Abstract
Effective attention and memory skills are fundamental to typical development and essential for achievement during the formal education years. It is critical to identify the specific mechanisms linking efficiency of attentional selection of an item and the quality of its memory retention. The present study capitalized on the spatial cueing paradigm to examine the role of selection via suppression in modulating children and adolescents’ memory encoding. By varying a single parameter, the spatial cueing task can elicit either a simple orienting mechanism (i.e., facilitation) or one that involves both target selection and simultaneous suppression of competing information (i.e., IOR). We modified this paradigm to include images of common items in target locations. Participants were not instructed to learn the items and were not told they would be completing a memory test later. Following the cueing task, we imposed a seven-minute delay and then asked participants to complete a recognition memory test. Results indicated that selection via suppression promoted recognition memory among 7-17 year-olds. Moreover, individual differences in the extent of suppression during encoding predicted recognition memory accuracy. When basic cueing facilitated orienting to target items during encoding, IQ was the best predictor of recognition memory performance for the attended items. In contrast, engaging suppression (i.e, IOR) during encoding counteracted individual differences in intelligence, effectively improving recognition memory performance among children with lower IQs. This work demonstrates that engaging selection via suppression during learning and encoding improves memory retention and has broad implications for developing effective educational techniques.
Keywords: selective attention, memory, intelligence, education
1. Introduction
Paying attention helps us form robust memories. Despite the centrality of these processes during development, identifying the mechanisms linking attention and memory within the context of individual differences in intelligence and developmental change has remained challenging. In the present study we focused on school-age children and adolescents to best expose these interactions during formal education years, when attentional strategies aimed at enhancing learning and memory might have lasting effects on achievement. We provide evidence that the nature of the underlying mechanism driving orienting has crucial implications for the efficacy of memory encoding for subsequent retrieval. Specifically, we show that selection mechanisms involving suppression have the power to boost memory encoding, effectively counteracting individual differences in intelligence.
Memory does not develop or function in isolation. Numerous studies have shown that effective attention allocation is necessary for successful memory encoding and retrieval. For example, memory performance suffers when attention is divided between two tasks (Craik, Govoni, Naveh-Benjamin, & Anderson, 1996; Fernandes & Moscovitch, 2000) or distracted by irrelevant stimuli (Wais, Rubens, Boccanfuso, & Gazzaley, 2010). Cowan and colleagues (Cowan, et al., 2005; Cowan, Fristoe, Elliott, Brunner, & Saults, 2006; Cowan, Nugent, Elliott, Ponomarev, & Saults, 1999) have shown that attention can influence both what information is selected for working memory as well as how much information can be retained in working memory. Recent work has also shown that cognitive control contributes to improved recognition memory performance by biasing selective attention towards task relevant versus task irrelevant information (Richter & Yeung, 2012).
Previous studies have also shown that cueing attention to relevant stimuli supports enhanced performance learning and visual short term memory tasks, both in adulthood (Hauer & MacLeod, 2005; Schmidt, Vogel, Woodman, & Luck, 2002) and during development (Astle, Nobre, & Scerif, 2012; Reid & Striano, 2005; Reid, Striano, Kaufman, & Johnson, 2004; Ross-Sheehy, Oakes, & Luck, 2011). For example, Astle, et al. (2012) presented children with an array of multiple objects and later asked them to recall whether a single item had been present in the array. Children showed a significant improvement in this short-term memory task when the location of the relevant item was cued prior to presentation of the of multiple item array, and, conversely, showed a significant deficit in visual short-term memory when an irrelevant location was cued prior to presentation of the object array. Critically, these examples reflect a well-established interaction between spatial attention and spatial working memory (Awh & Jonides, 2001; Chun, 2011; Fuster, 2000; Ikkai & Curtis, 2011). However, it remains unclear whether these effects extend to recognition memory processes that occur beyond the initial short-term representation and are classically relevant for building stable knowledge structures.
The present study extends previous work in several ways. First, we examined the role of selective attention in modulating memory encoding occurring at longer time scales, rather than focusing on short-term or working memory processes. Second, rather than treating attention as a unitary process, we instead compared the impact of different orienting mechanisms on memory encoding, allowing us to begin to tease apart the specific mechanisms of how selective attention influences memory encoding. Finally, we considered how these attention and memory interactions might vary depending on individual differences in intelligence and across a wide developmental range.
