affects the time scale of infectious process and thus can potentially influence the biomechanical changes of the middle ear for sound transmission. In our future studies on AOM induced by HI, the histological changes of the middle ear and mechanical properties of the middle ear soft tissues such as the TM, ossicular joints, and round window membrane will be included.
CONCLUSION
The roles of middle ear pressure, effusion, and structural changes in TM vibration loss in chinchilla AOM ears were quantified over the course of the disease (4 days and 8 days post inoculation). The effects of those three factors on TM mobility loss vary with the course of AOM. The middle ear pressure was the dominant factor on reduction of the TM mobility in 4D AOM ears, but showed little effect in 8D ears when MEE filled the tympanic cavity. The middle ear effusion was the primary factor on TM mobility loss for 8D ears, but affected the 4D ears only at high frequencies. After release of MEP and removal of MEE, there was residual TM mobility loss mainly at low frequencies in both 4D and 8D ears, which was associated with middle ear structural changes. More residual reduction of TM movement occurred in the early phase of the disease. This study establishes that the factors contributing to TM mobility loss in chinchilla middle ears infected with H. influenzae closely resemble those we previously reported in guinea pig middle ears infected with S. pneumoniae.
Research Highlights.
Middle ear pressure and effusion’s effect on TM mobility was assessed in 4 and 8 days AOM.
Middle ear pressure was a dominant factor of TM mobility loss in 4 days AOM.
Middle ear effusion was a dominant factor of TM mobility loss in 8 days AOM.
Effusion reduced TM mobility in 4 days ears only at high frequencies.
After removal of pressure and effusion, there was residual TM loss in both 4 and 8 days ears.
Acknowledgments
We thank Dr. Thomas W. Seale and Brett Cole in Department of Pediatrics at University of Oklahoma Health Science Center for their expert technical assistance on Haemophilus influenzae preparation. The authors also thank Dr. Seale and Dr. Mark Wood at Hough Ear Institute for editing this paper. This work was supported by NIH R01DC011585.
List of Abbreviation
- AOM
acute otitis media
- CFU
colony-forming unit
- HI
Haemophilus influenzae
- LDV
laser Doppler vibrometry
- MEE
middle ear effusion
- MEP
middle ear pressure
- SP
Streptococus pneumoniae
- SPL
sound pressure level
- TM
tympanic membrane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakaletz LO. Chinchilla as a robust, reproducible and polymicrobial model of otitis media and its prevention. Expert Rev Vaccines. 2009;8(8):1063–1082. doi: 10.1586/erv.09.63. [DOI] [PubMed] [Google Scholar]
- Bakaletz LO, Kennedy BJ, Novotny LA, Duquesne G, Cohen J, Lobet Y. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect Immun. 1999;67(6):2746–2762. doi: 10.1128/iai.67.6.2746-2762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone CD, Klein JO. Otitis media with effusion, atelectasis, and eustachian tube dysfuncion. In: Bluestone CD, Stool SE, editors. Pediatric Otolaryngology. W. B. Saunders Company; Philadelphia, PA: 1983. pp. 419–432. [Google Scholar]
- Bluestone CD, Stephenson JS, Martin LM. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11(8 Suppl):S7–11. doi: 10.1097/00006454-199208001-00002. [DOI] [PubMed] [Google Scholar]
- Browning GC, Granich MS. Surgical anatomy of the temporal bone in the chinchilla. Ann Otol Rhinol Laryngol. 1978;87(6 Pt 1):875–882. doi: 10.1177/000348947808700616. [DOI] [PubMed] [Google Scholar]
- Caye-Thomasen P, Hermansson A, Tos M, Prellner K. Pathogenesis of middle ear adhesions. Laryngoscope. 1996;106(4):463–469. doi: 10.1097/00005537-199604000-00013. [DOI] [PubMed] [Google Scholar]
- Caye-Thomasen P, Tos M. Polyp and fibrous adhesion formation in acute otitis media caused by non-typeable or type b Haemophilus influenzae or Moraxella catarrhalis. Acta Otolaryngol. 2000;120(7):810–814. doi: 10.1080/000164800750061642. [DOI] [PubMed] [Google Scholar]
