Abstract
The authors investigated coupling passive sampling technologies with ultraviolet irradiation experiments to study polycyclic aromatic hydrocarbon (PAH) and oxygenated PAH transformation processes in real-world bioavailable mixtures. Passive sampling device (PSD) extracts were obtained from coastal waters impacted by the Deepwater Horizon oil spill and Superfund sites in Portland, Oregon, USA. Oxygenated PAHs were found in the contaminated waters with our PSDs. All mixtures were subsequently exposed to a mild dose of ultraviolet B (UVB). A reduction in PAH levels and simultaneous formation of several oxygenated PAHs were measured. Site-specific differences were observed with UVB-exposed PSD mixtures. Environ Toxicol Chem 2014;33:XX–XX. © 2013 The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals, Inc. on behalf of SETAC. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial, and no modifications or adaptations are made.
Keywords: Photo-oxidation, Emerging contaminants, Oxy-PAH, Ultraviolet, Passive sampling
INTRODUCTION
Humans and organisms are exposed to complex mixtures of hazardous chemicals at Superfund sites or in waters impacted by oil spills. Characterizing exposure and associated risks in these scenarios is complicated by the fact that mixture composition changes over time. For example, natural ultraviolet B (UVB) radiation and technologies used for in situ contaminant remediation (chemical oxidation and bioremediation) are known to degrade components of chemical mixtures [1–4]. However, degradation of target chemicals produces new compounds that may have limited or no toxicological information [5,6]. Given these uncertainties, it is now recognized that more comprehensive sampling and analytical approaches are needed to fully understand the effects of chemical transformation processes on chemical fate, exposure estimates, and subsequent risks to human and environmental health [7–9].
Ultraviolet radiation is known to influence the toxicity of polycyclic aromatic hydrocarbons (PAHs). Studies conducted in a host of different biological models indicate that coexposure to UV radiation and PAHs markedly increased toxicity compared with PAH exposure alone [7,8,10–13]. It has also been shown that PAHs can be photomodified to ketone- and quinone-containing PAH (OPAH) derivatives in aqueous solutions [6], surfactant solutions [14], reaction chambers in the gas phase [15], and soils [16]. These OPAHs have been measured in a wide variety of environmental samples [17,18], and some display increased potency and unique biological activity relative to their PAH homologs [6,7,19,20]. Studies of the type described above are generally limited in scope, however, because they do not identify chemical agents associated with increased toxicity, they conduct irradiation experiments with only 1 or 2 representative compounds, they have uncertainty associated with PAH bioavailability and bioaccumulation, or they ignore mixture effects and interactions. These knowledge gaps limit our understanding of chemical fate at Superfund sites and oil spills. Using passive sampling devices (PSDs) to study real-world degradation processes in the presence of environmental stressors may provide a convenient and powerful strategy for addressing these unanswered questions.
Passive sampling devices concentrate the bioavailable fraction (freely dissolved concentration [Cfree]) of contaminants from water, air, and sediment [21]. Mechanisms that control partitioning from sampling media into PSDs are similar to those that control passive chemical uptake by organisms—namely, contaminant diffusion across biological membranes [21,22]. Physical properties of PSDs allow them to sequester contaminants at concentrations multiple orders of magnitude greater than those in the environment while nominally conserving chemical concentration ratios. Low-density polyethylene (LDPE) tubing has been used successfully to passively sample PAHs, the main toxic component of crude oil and a contaminant class commonly targeted for remediation at Superfund sites [23–25]. Furthermore, PSDs can be paired directly with bioassays to identify biological responses elicited by site-specific contaminant mixtures [26,27].
Although PSDs are established for measuring the bioavailable (Cfree) fraction of chemicals in water, further insights into the biogeochemical processes that alter Cfree, such as UVB light exposure, may provide a powerful new approach. The objectives of the present study were to 1) assess whether real-world mixtures of bioavailable PAHs, sampled using PSDs deployed at environmental sites, can be photodegraded; 2) illustrate that PSDs sequester OPAHs from contaminated aquatic environments; and 3) demonstrate that OPAHs are formed from PSD mixtures after exposure to UVB.
