Abstract
Aim
Human papillomavirus (HPV) is a pathogenic factor of squamous cell carcinoma in various mucosal locations, including anal carcinoma (ACA). It is also known that patients positive for HIV are at high risk of ACA. The goal of this study was to examine clinical outcome in ACA in relation to HPV/p16 positivity, histologic tumor differentiation, and HIV status. Patients with oropharyngeal cancers that are positive for HPV and show overexpression of p16 as well as having non-keratinizing/basaloid histology have been reported to have better outcomes following chemoradiation (CRT). However, such relationships in ACA remain unknown.
Methods
Forty-two patients with SCC of the anus treated with CRT between 1997 and 2009 were identified. The tumors were subclassified as either non-keratinizing (including basaloid) or keratinizing categories. HPV testing was performed using SPF10-PCR, and all cases were immunostained for p16.
Results
There were 23 men and 19 women; 43 % of men and 11 % of women were HIV-positive (p =0.04). Fifty-five percent of patients had local disease (stages I and II) and 41 % were stages III and IV, with 4 % stage unknown. All tumors were positive for high-oncogenic risk HPVs, and all were positive with p16 immunostain. Sixty-four percent of tumors were non-keratinizing/basaloid and 36 % were keratinizing. The keratinizing tumors were more common in HIV-positive patients (67 %), whereas non-keratinizing/basaloid tumors were more common in HIV-negative patients (77 %) (p =0.008). Thirty-one percent of patients had recurrence of disease, including 50 % HIV-positive patients and 23 % HIV-negative patients (p =0.09). There was no difference in the recurrence rate between non-keratinizing and keratinizing tumor subtypes (p =0.80). The 24-month recurrence-free survival for the cohort was 66 % (95 % CI=46 %, 81 %), with HIV-positive patients having worse recurrence-free survival compared to HIV-negative patients (HR=2.85, 95 % CI= 0.95, 8.53; p =0.06).
Conclusion
The regional and distant failure rate was not related to HPV/p16 positivity or histologic differentiation of ACA; however, HIV positivity appeared to be associated with a higher recurrence rate and worse recurrence-free survival.
Keywords: Anal carcinoma, Basaloid carcinoma, Keratinizing carcinoma, HPV, p16, HIV
Introduction
Anal carcinoma (ACA) is a relatively uncommon malignancy with a yearly incidence of 1.4/100,000 for men and 1.9/100,000 for women in the US [1]. Recent molecular studies of anal cancer have shown that up to 90 % of tumors are positive for high-oncogenic risk human papillomavirus (HPVs) and most frequently for HPV16 [2]. High-risk HPVs have been implicated in pathogenesis of cancers of various lower genital and oropharyngeal sites. Oropharyngeal squamous cell carcinomas that are positive for HPVs have been shown to have better prognosis than HPV-negative tumors [3]. In addition, immunohistochemical overexpression of p16 in tumor cells, a marker of HPV transcription, has been found to be prognostic of a more favorable outcome [4]. Paralleling the findings in oropharyngeal cancer, p16 positivity in vulvar cancer was shown to be associated with longer disease-free survival and overall survival using univariate analysis [5]. HPV and p16 positivity in squamous cell carcinoma is frequently related to a specific histopathologic tumor differentiation, namely non-keratinizing or basaloid tumor subtype. The study of oropharyngeal cancers by Chernock et al. [6] reported that non-keratinizing/basaloid tumors occurred in younger patients that were more often male and had better overall and disease-specific survival as compared to keratinizing carcinomas. The authors concluded that subclassification of oropharyngeal cancers by histologic type adds important clinical information [6].
Few studies have examined prognostic significance of HPV positivity, p16 expression, and histopathologic tumor differentiation in anal cancer. The goal of our study was to evaluate consecutive cases of invasive anal carcinoma for HPV and p16 positivity and tumor differentiation, as well as HIV status, to determine the prognostic significance of these clinicopathologic parameters.
Methods
Case Selection
The surgical pathology files of the Department of Pathology at Weill Medical College of Cornell University were searched from 1997 to 2009 to identify successive cases of anal carcinoma. A total of 42 patients with squamous cell carcinoma of the anus treated with chemoradiation were identified. Pathologic slides were reviewed by a single pathologist (ECP) and tumors categorized as either non-keratinizing (including basaloid) or keratinizing squamous cell carcinoma according to WHO classification [7]. The medical records for all patients were retrieved and analyzed. IRB approval has been obtained for this study.
p16 Immunostaining
The sections were subjected to heat-induced antigen retrieval and incubated in an automated stainer with p16 antibody (Dako, Glostrup, Denmark), at a dilution of 1:25, stained with diaminobenzidine chromogen and counterstained with hematoxylin. The staining was graded as 0—negative, 1—weak and focal nuclear or cytoplasmic blush, and 2—moderate to strong intensity nuclear and cytoplasmic staining with diffuse or patchy distribution.