- Dai C, Cheng T, Wood MW, Gan RZ. Fixation and detachment of superior and anterior malleolar ligaments in human middle ear: experiment and modeling. Hear Res. 2007;230(1–2):24–33. doi: 10.1016/j.heares.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Wood MW, Gan RZ. Combined effect of fluid and pressure on middle ear function. Hear Res. 2008;236(1–2):22–32. doi: 10.1016/j.heares.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes ML, Horsey E, Hiller NL, Buchinsky FJ, Hayes JD, Compliment JM, Hillman T, Ezzo S, Shen K, Keefe R, Barbadora K, Post JC, Hu FZ, Ehrlich GD. Strain-specific virulence phenotypes of Streptococcus pneumoniae assessed using the Chinchilla laniger model of otitis media. PLoS One. 2008;3(4):e1969. doi: 10.1371/journal.pone.0001969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan RZ, Dai C, Wood MW. Laser interferometry measurements of middle ear fluid and pressure effects on sound transmission. J Acoust Soc Am. 2006;120(6):3799–3810. doi: 10.1121/1.2372454. [DOI] [PubMed] [Google Scholar]
- Gan RZ, Nakmali D, Zhang X. Dynamic properties of round window membrane in guinea pig otitis media model measured with electromagnetic stimulation. Hear Res. 2013;301:125–136. doi: 10.1016/j.heares.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebink GS. Otitis media: the chinchilla model. Microbial drug resistance. 1999;5(1):57–72. doi: 10.1089/mdr.1999.5.57. [DOI] [PubMed] [Google Scholar]
- Gould JM, Matz PS. Otitis media. Pediatr Rev. 2010;31(3):102–116. doi: 10.1542/pir.31-3-102. [DOI] [PubMed] [Google Scholar]
- Guan X, Gan RZ. Effect of middle ear fluid on sound transmission and auditory brainstem response in guinea pigs. Hear Res. 2011;277(1–2):96–106. doi: 10.1016/j.heares.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Gan RZ. Mechanisms of Tympanic Membrane and Incus Mobility Loss in Acute Otitis Media Model of Guinea Pig. J Assoc Res Otolaryngol. 2013;14(3):295–307. doi: 10.1007/s10162-013-0379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Peake WT. Middle-ear characteristics of anesthetized cats. J Acoust Soc Am. 1967;41(5):1237–1261. doi: 10.1121/1.1910465. [DOI] [PubMed] [Google Scholar]
- Hanamure Y, Lim DJ. Anatomy of the chinchilla bulla and eustachian tube: I. Gross and microscopic study. Am J Otolaryngol. 1987;8(3):127–143. doi: 10.1016/s0196-0709(87)80035-2. [DOI] [PubMed] [Google Scholar]
- Hoa M, Syamal M, Sachdeva L, Berk R, Coticchia J. Demonstration of nasopharyngeal and middle ear mucosal biofilms in an animal model of acute otitis media. Ann Otol Rhinol Laryngol. 2009;118(4):292–298. doi: 10.1177/000348940911800410. [DOI] [PubMed] [Google Scholar]
- Hoberman A, Paradise JL, Rockette HE, Shaikh N, Wald ER, Kearney DH, Colborn DK, Kurs-Lasky M, Bhatnagar S, Haralam MA, Zoffel LM, Jenkins C, Pope MA, Balentine TL, Barbadora KA. Treatment of acute otitis media in children under 2 years of age. N Engl J Med. 2011;364(2):105–115. doi: 10.1056/NEJMoa0912254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerger J. Clinical experience with impedance audiometry. Archives of Otolaryngology. 1970;92(4):311–324. doi: 10.1001/archotol.1970.04310040005002. [DOI] [PubMed] [Google Scholar]
- Larsson C, Dirckx JJ, Decraemer WF, Bagger-Sjoback D, von Unge M. Pars flaccida displacement pattern in purulent otitis media in the gerbil. Otol Neurotol. 2003;24(3):358–364. doi: 10.1097/00129492-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Lee CY, Rosowski JJ. Effects of middle-ear static pressure on pars tensa and pars flaccida of gerbil ears. Hear Res. 2001;153(1–2):146–163. doi: 10.1016/s0378-5955(00)00269-0. [DOI] [PubMed] [Google Scholar]
- Lin J, Tsuboi Y, Pan W, Giebink GS, Adams GL, Kim Y. Analysis by cDNA microarrays of altered gene expression in middle ears of rats following pneumococcal infection. Int J Pediatr Otorhinolaryngol. 2002;65(3):203–211. doi: 10.1016/s0165-5876(02)00130-1. [DOI] [PubMed] [Google Scholar]
- Long JP, Tong HH, Shannon PA, DeMaria TF. Differential expression of cytokine genes and inducible nitric oxide synthase induced by opacity phenotype variants of Streptococcus pneumoniae during acute otitis media in the rat. Infect Immun. 2003;71(10):5531–5540. doi: 10.1128/IAI.71.10.5531-5540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Lu H, Dai C, Gan RZ. A comparison of Young’s modulus for normal and diseased human eardrums at high strain rates. Int J of Experimental and Computational Biomechanics. 2009;1(1):1–22. [Google Scholar]