Selective attention reflects a continual balance between two primary components – enhanced processing of attended stimuli and concurrent suppression of irrelevant or unattended information (Desimone & Duncan, 1995; Kastner & Ungerleider, 2000). Together, this dual excitation and suppression resolves the conflict between the numerous stimuli that are continually competing for our attentional resources. Previous research has shown that these processes are associated with differential activity in visual cortex, with enhanced signal associated with information appearing in attended locations and suppression of the signal associated with information appearing in unattended or competing locations (Brefczynski & DeYoe, 1999; Corbetta, Miezin, Dobmeyer, Shulman, & Petersen, 1991; Gandhi, Heeger, & Boynton, 1999; Kastner, Pinsk, De Weerd, Desimone, & Ungerleider, 1999; Pestilli & Carrasco, 2005; Slotnick, Schwarzbach, & Yantis, 2003; Smith, Singh, & Greenlee, 2000). However, to our knowledge, no one has considered the impact of this modulation of visual cortex activity on memory encoding of the attended items.
Within this framework, attention orienting can be driven by different underlying mechanisms, some of which elicit the suppression component of selective attention while others do not (Posner & Cohen, 1984; Tipper, 1985). As such, the nature of the selection mechanisms underlying visual orienting, and particularly whether suppression is involved, may have important implications for subsequent encoding of the attended information. Our working hypothesis is that relative to selection powered by excitation alone, concurrent suppression at the unattended location should generate a signal for the attended information that is more robust and less susceptible to interference, thus supporting enhanced encoding for subsequent retrieval.
The present study utilized the spatial cueing paradigm (Posner, 1980) to examine the role of selection via suppression in modulating children and adolescents’ recognition memory. In this task, attention is engaged at a central location while a cue flashes in the periphery. After a delay of varying length, a target appears in the same cued location or in the opposite, non-cued location. Following a very short cue-to-target delay (< 250 ms) individuals typically respond faster to targets appearing in the cued location. This facilitation effect reflects a mechanism in which attention is reflexively drawn to the peripheral cue and remains engaged at the cued location when the target appears (Posner, 1980; Posner & Cohen, 1984). In contrast, following a longer (> 250 ms) cue-to-target delay, attention instead becomes suppressed at the cued location and individuals respond faster to targets appearing in the opposite, non-cued location, an effect termed inhibition of return (IOR) (Klein, 2000; Posner, Rafal, Choate, & Vaughan, 1985). Unlike facilitation, IOR reflects a mechanism in which attention is enhanced at the non-cued location and concurrently suppressed at the cued location. Although traditional spatial cueing tasks use a single target, IOR nonetheless elicits a suppression effect that is similar to that observed when competing stimuli are present (McDonald, Ward, & Kiehl, 1999).
Thus, by varying a single timing parameter (the cue-to-target delay, see Figure 1), the spatial cueing task can elicit either a basic orienting mechanism that involves excitation alone (i.e., facilitation) or one that involves both excitation and suppression (i.e., IOR). In the present study we capitalized on this nuance to directly compare children and adolescents’ encoding and subsequent recognition memory in the context of basic excitation vs. concurrent excitation and suppression. We modified the classic task by placing common object images for encoding in the attended locations. Following the spatial cueing/encoding phase, participants were tested on a standard recognition memory task. We predicted that the additional suppression component of IOR would promote memory encoding, which would be reflected in enhanced recognition accuracy at test, whereas eliciting the facilitation mechanism would have little impact on participants’ recognition memory accuracy. All presentation parameters were equated across the two conditions; thus, any differences in encoding efficacy would be attributable to differences in the underlying attention mechanisms.
Figure 1.
Examples of object images used as target stimuli for encoding.
Finally, we examined whether the facilitation and IOR orienting mechanisms differentially affected memory encoding at different points in development and/or based on individual differences in intelligence (IQ). IQ has been repeatedly related to attention and memory processes (Cowan, et al., 2006; Engle, Tuholski, Laughlin, & Conway, 1999; Kane & Engle, 2000; Lahaderne, 1968; Reber, Walkenfeld, & Hernstadt, 1991; Rose, Feldman, Jankowski, & Van Rossem, 2012), with higher IQ associated with improved memory performance. However, given that attention is not uniform, it is unclear whether this relationship between IQ and recognition memory may be moderated by the nature of the attention mechanism that is engaged during encoding. Furthermore, this question may be especially relevant when considering whether specific attention strategies may be most beneficial for boosting learning and memory in formal education settings.