MATERIALS AND METHODS
Site description and test mixtures
Test mixtures used for UVB irradiation were of 2 types: a standard mixture of 16 US Environmental Protection Agency priority pollutant PAHs [13] and environmental PSD extracts, in which PSDs were composed of LDPE. The PSDs were deployed in Portland Harbor (OR, USA), a 9-mile section of the Willamette River designated a Superfund site in 2000 because of high levels of PAHs, polychlorinated biphenyls, dioxins, pesticides, and metals in sediments and water [28]. The PSDs were deployed in the Willamette River on the west bank at river miles (RM) 6.5 and 7 (Supplemental Data, Figure S1), in September 2010 and October 2009 respectively [27]. Further site characterization and sampling details have been described by Allan et al. [27]. Other PSDs were deployed at coastal sites in the Gulf of Mexico during and after the Deepwater Horizon oil spill [29]. Gulf coast extracts used in the present study came from 2 sites: Grand Isle, Louisiana, USA (June 2010) and Gulf Breeze, Florida, USA (April 2011; Supplemental Data, Figure S2). The site at Grand Isle was located closer to the source of the spill and was visibly impacted by oil during the June 2010 sampling event, whereas the Gulf Breeze site was more protected from direct oiling. Both sites were impacted by local urban and industrial activities. Further description of sampling and site characterizations have been detailed by Allan et al. [29].
Field-exposed PSDs were extracted into hexane using methods detailed elsewhere [27,29] and concentrated to 1 mL final volume under filtered nitrogen. The summed concentrations of 33 PAHs in PSD extracts from Florida, Portland Harbor RM 6.5 west (w), Portland Harbor RM 7w, and Louisiana were 470 ng/mL, 20 000 ng/mL, 88 000 ng/mL, and 240 000 ng/mL, respectively [27,29]. The 4 sites also had vastly different PAH mixture chemical profiles [27,29]. For the UVB experiment, standard test mixtures of 16 priority pollutant PAHs were prepared by diluting purchased stock solutions (AccuStandard) to 1000 ng/mL in n-hexane for each individual PAH. This produced a solution with a summed PAH concentration of 16 000 ng/mL, which was within the range of field samples. Transportation and laboratory quality control samples were used throughout the present study to ensure data quality. Further details on quality control, PSD extraction, and instrumental analysis are provided in the Supplemental Data and Allan et al. [27,29].
UV irradiation conditions and analysis
Ultraviolet irradiation experiments were conducted using 2 parallel 1.83-m fixed-wavelength UVB bulbs (Philips) with a Lithonid Lighting fluorescent fixture (Underwriters Labs). Radiation was emitted at a wavelength of 313 nm with an irradiance of 230 µW cm−2, a level similar to summer environmental conditions in the United States [10–12]. Test mixtures (1 mL) were contained on 4-mL watch glasses placed 22.5 cm below the UV source. All test sets were open to the atmosphere during exposure to UVB. Mixtures of priority pollutant PAHs were irradiated in triplicate for 0 min, 3 min, 10 min, and 30 min, similar to methods described by Fasnacht and Blough [30]. Each PSD mixture was irradiated for 30 min only. Duplicate PSD extracts from Portland Harbor RM 7w were irradiated to assess the repeatability of the whole analytical approach. Sample volumes were maintained through replenishment with n-hexane. For the purposes of the present study, mixtures were kept in n-hexane to reduce solubility issues with water; to allow for the analytically preferable low-volume, high-concentration setup; and to provide evidence that OPAH formation was possible with this UVB setup with PSD extracts. Negative controls, included with each replicate experiment, were placed under UV lights for the duration of experiments and consisted of an aliquot of the priority pollutant PAH test mixture open to the atmosphere but covered with aluminum foil.