HPV Testing
HPV testing was performed on available cases. Tissue digestion and DNA release with proteinase K were performed using standard methods. Broad-spectrum HPV DNA amplification was performed using the SPF10 PCR-DEIA-LiPA25, version 1 (Labo Biomedical Products, Rijswijk, The Netherlands), as described previously [8] and according to the manufacturer’s instructions. Briefly, the PCR conditions were as follows: activation of AmpliTaq Gold for 9 min at 94 °C was followed by 40 cycles of 30 s at 94 °C, 45 s at 52 °C, and 45 s at 72 °C with a final extension of 5 min at 72 °C. Each experiment was performed with a separate positive and negative control. All HPV-positive samples were further genotyped using HPV LiPA25, version 1 (Labo Biomedical Products, Rijswijk, The Netherlands). The system is a reverse hybridization method that can identify 15 high-risk HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68/73, and ten low-risk HPV types 6, 11, 34, 40, 42, 43, 44, 54, 70, and 74 [8].
Statistical Analyses
Descriptive statistics (including mean, standard deviation, median, range, frequency, and percent) were calculated to characterize the study cohort. The two-sample t test was used to compare age at diagnosis between groups of interest (i.e., HIV status) and the Pearson’s chi-square test or Fisher’s exact was used, as appropriate, to assess relationships between categorical variables of interest (i.e., gender, HIV status, stage, histologic tumor type, and tumor recurrence). Kaplan–Meier survival analysis was performed to estimate recurrence-free survival (RFS) for the cohort, and 24-month RFS was reported from the survival curve. The log-rank test was used to compare RFS between levels of categorical variables of interest. All p values are two-sided with statistical significance evaluated at the 0.05 alpha level. Ninety-five percent confidence intervals (95 % CI) for 24-month RFS and univariate hazard ratios (HR) were calculated to assess the precision of the obtained estimates. All analyses were performed in SPSS Version 21.0 (SPSS Inc., Chicago, IL).
Results
Clinicopathologic Data
Of a total of 42 patients identified in the computerized charts, 23 were men and 19 were women, with 43 % of men and 11 % of women being positive for HIV (p =0.04) (Table 1). The average age for all patients was 59 (range, 36 to 88)years; however, the patients positive for HIV were on average two decades younger (44 years) than the HIV-negative patients (65 years) (p <0.0001). Fifty-five percent of patients had local disease (stages I and II) and 41 % presented with advanced tumor spread (stages III and IV). The stage at presentation was similar between men and women (men: stage III/IV=34 %; women: stage III/IV=48 %; p =0.55). The median radiation dose was 45 (range, 10 to 59)Gy. The most common chemotherapy regimen was 5-fluorouracil and mitomycin C. The median follow-up was 36 (range, 1 to 120)months. Overall, 31 % of patients had tumor recurrence and the rate of recurrence was higher for men compared to women (39 vs. 21 %, respectively, p =0.21). The stage at presentation was similar between HIV-positive and HIV-negative patients (p =0.34) (Table 2); however, the rate of recurrence in HIV-positive patients was twice as high as the rate in HIV-negative patients, 50 vs. 23 %, respectively (p =0.09).
Table 1.