2. Method
2.1 Participants
Seventy-six children and adolescents (range 7-17 years, 33 M, 43 F; MAge = 10.72 years, SD = 2.58 years) participated in a single testing session. There were no age differences across the facilitation and IOR conditions. An additional 7 participants were tested but excluded because they did not complete both the IQ assessment and the spatial cueing task. Participants were recruited from the community via advertisements. Based on parental report, 86.8% of participants were Caucasian, 9.2% were Hispanic, 1.3% were African-American, 1.3% were Asian, and 1.3% were Other/Unknown. Prior to enrollment, we screened participants via parental report to ensure that participants did not have a personal history of diagnosed psychiatric disorders (Tourette’s, ADHD, Autism, Obsessive Compulsive Disorder, Schizophrenia, Panic and Anxiety Disorders, Major Depression), uncorrected visual or auditory impairments, or preterm birth. Families were compensated for participation. Parents gave informed consent and children provided assent in accordance with the Institutional Review Board before the test session began.
2.2 Materials
2.2.1 Eye tracking apparatus
Eye movements were recorded using a remote eye tracker (SensoMotoric Instruments RED system). At the beginning of the test session, each participant’s point-of-gaze (POG) was calibrated using a 5-point protocol provided by the SMI Experiment Center software. Average deviation was 0.72° (SD = 0.48°), suitable for assessing eye movements to the left and right periphery during the spatial cueing portion of the task.
2.2.2 IQ Assessment
Each participant completed the two-subtest version (Vocabulary and Matrix Reasoning) of the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999). The two-subtest version yields a full-scale IQ score that summarizes the individual’s cognitive functioning. The Vocabulary subtest yields a verbal IQ score that indexes the individual’s verbal knowledge. The Matrix Reasoning subtest yields a non-verbal IQ score that indexes the individual’s non-verbal reasoning skills. IQ scores were similar across the facilitation (M = 120.08, SD = 12.21, range = 90 - 141) and IOR conditions (M = 121.27, SD = 17.08, range = 86 - 151).
2.2.3. Spatial cueing task
Stimuli for the spatial cueing/encoding phase included a central fixation (4.5 cm2), peripheral cue (1.5 cm2), and multiple target stimuli (each 8.5 cm2). Target items for valid cueing trials consisted of black line drawings depicting everyday objects against a white background (Figure 1). All of the target images were drawn from the International Picture Naming Project database (Szekely, et al., 2004). Example target objects are shown in Figure 1. Mean age of acquisition for the target image labels was 2.2 years (SD = 0.90 years), giving us confidence that they are familiar to all 7-17 year-olds in our sample. Two sets of 30 object pictures were used; one set provided targets for the spatial cueing task and the second set served as novel images during the recognition memory test. Set order was counterbalanced across participants.
Spatial cueing effects are typically assessed by comparing reaction times to targets appearing in the cued versus non-cued locations. Thus, it was necessary to include additional trials with a stimulus (the foil) that appeared in the location opposite the expected attention bias. The foil was a blank white square that was the same size as the targets (8.5 cm2). The use of a blank square as the foil stimulus ensured that the foil trials would not introduce interference from additional complex visual stimuli during encoding. All targets and foils appeared to the left or right of the central fixation stimulus. Each target appeared once on the left and once on the right and the foil appeared 30 times on both the left and right, generating a total of 60 target trials and 60 foil trials. Order of trial type and left/right presentation was randomized for each participant.
All 60 object images were presented during the recognition memory test. One set of pictures served as the old, to-be-remembered images and the second set served as the novel images. Test stimuli were presented once in the center of the screen in random order.