Pre- and post-UV-irradiated samples were analyzed for 33 PAHs by using a gas chromatography–mass spectrometry (GC-MS) method described by Forsberg et al. [31] and 22 OPAHs using a GC-MS method modified from Layshock et al. [18]. Instrumental details are further described in Supplemental Data, Table S1. Briefly, each analyte was positively identified by retention time, target ion, and 2 unique confirmation ion masses. Confirmation ions occurred at the same retention time as target ions and had to be within ±35% of expected values to be considered confirmatory. The PAHs and OPAHs were quantified using internal standard calibration with calibration curves composed of 7 to 9 points and coefficients of determination (R2) that were in general greater than 0.99. Data interpretation was performed in SigmaPlot for Windows 11.0 (Systat Software) and Microsoft Excel 2007. One-way analyses of variance (ANOVAs) and two-sample t tests were used to examine treatment-related differences in analyte levels. Statistical analyses were considered significant at p ≤ 0.05 and suggestive at >0.05 and <0.1.
RESULTS AND DISCUSSION
Changes in test mixture PAH concentrations were observed using UVB irradiation durations and intensities that were much less than environmental conditions present at our field sites [10–12] and irradiance levels similar to, or lower than, those used in remediation based studies [32,33]. Levels of pyrene, benz[a]anthracene, indeno[1,2,3-cd]perylene, and dibenz[a,h]anthracene were significantly decreased relative to negative controls after 30 min of irradiation (p < 0.05), with average decreases ranging from 55 ng/mL to 110 ng/mL, or 11% to 15% (Supplemental Data, Figure S3). Although rate constants were not calculated, PAH photodegradation appeared to occur more slowly than reported by Fasnacht and Bluogh [30] and likely is due to differences in irradiance intensities between studies and lower levels of oxygen in the present study's n-hexane test system [34]. The degradation percentage of individual PAHs in PSD and priority pollutant PAH test mixtures was similar at the 30-min time point (Supplemental Data, Table S2 and Figure S3). Additionally, duplicate irradiation experiments conducted with Portland Harbor RM 7w PSD extracts generated relative difference percentages that were generally less than 25% for PAHs. There was no indication of significant UVB-mediated PAH degradation in standard solutions at 0 min, 3 min, or 10 min.
Several OPAHs were detected in priority pollutant PAH test mixtures after 30 min of UVB irradiation (Figure 1 and Table 1). Among 22 OPAHs monitored, 3 were identified, including 9-fluorenone (9-FLUO), 9,10-anthraquinone (9,10-ANTQ), and 7,12-benz[a]anthracenequinone (BaANCQ), concentrations of which ranged from 20 ng/mL to 50 ng/mL. All OPAHs detected in this experiment have structures that correspond to homolog PAHs present in priority pollutant PAH test mixtures, namely, fluorene, anthracene, and benz[a]anthracene. Interestingly, anthracene and benz[a]anthracene were significantly decreased after 30 min of UVB exposure. Results from the current n-hexane experiments compared well with results reported for simulated solar radiation-induced photodegradation of anthracene in aqueous solutions [6], suggesting that UV exposure in n-hexane conserved dominant PAH transformation pathways. Other OPAHs might have formed but were not identified because of the limited number of commercially available OPAH reference standards. Potential routes to identifying other OPAHs not reported in the present study could include the use of high-resolution time-of-flight mass spectrometry and/or more complex GC × GC chromatographic methods.
Figure 1.
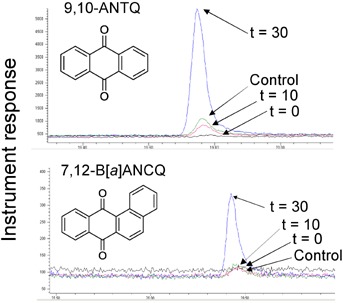
Overlay of gas chromatography–mass spectrometry selected ion monitoring total ion chromatograms demonstrating ultraviolet B (UVB)-induced formation of oxygenated polycyclic aromatic hydrocarbons (PAHs) from UVB-irradiated PAH standard solutions after 0 min, 10 min, and 30 min. The PAH homologues of 9,10-anthraquinone (9,10-ANTQ) and 7,12-benz[a]anthraquinone (7,12-BaANCQ) were present in PAH standards prior to UVB irradiation, namely, anthracene and benz[a]anthracene.