Clinicopathologic features of consecutive 42 patients with diagnosis of anal cancer
| Clinical features | Male (N =23) | Female (N =19) | Overall (N =42) | P value | |||
|---|---|---|---|---|---|---|---|
| Age (mean±SD) | 58±15 | 61±11 | 59±13 | ||||
| Age of HIV-positive patients (mean±SD) | 44±7 | 47±9 | 44±7 | <0.0001a | |||
| Age of HIV-negative patients (mean±SD) | 69±10 | 62±10 | 65±10 | ||||
| N | % | N | % | N | % | ||
| HIV+ | 10 | 43 % | 2 | 11 % | 12 | 29 % | 0.04b |
| HIV− | 13 | 57 % | 17 | 89 % | 30 | 71 % | |
| Stage | |||||||
| I | 2 | 9 % | 5 | 26 % | 7 | 17 % | 0.55c |
| II | 11 | 48 % | 5 | 26 % | 16 | 38 % | |
| III | 7 | 30 % | 6 | 32 % | 13 | 31 % | |
| IV | 1 | 4 % | 3 | 16 % | 4 | 10 % | |
| Unknown | 2 | 9 % | 0 | 0 % | 2 | 4 % | |
| Outcome | |||||||
| Tumor recurrence + | 9 | 39 % | 4 | 21 % | 13 | 31 % | 0.21d |
| Tumor recurrence − | 14 | 61 % | 15 | 79 % | 29 | 69 % | |
| Histopathologic features | |||||||
| HPV positivity | 23 | 100 % | 19 | 100 % | 42 | 100 % | |
| p16 positivity | 23 | 100 % | 19 | 100 % | 42 | 100 % | |
| Histologic tumor type | |||||||
| Non-keratinizing | 12 | 52 % | 15 | 79 % | 27 | 64 % | 0.07e |
| Keratinizing | 11 | 48 % | 4 | 21 % | 15 | 36 % | |
T test for difference in age between HIV-positive vs. HIV-negative patients
Fisher’s exact test for difference in HIV positivity between men and women
Pearson’s chi-square test for difference in local tumors (stage I/II) and advanced tumors (stage III/IV) between men and women
Pearson’s chi-square test for difference in rate of recurrence between men and women
Pearson’s chi-square test for the difference in tumor histologic type distribution between men and women
Table 2.
Clinicopathologic features of HIV-positive and HIV-negative patients with anal cancer
| HIV+ (N =12) | HIV− (N =30) | P value | |||
|---|---|---|---|---|---|
| Stage | N | % | N | % | |
| I | 2 | 17 % | 5 | 17 % | 0.34a |
| II | 3 | 25 % | 13 | 43 % | |
| III | 5 | 42 % | 8 | 27 % | |
| IV | 1 | 8 % | 3 | 10 % | |
| Unknown | 1 | 8 % | 1 | 4 % | |
| Outcome | |||||
| Tumor recurrence + | 6 | 50 % | 7 | 23 % | 0.09b |
| Tumor recurrence − | 6 | 50 % | 23 | 77 % | |
| Histologic tumor type | |||||
| Non-keratinizing | 4 | 33 % | 23 | 77 % | 0.008c |
| Keratinizing | 8 | 67 % | 7 | 23 % | |
Pearson’s chi-square test for difference in local tumors (stage I/II) and advanced tumors (stage III/IV) between HIV-positive and HIV-negative patients
Pearson’s chi-square test for difference in tumor recurrence between HIV-positive and HIV-negative patients
Pearson’s chi-square test for difference in tumor histologic type distribution between HIV-positive and HIV-negative patients
HPV Detection, p16 Immunostaining, and Histopathologic Review
All tumors were positive for high-oncogenic risk HPV. HPV16 was the most common genotype and accounted for 73 % of cases. It was followed by other types, including HPV18–26 %, HPV31–13 %, HPV52–13 %, HPV39–6 %, HPV51–6 %, and HPV66–6 %. Multiple HPV types were detected in 75 % of tumors of HIV-positive patients and 14 % of HIV-negative patients. All tumors were strongly and diffusely positive with p16 immunostain. Sixty-four percent of tumors were non-keratinizing/basaloid, and 36 % were keratinizing type (Table 1). The keratinizing tumors were more common in HIV-positive patients (67 %), whereas non-keratinizing/basaloid tumors were more common in HIV-negative patients (77 %) (p =0.008) (Table 2). The stage at presentation was similar between the tumor types (non-keratinizing: stage III/IV=41 %; keratinizing: stage III/IV= 40 %; p =0.80) (Table 3), and the rate of recurrence was not significantly different between keratinizing and non-keratinizing carcinoma (33 vs. 30 %, respectively, p =0.80).
Table 3.