2.3 Procedure
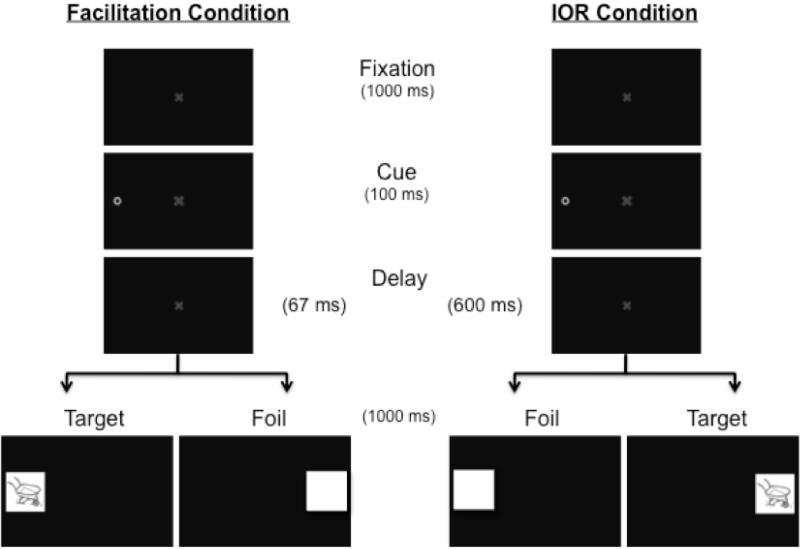
The facilitation and IOR conditions were age-matched but participants were otherwise randomly assigned to the two conditions. The spatial cueing task began after calibration of the participant’s POG was complete. A crosshair appeared at the beginning of each spatial cueing/encoding trial to orient participants’ attention to the center of the screen. After 300 ms, the central fixation appeared and remained on screen for 1000 ms (Figure 2). At this time, the cue appeared in the left or right periphery and remained on screen for 100 ms. After a delay of 67 ms (facilitation condition) or 600 ms (IOR condition), a target or foil stimulus appeared on the left or right side (i.e., in the cued or non-cued location). Target images always appeared in the location of the predicted attention bias (i.e., the cued location in the facilitation condition, the non-cued location in the IOR condition). The central fixation remained visible through the cue presentation and subsequent delay and disappeared at target onset. Targets remained on screen for 1000 ms, followed by an inter-trial interval of 2 seconds. Note that targets and foils never appeared at the same time; thus there were no distracting stimuli when the targets were present.
Figure 2.
Schematic depiction of the spatial cueing/encoding task.
Participants were explicitly instructed to fixate the central stimulus and avoid looking at the peripheral cue. In addition, participants were asked to look directly at the target/foil when it appeared while using key presses to indicate whether the stimulus was on the left or right side of the screen. Participants were not asked to study or memorize the targets and were not told about the subsequent recognition task at any point during the spatial cueing/encoding phase.
After completing the spatial cueing task, participants were given a 7-minute break. During this time they reiterated the spatial cueing instructions to ensure understanding. In addition, the researchers administered a questionnaire about the participant’s everyday activities in order to fill the time without giving any indication of the upcoming recognition memory test.
During the recognition memory test, participants used key presses to indicate whether the target picture was old or new. Test stimuli remained on screen until the participant responded. POG was not recorded during this portion of the task.
2.4 Data Processing
The primary variables of interest for the spatial cueing/encoding phase included saccade latencies to the cued and non-cued locations and duration of looking at the targets. Initial processing of the eye movement record was conducted using the native SMI BeGaze analysis software. The screen was divided into three equivalent areas of interest (AOIs) corresponding to the central, left, and right stimulus locations. These AOIs were defined as a 16.2 cm × 29.8 cm region of space over each of these locations. Usable looks were defined as segments of the data in which the POG remained within 2.9 cm2 (2°) for at least 100 ms. Saccade latencies were computed based on the time at which a look lasting more than 100 ms first entered the relevant AOI. Duration of looking was computed by summing the duration of all looks that occurred within the AOI following target onset.
Individual spatial cueing trials were discarded if there was no eye tracking data available, if the participant looked at the cue prior to target onset, or if the participant did not orient to the target location. There were no differences across the two conditions in rates of missing data (M = 3.3%, SD = 9.2%) looking to the cue (M = 9.6 % of trials, SD = 8.0%) or failing to orient to the target (M = 8.2%, SD = 8.6%). Trials were further filtered to exclude those with latencies that were less than 100 ms or greater than 2 SD above the individual mean latency. In addition to the raw latency to target values, we generated a facilitation/IOR score for each individual by subtracting their mean latency to the non-cued location from their mean latency to the cued location. All analyses utilized the absolute value of these facilitation/IOR scores so that stronger facilitation and IOR effects would both be indicated by more positive values.
Finally, for the test phase of the task we computed recognition memory scores for each participant based on his/her accuracy in discriminating old versus new test items. These discrimination (d’) scores were derived by subtracting the normalized proportion of “false alarms” from the normalized proportion of “hits”.