Table 1.
Oxygenated polycyclic aromatic hydrocarbons (OPAH) concentrations (ng/mL) and percentage of change in concentrations (% Δ) for standard test mixtures, Superfund, and Gulf of Mexico passive sampling device extracts before (C0) and after (C30) 30 min of ultraviolet B exposure
| PP PAH standard (average, n = 3) |
Portland Harbor Superfunda |
Gulf of Mexicob |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RM 7w (average, n = 2) |
RM 6.5w (n = 1) |
Louisiana (n = 1) |
Florida (n = 1) |
||||||||||||
| OPAH | C0 | C30 | % Δc | C0 | C30 | % Δc | C0 | C30 | % Δc | C0 | C30 | % Δc | C0 | C30 | % Δc |
| 9-FLUO | 13.9 | 20.0 | 43 | 26.4 | 29.4 | 11 | 38.7 | 39.3 | NC | ND | ND | NC | 12.8 | 12.7 | NC |
| 9,10-ANTQ | 29.6 | 49.3 | 67 | 176 | 149 | −15 | 292 | 321 | 10 | 4380 | 3330 | −24 | 45.2 | 40.8 | −10 |
| CPdefPHEO | 26.8 | 26.5 | NC | 78.1 | 91.0 | 16 | 143 | 158 | 10 | ND | ND | NC | 28.3 | 28.5 | NC |
| BaFLUO | ND | ND | NC | 192 | 271 | 41 | 752 | 1000 | 33 | 2280 | 2450 | NC | 33.8 | 34.0 | NC |
| AANEQ | ND | ND | NC | 44.5 | 45.8 | NC | 60.0 | ND | −100 | ND | ND | NC | ND | ND | NC |
| BaANCQ | ND | 46.9 | 100 | 81.9 | 95.6 | 17 | 158 | 223 | 41 | 586 | 543 | NC | 45.0 | 43.0 | NC |
| 5,12-NAPQ | ND | ND | NC | ND | ND | NC | 124 | 161 | 30 | ND | ND | NC | ND | ND | NC |
Samples collected from the Willamette River at river miles 7 west (w) and 6.5w; 7w represents the average of PSD field duplicates [27].
Samples collected from Grand Isle, Louisiana, USA, and Gulf Breeze, Florida, USA, coastal waters during and after the Deepwater Horizon oil spill [29].
% Δ = 100 × [(C30/C0) − 1]; percentage change, where C0 = negative control in PP PAH standards exposures and non-UV-exposed extracts for PSD exposures.
PP PAH = US Environmental Protection Agency priority pollutant polyaromatic hydrocarbon; RM = river mile; 9-FLUO = 9-fluorenone; 9,10-ANTQ = 9,10-anthraquinone; CPdefPHEO = 4H-cyclopenta[def]phenanthrene-4-one; BaFLUO = benzo[a]-11-fluorenone; AANEQ = aceanthrenequinone; BaANCQ = benz[a]anthracenequinone; 5,12-NAPQ = 5,12-napthacenequinone. NC = change was less than 10% between C0 and C30; ND = not detected in C0 or C30.
Many OPAHs were detected directly in PSD extracts, illustrating their potential importance in exposure assessment. The OPAHs identified included 9-FLUO, 9,10-ANTQ, 4H-cyclopenta[def]phenanthrene-4-one (CPdefPHEO), benzo[a]-11-fluorenone (BaFLUO), AANEQ, and BaANCQ; an additional OPAH, 5,12-napthacenequinone (5,12-NAPQ), was present at the Portland Harbor RM6.5w site. The log octanol–water partition coefficient (KOW) for OPAHs detected in PSDs ranged from 3.6 to 4.7, the same as many PAHs that are readily sampled using LDPE PSDs [21]. Low concentrations of OPAHs were detected in the Florida sample, which was collected 1 yr after the Deepwater Horizon oil spill (Table 1). In contrast, the Gulf of Mexico Louisiana site had very large OPAH concentrations during the oil spill. For example, 9,10-ANTQ was 400-fold higher in Louisiana during the Deepwater Horizon oil spill than in Florids 1 yr later. In addition, BaFLUO and BaANCQ were present in Louisiana PSD extracts. A more detailed report on passive sampling and its use for studying OPAH environmental occurrence is in preparation.