- MacArthur CJ, Hefeneider SH, Kempton JB, Parrish SK, McCoy SL, Trune DR. Evaluation of the mouse model for acute otitis media. Hear Res. 2006;219(1–2):12–23. doi: 10.1016/j.heares.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Mason KM, Munson RS, Jr, Bakaletz LO. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect Immun. 2003;71(6):3454–3462. doi: 10.1128/IAI.71.6.3454-3462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhus A, Ryan AF. A mouse model for acute otitis media. APMIS. 2003;111(10):989–994. doi: 10.1034/j.1600-0463.2003.1111012.x. [DOI] [PubMed] [Google Scholar]
- Morton DJ, Bakaletz LO, Jurcisek JA, VanWagoner TM, Seale TW, Whitby PW, Stull TL. Reduced severity of middle ear infection caused by nontypeable Haemophilus influenzae lacking the hemoglobin/hemoglobin-haptoglobin binding proteins (Hgp) in a chinchilla model of otitis media. Microb Pathog. 2004;36(1):25–33. doi: 10.1016/j.micpath.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Morton DJ, Hempel RJ, Seale TW, Whitby PW, Stull TL. A functional tonB gene is required for both virulence and competitive fitness in a chinchilla model of Haemophilus influenzae otitis media. BMC research notes. 2012;5:327. doi: 10.1186/1756-0500-5-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima HH, Ravicz ME, Merchant SN, Peake WT, Rosowski JJ. Experimental ossicular fixations and the middle ear’s response to sound: evidence for a flexible ossicular chain. Hear Res. 2005A;204(1–2):60–77. doi: 10.1016/j.heares.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Nakajima HH, Ravicz ME, Rosowski JJ, Peake WT, Merchant SN. Experimental and clinical studies of malleus fixation. The Laryngoscope. 2005B;115(1):147–154. doi: 10.1097/01.mlg.0000150692.23506.b7. [DOI] [PubMed] [Google Scholar]
- Naguib MB, Hunter RE, Henley CM. Cochlear polyamines: markers of otitis media-induced cochlear damage. Laryngoscope. 1994;104(8 Pt 1):1003–1007. doi: 10.1288/00005537-199408000-00015. [DOI] [PubMed] [Google Scholar]
- Paradise JL, Smith CG, Bluestone CD. Tympanometric detection of middle ear effusion in infants and young children. Pediatrics. 1976;58(2):198–210. [PubMed] [Google Scholar]
- Ravicz ME, Rosowski JJ, Merchant SN. Mechanisms of hearing loss resulting from middle-ear fluid. Hear Res. 2004;195(1–2):103–130. doi: 10.1016/j.heares.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Reid SD, Hong W, Dew KE, Winn DR, Pang B, Watt J, Glover DT, Hollingshead SK, Swords WE. Streptococcus pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J Infect Dis. 2009;199(6):786–794. doi: 10.1086/597042. [DOI] [PubMed] [Google Scholar]
- Rosowski JJ, Lee CY. The effect of immobilizing the gerbil’s pars flaccida on the middle-ear’s response to static pressure. Hear Res. 2002;174(1–2):183–195. doi: 10.1016/s0378-5955(02)00655-x. [DOI] [PubMed] [Google Scholar]
- Rosowski JJ, Nakajima HH, Merchant SN. Clinical utility of laser-Doppler vibrometer measurements in live normal and pathologic human ears. Ear Hear. 2008;29(1):3–19. doi: 10.1097/AUD.0b013e31815d63a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC, Robles L, Shivapuja BG. Middle-ear response in the chinchilla and its relationship to mechanics at the base of the cochlea. J Acoust Soc Am. 1990;87(4):1612–1629. doi: 10.1121/1.399409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Bakaletz LO. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect Immun. 1994;62(5):1710–1718. doi: 10.1128/iai.62.5.1710-1718.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JL, Chevallier KM, Koka K, Gabbard SA, Tollin DJ. Conductive hearing loss induced by experimental middle-ear effusion in a chinchilla model reveals impaired tympanic membrane-coupled ossicular chain movement. J Assoc Res Otolaryngol. 2013;14(4):451–464. doi: 10.1007/s10162-013-0388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Unge M, Decraemer WF, Bagger-Sjoback D, Dirckx JJ. Displacement of the gerbil tympanic membrane under static pressure variations measured with a real-time differential moire interferometer. Hear Res. 1993;70(2):229–242. doi: 10.1016/0378-5955(93)90161-s. [DOI] [PubMed] [Google Scholar]
- von Unge M, Decraemer WF, Bagger-Sjoback D, Van den Berghe D. Tympanic membrane changes in experimental purulent otitis media. Hear Res. 1997;106(1–2):123–136. doi: 10.1016/s0378-5955(97)00008-7. [DOI] [PubMed] [Google Scholar]
- Vrettakos PA, Dear SP, Saunders JC. Middle ear structure in the chinchilla: a quantitative study. Am J Otolaryngol. 1988;9(2):58–67. doi: 10.1016/s0196-0709(88)80009-7. [DOI] [PubMed] [Google Scholar]