3. Results
3.1 Spatial cueing
We first verified that our task elicited the predicted orienting and selection effects. Five participants from the IOR condition were excluded from this and subsequent analyses because their accuracy during the spatial cueing phase (i.e., indicating whether the target appeared on the left or right side of the screen) was more than 2 SD below the group mean, indicating poor motivation/attention during the task. The final sample included 39 participants in the facilitation condition and 32 participants in the IOR condition. Mean saccade latencies were entered into a Trial type (cued, non-cued) × Condition (facilitation, IOR) ANCOVA with Age as a covariate. Results indicated a main effect of Age (F(1,68) = 6.21, p = .015), with mean saccade latencies becoming faster with age. In addition, there was a significant Trial type × Condition interaction (F(1,68) = 65.40, p < .001). Follow-up analyses verified that participants in the facilitation condition showed the expected excitation of attention at the cued location, with faster latencies to the cued location (M = 440.77 ms, SD = 134.97 ms) relative to the non-cued location (M = 466.30 ms, SD = 133.37 ms; F(1,38) = 6.71, p = .014; η2 = .15). Participants in the IOR condition demonstrated the expected inhibition of attention at the cued location, with slower latencies to the cued location (M = 523.65 ms, SD = 105.33 ms) compared to the non-cued location (M = 439.71 ms, SD = 117.51 ms; F(1,31) = 94.50, p < .001; η2 = .75). Trial type did not interact with Age in either condition. Thus, our task elicited the expected attentional selection effects with no developmental differences in these effects.
3.2 Recognition Memory
On average, participants correctly identified 76.1% (SD = 16.5%) of the old target images and 84.7% (SD = 16.2%) of the new images. Our primary aim was to examine whether the facilitation and IOR attention mechanisms differentially impacted recognition memory. As discussed, IQ and memory are linked in the literature (Reber, et al., 1991; Rose, et al., 2012). As such, we utilized simultaneous multiple regression to model continuous Age and IQ predictors of recognition memory scores (d’). Preliminary examination of standardized residuals indicated four outliers (two from each condition); these data were excluded to preserve model assumptions, leaving an N of 37 in the facilitation condition and 30 in the IOR condition. Predictors included Age, Condition, facilitation/IOR score, and IQ. As described earlier, the facilitation/IOR score reflected the difference in reaction time to targets in the cued versus non-cued locations. As such, this score indicated the extent to which a participant showed the excitation and suppression effects during the attention task. In addition to these main factors, we included the Condition × Facilitation/IOR score interaction term to determine whether the relationship between the strength of cueing effects and recognition memory was moderated by the nature of the cueing effect (i.e., facilitation vs. IOR). Similarly, we included the Condition × IQ and Condition × Age interaction terms to determine whether these factors were differentially related to recognition memory in the facilitation versus IOR conditions.
The overall model accounted for a significant proportion of variance in d’ (R2 = .26, F(7,59) = 2.91, p = .01). Regression coefficients are presented in Table 1. Results showed that, when accounting for the variance contributed by age, IQ, etc., the IOR condition was associated with higher memory scores relative to the facilitation condition (t(59) = 2.05, p = .045), confirming our initial hypothesis. IQ and facilitation/IOR score were also significant predictors of d’ (tIQ(59) = 2.46, p = .017; tfac/IOR score(59) = 2.00, p = .05), with higher scores predicting enhanced recognition memory performance. Age was not a significant predictor of d’, indicating similar recognition scores across our age range.
Table 1. Predictors of recognition memory scores (d’) – Full model.