Oxygenated PAHs were formed or displayed increased concentrations in UVB-irradiated PSD extracts (Table 1). Notable increases in CPdefPHEO, BaFLUO, and BaANCQ concentrations were observed in all irradiated Portland Harbor Superfund PSD extracts. The largest increases in concentrations at all Superfund sites were for BaFLUO and BaANCQ compared with the pre-UVB concentrations. In contrast, there was little or no change in OPAH concentrations in the Gulf of Mexico samples. This probably is due to a combination of environmental factors, including differences in sunlight intensity in the Gulf compared with Portland Harbor, which led to different mixture compositions as noted above. Waters from the Gulf sites had large OPAH concentrations compared with other sites prior to in-laboratory UVB exposure. Although anthracene is a component of the nonweathered Deepwater Horizon oil [23], anthracene was not detected in our original PSD extracts (Supplemental Data, Table S2). However, 9,10-ANTQ was present at more than 2200 ng/mL, suggesting that transformations had already occurred in the Gulf prior to sampling. Because parent PAHs that are susceptible to UVB transformations were no longer present in the PSD Gulf extracts, further UVB exposures did not produce additional or increased OPAHs. Additional differences in the extent of OPAH formation between sites may be related to other chemical constituents present in PSD extracts, such as humic substances and other photochemically active compounds [34,35].
Previous investigations have reported that OPAHs can form from single PAHs in the presence of electromagnetic radiation [6,14–16,36]. For example, Brack et al. [6] demonstrated that aqueous solutions of anthracene can undergo photomodification in simulated solar radiation to produce several oxygenated PAHs, including 9,10-ANTQ and 1,4-anthracenequinone, where the proposed reaction mechanism proceeded via a Diels–Alder cycloaddition with a singlet oxygen dienophile [6]. All OPAHs identified in the present study—namely, BaANCQ, 9-FLUO, 9,10-ANTQ, and BaFLUO—have been measured in environmental samples or have been demonstrated to form from irradiated PAHs [6,17]. The OPAHs observed in the present study are consistent with the same chemical species shown to dominate many environmental OPAH chemical abundance profiles [17,37], suggesting that the coupled PSD–UV irradiation approach described in the present study conserved environmentally relevant transformation processes.
Coupling PSDs to UVB is a demonstration of a new approach. Although aqueous exposures are feasible, lipophilic n-hexane was used because it is an ideal solvent for extracting contaminants out of PSDs and is well suited to sample concentration. Furthermore, many real-world aquatic PAH–UV exposures occur in the presence of organic phases, such as dissolved organic carbon. Biota accumulate PAHs into lipophilic tissues, where they can be photosensitized by UV light to result in phototoxicity [38]. Additionally, quantitative structure–activity relationship studies conducted by Newsted and Giesy [39] and further refined by Makenyan et al. [40] show that the phototoxicity of PAHs is largely described by the structural stability of the PAH molecule, described by the HOMO–LUMO gap, which is independent of solvent [38]. The fact that phototoxicity studies equate biological effect to absorbed doses suggests that it would be useful to have experimental approaches capable of employing a broad array of solvent systems with characteristics similar to these lipophilic environments. The paired PSD–UV approach described here has this capacity.
It is difficult to find advancing technologies that broaden our understanding of exposures to emerging contaminants such as OPAHs. Coupling PSD technologies to UV addresses some of these issues. PSD extracts provide chemical mixtures that better reflect site-specific exposure scenarios, and the solvent system of PSD extracts can be exchanged prior to irradiation experiments to simulate different exposure environments. Importantly, the approach allows for the identification and quantification of new chemicals. Thus, pairing PSD technologies with UV irradiation may help researchers better characterize changes in chemical exposure on a site-specific basis, allow for the identification of nonregulated toxicants characterized by little or no toxicity data, and extend the application of existing mixture toxicity evaluation methods.