Clinical features of patients with non-keratinizing and keratinizing anal cancer
| Non-keratinizing (N =27) | Keratinizing (N =15) | P value | |||
|---|---|---|---|---|---|
| Stage | N | % | N | % | |
| I | 4 | 15 % | 3 | 20 % | 0.80a |
| II | 10 | 37 % | 6 | 40 % | |
| III | 8 | 30 % | 5 | 33 % | |
| IV | 3 | 11 % | 1 | 7 % | |
| Unknown | 2 | 7 % | 0 | 0 % | |
| Outcome | |||||
| Tumor recurrence + | 8 | 30 % | 5 | 33 % | 0.80b |
| Tumor recurrence − | 19 | 70 % | 10 | 67 % | |
Pearson’s chi-square test for difference in local tumors (stage I/II) and advanced tumors (stage III/IV) between patients with keratinizing and non-keratinizing tumors
Pearson’s chi-square test for difference in tumor recurrence between patients with keratinizing and non-keratinizing tumors
Recurrence-Free Survival
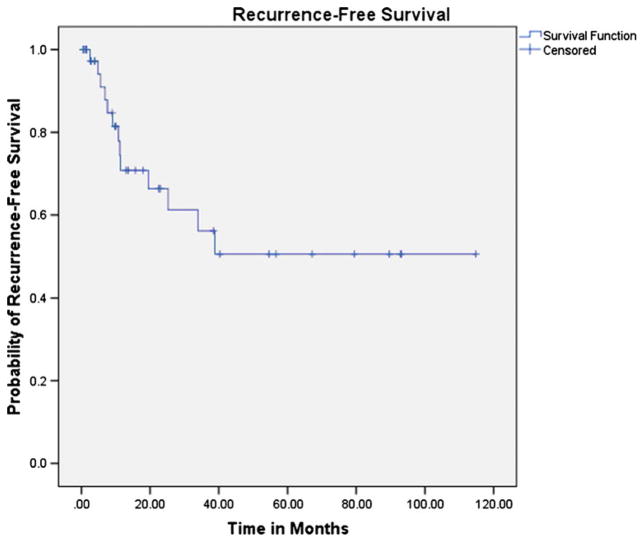
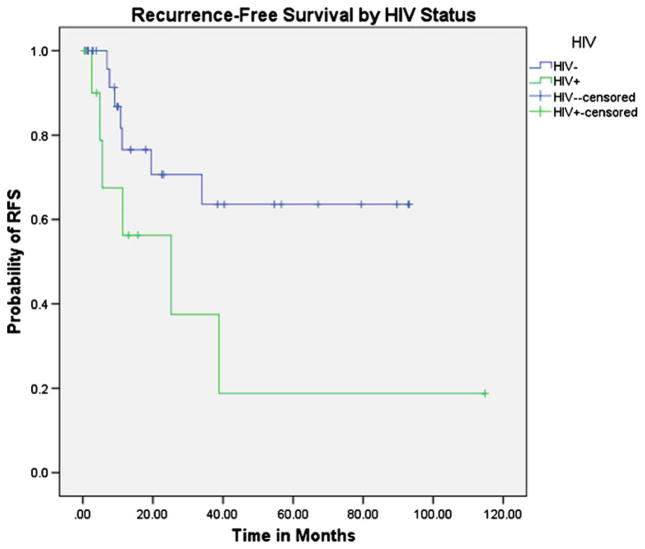
The 24-month RFS for the cohort was 66 % (95 % CI=46 %, 80 %) (Fig. 1). HIV-positive patients had worse RFS compared to HIV-negative patients (HR=2.85, 95 % CI=0.95, 8.53; p =0.06) (Fig. 2). Males tended to have worse RFS compared to females (HR=2.33, 95 % CI=0.71, 7.60; p =0.16), and patients with advanced stage (III/IV) had worse RFS compared to patients with local disease (I/II) (HR=3.35, 95 % CI=1.06, 10.58; p =0.04). Patients with keratinizing versus non-keratinizing tumors had near identical RFS (HR=0.94, 95 % CI=0.31, 2.90; p =0.92).
Fig. 1.
Kaplan–Meier analysis of recurrence-free survival, entire cohort. N =42 patients, 13 recurrences; 24-month recurrence-free survival=66 % (95 % CI=46, 80 %)
Fig. 2.
Kaplan–Meier analysis of recurrence-free survival by HIV status. HIV-positive: N =12 patients, six recurrences. HIV-negative: N =30 patients, seven recurrences. P =0.06 by log-rank test. Hazard ratio (for HIV+)=2.85, 95 % CI=0.95, 8.53; p =0.06
Discussion
The results of our study demonstrate that patients with higher stage of anal carcinoma, HIV positivity, and male gender have worse recurrence-free survival. However, pathologic characteristics of the tumor, such as histologic tumor type, HPV positivity, and p16 positivity have no bearing on clinical outcome.