| Variable | B (SE) | Tolerance |
|---|---|---|
| Constant | −0.47 (1.10) | |
| Age | −0.02 (0.04) | 0.71 |
| Condition (Facilitation, IOR) | 4.52* (2.21) | 0.01 |
| Facilitation/IOR Score | 0.004* (0.002) | 0.64 |
| IQ | 0.02* (0.01) | 0.82 |
| Condition × Age | −0.06 (0.08) | 0.05 |
| Condition × IQ | −0.04* (0.02) | 0.01 |
| Condition × Facilitation/IOR Score | 0.01* (0.004) | 0.37 |
|
| ||
| R 2 | 0.27* | |
| N | 67 | |
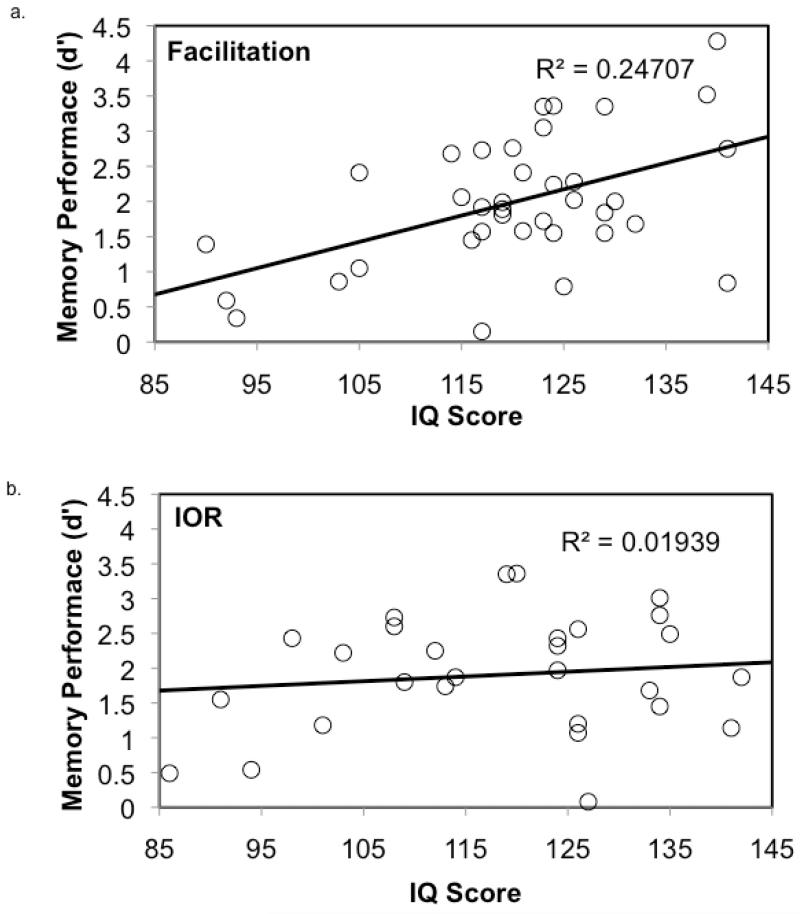
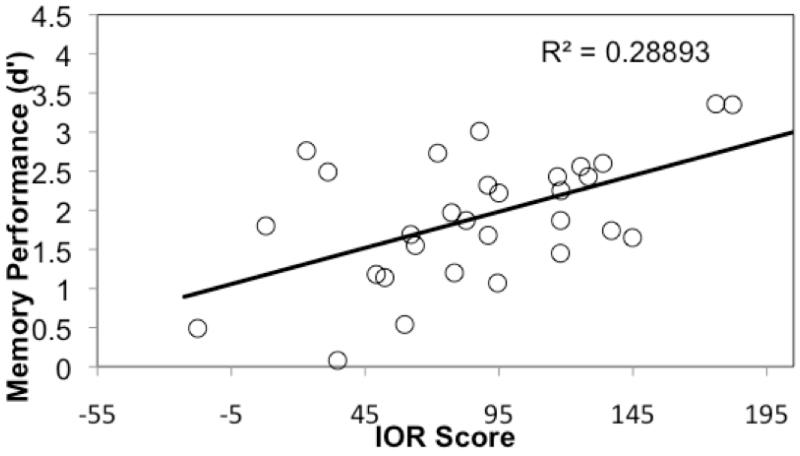
In addition to these main effects, the Condition × IQ interaction was a significant predictor of d’ (t(59) = −2.65, p = .014), as was the Condition × facilitation/IOR score interaction (t(59) = 2.13, p = .038). Follow-up analyses (Table 2) indicated that IQ was a significant predictor of memory scores in the facilitation condition (t(33) = 3.13, p = .004; Figure 3a); however, the extent of attentional excitation (i.e., facilitation score) was not predictive of memory performance (t(33) = −0.09, p = .931). Data revealed a different pattern of results in the IOR condition. Here, the effects of IQ on memory were attenuated (t(26) = −0.11, p = .917; Figure 3b) whereas the extent of attentional inhibition (i.e., IOR score) significantly predicted memory performance (t(26) = 3.09, p = .005, Figure 4). There was no direct relationship between IQ and memory scores in the IOR condition, confirming that IOR score was not simply mediating the effect of IQ on memory performance. These data indicate that basic orienting is unrelated to memory performance and intelligence is the best predictor of recognition memory scores when attention during encoding is minimal. However, when suppression is engaged during the IOR condition, basic individual differences in intelligence are diminished and the extent of inhibition becomes primary in driving memory. These findings were consistent across all ages in our sample.