Acknowledgments
The project described was supported in part by Award P42 ES016465 and the associated Analytical Chemistry Facility Core, P30 ES000210 and R21 ES020120, from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institute of Health. We would like to thank Grand Isle State Park and Gulf Shores National Seashore. We appreciate valuable help from L. Tidwell, K. Hobbie, and M. McCartney. We would also like to thank P. Hoffman for his assistance with UV experiments and G. Wilson for his help with instrumental analysis.
SUPPLEMENTAL DATA
Tables S1–S2.
Figures S1–S3. (1 MB DOC).
Supporting Information
All Supplemental Data may be found in the online version of this article.
Supplemental Data.
REFERENCES
- 1.United States Environmental Protection Agency. 1998. Field applications of in situ remediation technologies: Chemical oxidation. EPA 542/R-98/008. Washington, DC.
- 2.Ferrarese E, Andreottola G, Oprea IA. Remediation of PAH-contaminated sediments by chemical oxidation. J Hazard Mater. 2008;152:128–139. doi: 10.1016/j.jhazmat.2007.06.080. [DOI] [PubMed] [Google Scholar]
- 3.Gan S, Lau EV, Ng HK. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs) J Hazard Mater. 2009;172:532–549. doi: 10.1016/j.jhazmat.2009.07.118. [DOI] [PubMed] [Google Scholar]
- 4.O'Mahony MM, Dobson ADW, Barnes JD, Singleton I. The use of ozone in the remediation of polycyclic aromatic hydrocarbon contaminated soil. Chemosphere. 2006;63:307–314. doi: 10.1016/j.chemosphere.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 5.McConkey BJ, Duxbury CL, Dixon DG, Greenberg BM. Toxicity of a PAH photooxidation product to the bacteria photobacterium phosphoreum and the duckweed Lemna gibba: Effects of phenanthrene and its primary photoproduct, phenanthrenequinone. Environ Toxicol Chem. 1997;16:892–899. [Google Scholar]
- 6.Brack W, Altenburger R, Küster E, Meissner B, Wenzel K-D, Schüürmann G. Identification of toxic products of anthracene photomodification in simulated sunlight. Environ Toxicol Chem. 2003;22:2228–2237. doi: 10.1897/02-450. [DOI] [PubMed] [Google Scholar]
- 7.Lampi MA, Gurska J, McDonald KIC, Xie F, Huang X-D, Dixon DG, et al. Photoinduced toxicity of polycyclic aromatic hydrocarbons to Daphnia magna: Ultraviolet-mediated effects and the toxicity of polycyclic aromatic hydrocarbon photoproducts. Environ Toxicol Chem. 2006;25:1079–1087. doi: 10.1897/05-276r.1. [DOI] [PubMed] [Google Scholar]
- 8.Incardona JP, Vines CA, Linbo TL, Myers MS, Sloan CA, Anulacion BF, Boyd D, Collier TK, Morgan S, Cherr GN, Scholz NL. Potent phototoxicity of marine bunker oil to translucent herring embryos after prolonged weathering. PLoS One. 2012;7:e30116. doi: 10.1371/journal.pone.0030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute of Environmental Health Sciences. 2012. 2012–2017 strategic plan—Advancing science, improving health: A plan for environmental health research. NIH Publication No. 12-7935. US Department of Health and Human Services and National Institutes of Health, Research Triangle Park, NC.