Most patients with anal cancer treated with chemoradiotherapy have an excellent prognosis with overall survival rates of 60 to 75 %; however, a proportion of patients has poor outcome. The most important prognostic factor for anal carcinoma is the stage and tumor size. In addition, gender was shown to be an independent predictor for local control as well as survival, with men having significantly lower rates of disease-free survival than women [9]. The initial, small studies of HIV-positive patients with anal carcinoma reported that treatment response rate, overall survival, and local control seemed to be comparable with that of HIV-negative population [10–13]; however, more recent, larger, and controlled studies have reported worse outcome in HIV-positive patients. As compared with HIV-negative patients, HIV-positive patients showed impaired tolerance to chemoradiotherapy, a lower survival rate, and a higher rate of local failure [14–17]. The results of our current study confirm these recent findings.
A large majority of anal cancers are positive for HPV. A recent systematic literature review included 992 cases of ACA and reported 72 % positivity for HPV 16 or 18, as assessed by PCR or hybrid capture [18]. The rate of positivity may be related to the method of HPV detection, as recent small study of 29 patients using a PCR method similar to our study found 100 % HPV positivity of the cancer cases [19]. That result matches our current study in which we have found 100 % HPV positivity of all 42 cases of ACA.
The overexpression of the cell cycle regulator p16 has been demonstrated in HPV-associated squamous cell cancers of the head and neck as well as the vulva and cervix [4, 5, 20]. The current study found that 100 % of tumors were positive for p16 overexpression, showing perfect correlation with the results of HPV detection. This finding confirms that p16 immunostaining is a reliable surrogate marker HPV in anal cancer samples.
Tumor histopathology was shown to be important prognostic factor in the oropharyngeal location. Our study failed to identify a relationship between tumor differentiation (keratinizing vs. non-keratinizing) and prognosis in anal cancer. In oropharyngeal and vulvar location, the tumors that are HPV positive are almost invariably of basaloid (non-keratinizing) morphology, whereas HPV-negative tumors are of keratinizing histologic subtype. This study, however, uncovered that HPV-positive tumors may show keratinizing differentiation, and this is mainly seen in the HIV-positive group of patients. This observation of development of HPV-positive keratinized tumors in immunosuppressed patients has not been reported before.
There are some limitations to this study that must be noted. This is a small retrospective study and is subject to all of the attendant biases. Specifically, the reason for the decreased local control and survival in HIV-positive patients is unclear. HIV-positive patients were not typically planned to receive a lower dose of radiotherapy but may have been prescribed decreased intensity chemotherapy. More commonly, patients may have tolerated standard therapy more poorly, requiring a decrease in chemotherapy dose or a radiotherapy treatment break. It is expected that tolerance will improve as radiotherapy techniques evolve, with the wider use of intensity-modulated radiation therapy for squamous cell carcinoma of the anal canal [21]. Another possibility is that the disease is more aggressive in HIV patients. A prospective study could be helpful in answering this question.
In conclusion, this study provides evidence that HPV and p16 positivity do not affect clinical outcome in patients with squamous cell carcinoma of the anal canal. However, HIV positivity appears to be correlated with poorer outcome in our cohort.
Acknowledgments
We would like to express gratitude to Delft Diagnostic Laboratory for HPV analysis. Dr. Paul Christos was partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1-TR000457-06).
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Contributor Information
Joshua E. Meyer, Fox Chase Cancer Center, Philadelphia, PA, USA
Vinicius J. A. Panico, Irmandade Da Santa Casa De Misericórdia De São Paulo, São Paulo, Brazil
Heloisa M. F. Marconato, Universidade Estadual de Londrina, Londrina-PR, Brazil
David L. Sherr, Rosetta Radiology, New York, NY, USA
Paul Christos, Department of Pathology, Weill Medical College of Cornell University, 525 E 68th Street, ST-1041, New York, NY 10065, USA.
Edyta C. Pirog, Email: ecpirog@med.cornell.edu, Department of Pathology, Weill Medical College of Cornell University, 525 E 68th Street, ST-1041, New York, NY 10065, USA
References
- 1.United States Cancer Statistics (USCS) Cancers By Race and Ethnicity. 2008 http://apps.nccd.cdc.gov/uscs/cancersbyraceandethnicity.aspx.