- 10.Peachey RBJ. The synergism between hydrocarbon pollutants and UV radiation: A potential link between coastal pollution and larval mortality. J Exp Mar Biol Ecol. 2005;315:103–114. [Google Scholar]
- 11.Pelletier MC, Burgess RM, Ho KT, Kuhn A, McKinney RA, Ryba SA. Phototoxicity of individual polycyclic aromatic hydrocarbons and petroleum to marine invertebrate larvae and juveniles. Environ Toxicol Chem. 1997;16:2190–2199. [Google Scholar]
- 12.Ankley GT, Collyard SA, Monson PD, Kosian PA. Influence of ultraviolet light on the toxicity of sediments contaminated with polycyclic aromatic hydrocarbons. Environ Toxicol Chem. 1994;13:1791–1796. [Google Scholar]
- 13.Yan J, Wang L, Fu PP, Yu H. Photomutagenicity of 16 polycyclic aromatic hydrocarbons from the USEPA priority pollutant list. Mutat Res Genet Toxicol Environ Mutagen. 2004;557:99–108. doi: 10.1016/j.mrgentox.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigman ME, Schuler PF, Ghosh MM, Dabestani RT. Mechanism of pyrene photochemical oxidation in aqueous and surfactant solutions. Environ Sci Technol. 1998;32:3980–3985. [Google Scholar]
- 15.Lee J, Lane DA. Formation of oxidized products from the reaction of gaseous phenanthrene with the OH radical in a reaction chamber. Atmos Environ. 2010;44:2469–2477. [Google Scholar]
- 16.Fatiadi AJ. Effects of temperature and of ultraviolet radiation on pyrene absorbed on garden soil. Environ Sci Technol. 1967;1:570–572. doi: 10.1021/es60007a003. [DOI] [PubMed] [Google Scholar]
- 17.Lundstedt S, White PA, Lemieux CL, Lynes KD, Lambert IB, Öberg L, Haglund P, Tysklind M. Sources, fate, and toxic hazards of oxygenated polycyclic aromatic hydrocarbons (PAHs) at PAH-contaminated sites. Ambio. 2007;36:475–485. doi: 10.1579/0044-7447(2007)36[475:sfatho]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Layshock JA, Wilson G, Anderson KA. Ketone and quinone-substituted polycyclic aromatic hydrocarbons in mussel tissue, sediment, urban dust, and diesel particulate matrices. Environ Toxicol Chem. 2010;29:2450–2460. doi: 10.1002/etc.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren L, Huang XD, McConkey BJ, Dixon DG, Greenberg BM. Photoinduced toxicity of three polycyclic aromatic hydrocarbons (fluoranthene, pyrene, and naphthalene) to the duckweed Lemna gibba l. G-3. Ecotoxicol Environ Saf. 1994;28:160–171. doi: 10.1006/eesa.1994.1042. [DOI] [PubMed] [Google Scholar]
- 20.Knecht AL, Goodale BC, Truong L, Simonich MT, Swanson AJ, Matzke MM, Anderson KA, Waters KM, Tanguay RL. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol Appl Pharmacol. 2013;271:266–275. doi: 10.1016/j.taap.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huckins JN, Petty JD, Booij K. Monitors of Organic Chemicals in the Environment: Semipermeable Membrane Devices. New York, NY, USA: Springer; 2006. [Google Scholar]
- 22.Mayer P, Tolls J, Hermens JLM, Mackay D. Equilibrium sampling devices. Environ Sci Technol. 2003;37:184A–191A. doi: 10.1021/es032433i. [DOI] [PubMed] [Google Scholar]
- 23.Diercks A-R, Highsmith RC, Asper VL, Joung D, Zhou Z, Guo L, Shiller AM, Joye SB, Teske AP, Guinasso N, Wade TL, Lohrenz SE. Characterization of subsurface polycyclic aromatic hydrocarbons at the Deepwater Horizon site. Geophys Res Lett. 37:L20602. [Google Scholar]
- 24.Sower GJ, Anderson KA. Spatial and temporal variation of freely dissolved polycyclic aromatic hydrocarbons in an urban river undergoing superfund remediation. Environ Sci Technol. 2008;42:9065–9071. doi: 10.1021/es801286z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson KA, Sethajintanin D, Sower G, Quarles L. Field trial and modeling of uptake rates on in situ lipid-free polyethylene membrane passive sampler. Environ Sci Technol. 2008;42:4486–4493. doi: 10.1021/es702657n. [DOI] [PubMed] [Google Scholar]
- 26.Hillwalker WE, Allan SE, Tanguay RL, Anderson KA. Exploiting lipid-free tubing passive samplers and embryonic zebrafish to link site specific contaminant mixtures to biological responses. Chemosphere. 2010;79:1–7. doi: 10.1016/j.chemosphere.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allan SE, Smith BW, Tanguay RL, Anderson KA. Bridging environmental mixtures and toxic effects. Environ Toxicol Chem. 2012;31:2877–2887. doi: 10.1002/etc.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lower Willamette Group. 2009. Portland Harbor RI/FS remedial investigation report. IC09-0003, Vol 1–14. Portland, OR, USA.
- 29.Allan SE, Smith BW, Anderson KA. Impact of the Deepwater Horizon oil spill on bioavailable polycyclic aromatic hydrocarbons in Gulf of Mexico coastal waters. Environ Sci Technol. 2012;46:2033–2039. doi: 10.1021/es202942q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fasnacht MP, Blough NV. Aqueous photodegradation of polycyclic aromatic hydrocarbons. Environ Sci Technol. 2002;36:4364–4369. doi: 10.1021/es025603k. [DOI] [PubMed] [Google Scholar]
- 31.Forsberg ND, Wilson GR, Anderson KA. Determination of parent and substituted polycyclic aromatic hydrocarbons in high-fat salmon using a modified QuEChERS extraction, dispersive SPE and GC–MS. J Agric Food Chem. 2011;59:8108–8116. doi: 10.1021/jf201745a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rababah A, Matsuzawa S. Treatment system for solid matrix contaminated with fluoranthene. II—Recirculating photodegradation technique. Chemosphere. 2002;46:49–57. doi: 10.1016/s0045-6535(01)00090-x. [DOI] [PubMed] [Google Scholar]
- 33.Dong D, Li P, Li X, Xu C, Gong D, Zhang Y, Zhao Q, Peng L. Photocatalytic degradation of phenanthrene and pyrene on soil surfaces in the presence of nanometer rutile TiO2 under UV-irradiation. Chem Eng J. 158:378–383. [Google Scholar]
- 34.Clark CD, De Bruyn WJ, Ting J, Scholle W. Solution medium effects on the photochemical degradation of pyrene in water. J Photochem Photobiol A. 2007;186:342–348. [Google Scholar]
- 35.Zeng K, Hwang H-M, Yuzuri H. Effect of dissolved humic substances on the photochemical degradation rate of 1-aminopyrene and atrazine. Int J Mol Sci. 2002;3:1048–1057. [Google Scholar]
- 36.Barbas JT, Sigman ME, Dabestani R. Photochemical oxidation of phenanthrene sorbed on silica gel. Environ Sci Technol. 1996;30:1776–1780. [Google Scholar]
- 37.Albinet A, Leoz-Garziandia E, Budzinski H, Villenave E, Jaffrezo JL. Nitrated and oxygenated derivatives of polycyclic aromatic hydrocarbons in the ambient air of two french alpine valleys: Part 1: Concentrations, sources and gas/particle partitioning. Atmos Environ. 2008;42:43–54. [Google Scholar]
- 38.Ankley GT, Burkhard LP, Cook PM, Diamond SA, Erickson RJ, Mount DR, Douben PET. PAHs: An Ecotoxicological Perspective. Chichester, UK: John Wiley & Sons; 2003. Assessing risks from photoactivated toxicity of PAHs to aquatic organisms; pp. 275–296. [Google Scholar]
- 39.Newsted JL, Giesy JP. Predictive models for photoinduced acute toxicity of polycyclic aromatic hydrocarbons to Daphnia magna, Strauss (Cladocera, Crustacea) Environ Toxicol Chem. 1987;6:445–461. [Google Scholar]
- 40.Mekenyan OG, Ankley GT, Veith GD, Call DJ. QSARs for photoinduced toxicity: I. Acute lethality of polycyclic aromatic hydrocarbons to Daphnia magna. Chemosphere. 1994;28:567–582. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data.