- 2.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papilloma-virus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626–36. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 3.Ukpo OC, Flanagan JJ, Ma XJ, Luo Y, Thorstad WL, Lewis JS., Jr High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–50. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JS, Jr, Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, et al. p16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–96. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tringler B, Grimm C, Dudek G, Zeillinger R, Tempfer C, Speiser P, et al. p16INK4a expression in invasive vulvar squamous cell carcinoma. Appl Immunohistochem Mol Morphol. 2007;15:279–83. doi: 10.1097/01.pai.0000213118.81343.32. [DOI] [PubMed] [Google Scholar]
- 6.Chernock RD, El-Mofty SK, Thorstad WL, Parvin CA, Lewis JS., Jr HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3:186–94. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenger C, Fritsch M, Marti MC, Parc R. Tumours of the anal canal. In: Hamilton SR, Aaltonen LA, editors. World Health Organisation classification of tumours. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press; 2000. pp. 145–55. [Google Scholar]
- 8.Quint WG, Scholte G, van Doorn LJ, Kleter B, Smits PH, Lindeman J. Comparative analysis of human papillomavirus infections in cervical scrapes and biopsy specimens by general SPF(10) PCR and HPV genotyping. J Pathol. 2001;194:51–8. doi: 10.1002/path.855. [DOI] [PubMed] [Google Scholar]
- 9.Das P, Crane CH, Eng C, Ajani JA. Prognostic factors for squamous cell cancer of the anal canal. Gastrointest Cancer Res. 2008;2:10–4. [PMC free article] [PubMed] [Google Scholar]
- 10.Peddada AV, Smith DE, Rao AR, et al. Chemotherapy and low-dose radiotherapy in the treatment of HIV-infected patients with carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 1997;37:1101–5. doi: 10.1016/s0360-3016(96)00596-2. [DOI] [PubMed] [Google Scholar]
- 11.Blazy A, Hennequin C, Gornet JM, et al. Anal carcinomas in HIV-positive patients: high-dose chemoradiotherapy is feasible in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2005;48:1176–81. doi: 10.1007/s10350-004-0910-7. [DOI] [PubMed] [Google Scholar]
- 12.Edelman S, Johnstone PA. Combined modality therapy for HIV-infected patients with squamous cell carcinoma of the anus: outcomes and toxicities. Int J Radiat Oncol Biol Phys. 2006;66:206–11. doi: 10.1016/j.ijrobp.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 13.Wexler A, Berson AM, Goldstone SE, Waltzman R, Penzer J, Maisonet OG, et al. Invasive anal squamous-cell carcinoma in the HIV-positive patient: outcome in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2008;51:73–81. doi: 10.1007/s10350-007-9154-7. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Sarani B, Orkin BA, et al. HIV-positive patients with anal carcinoma have poorer treatment tolerance and outcome than HIV-negative patients. Dis Colon Rectum. 2001;44:1496–502. doi: 10.1007/BF02234605. [DOI] [PubMed] [Google Scholar]
- 15.Munoz-Bongrand N, Poghosyan T, Zohar S, Gerard L, Chirica M, Quero L, et al. Anal carcinoma in HIV-infected patients in the era of antiretroviral therapy: a comparative study. Dis Colon Rectum. 2011;54:729–35. doi: 10.1007/DCR.0b013e3182137de9. [DOI] [PubMed] [Google Scholar]
- 16.Oehler-Jänne C, Huguet F, Provencher S, Seifert B, Negretti L, Riener MO, et al. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol. 2008;26:2550–7. doi: 10.1200/JCO.2007.15.2348. [DOI] [PubMed] [Google Scholar]
- 17.Gervaz P, Calmy A, Durmishi Y, Allal AS, Morel P. Squamous cell carcinoma of the anus-an opportunistic cancer in HIV-positive male homosexuals. World J Gastroenterol. 2011;17:2987–91. doi: 10.3748/wjg.v17.i25.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papilloma-virus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009;124:2375–83. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- 19.Lu DW, El-Mofty SK, Wang HL. Expression of p16, Rb, and p53 proteins in squamous cell carcinomas of the anorectal region harboring human papillomavirus DNA. Mod Pathol. 2003;16:692–9. doi: 10.1097/01.MP.0000077417.08371.CE. [DOI] [PubMed] [Google Scholar]
- 20.Lindel K, Burri P, Studer HU, Altermatt HJ, Greiner RH, Gruber G. Human papillomavirus status in advanced cervical cancer: predictive and prognostic significance for curative radiation treatment. Int J Gynecol Cancer. 2005;15:278–84. doi: 10.1111/j.1525-1438.2005.15216.x. [DOI] [PubMed] [Google Scholar]
- 21.Kachnic LA, Winter K, Myerson RJ, Goodyear MD, Willins J, Esthappan J, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluoruracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]