Table 2. Predictors of recognition memory scores (d’) per condition.
| Condition | Variable | B (SE) | Tolerance |
|---|---|---|---|
| Facilitation | Constant | −2.56 (1.42) | |
| Age | 0.01 (0.06) | 0.97 | |
| Facilitation Score | −0.0003 (0.003) | 0.93 | |
| IQ | 0.04* (0.01) | 0.87 | |
|
| |||
| R 2 | 0.25* | ||
| N | 37 | ||
|
| |||
| IOR | Constant | 0.88 (0.96) | |
| Age | −0.04 (0.06) | 0.81 | |
| IOR Score | 0.002* (0.008) | 0.95 | |
| IQ | 0.009 (0.003) | 0.81 | |
|
| |||
| R 2 | 0.31* | ||
| N | 30 | ||
Figure 3.
IQ predicts memory performance in the facilitation condition (a) but not in the IOR condition (b).
Figure 4.
The extent of inhibition, as measured by IOR score, predicts memory performance in the IOR condition.
Finally, we conducted supplementary control analyses to ensure that any difference in recognition memory scores across the facilitation and IOR conditions could not be attributed to simple differences in exposure to the target images during encoding. There were no differences in time spent looking to the target images across the facilitation (M = 500.66 ms, SD = 139.19 ms) and IOR conditions (M = 455.45 ms, SD = 107.22 ms), verifying that differential recognition memory across the two conditions was not due to differences in exposure to the target images during encoding.
4. Discussion
The present study compared the impact of two different orienting mechanisms on children and adolescents’ recognition memory, specifically asking whether the concurrent suppression associated with IOR would enhance memory encoding beyond excitation (i.e., facilitation) alone. Varying these selective attention mechanisms resulted in differential encoding, as IOR was associated with improved recognition memory relative to facilitation. Within the IOR condition, individuals who showed the strongest IOR/suppression were most accurate in the recognition memory test, further underscoring the important role of suppression in modulating memory encoding. In contrast, basic orienting/excitation was unrelated to recognition memory performance in the facilitation condition. This finding was consistent across all ages in our sample. These results support the hypothesis that selection mechanisms involving both excitation and suppression promote learning and memory by generating a more robust signal for the attended information during encoding. To our knowledge, this is the first evidence of selection via suppression modulating the efficacy of memory encoding among children and adolescents.
To reiterate, the only difference in the encoding phase between the facilitation and IOR conditions involved the nature of the orienting mechanism that generated the eye movement to that location. The cue and target durations, task demands, target items, and delay between the spatial cueing and recognition memory tasks were all identical. The only procedural difference was the longer cue-target delay length in the IOR condition. It is unclear whether this longer delay would lead to improved encoding or increased forgetting; however, data from a related study (Markant & Amso, 2013) indicated that the enhanced encoding in the IOR condition could not be attributed to this timing difference. Furthermore, in the present study, individual differences in the strength of IOR were positively related to recognition memory accuracy. Thus, the extent of suppression modulated memory encoding even among individuals who were in the same condition and experienced the same cue-target delay length. This result confirms that it was the suppression associated with IOR, rather than total trial duration, that promoted enhanced encoding and recognition memory in the IOR condition relative to the facilitation condition.
We also verified that participants spent the same amount of time looking at the target items across the facilitation and IOR conditions. Again, this confirms that differential encoding efficacy across the two conditions cannot be attributed to simple differences in look durations to the target images. Moreover, this result demonstrates that overt measures of looking can be dissociated from the underlying attention mechanisms that drive orienting. Participants in the facilitation and IOR conditions showed the same eye movement patterns and looked at the target images for the same amount of time yet engaged in differential processing of the target information. This kind of dissociation between overt looking and underlying attention processing has been demonstrated repeatedly in Richards and colleagues’ studies of sustained attention in infancy, in which heart rate measures reveal multiple phases of attention and inattention within a single bout of looking (see Richards, 2010 for review). The present study similarly highlights the importance of examining underlying attention mechanisms rather than considering only overt measures of orienting.
The present results are consistent with the neural mechanisms mediating selective attention. As noted earlier, much work has shown that frontoparietal selective attention networks drive differential activity in visual cortex, with enhanced activity for items/locations that are attended and suppressed activity for competing items that are irrelevant or unattended (Brefczynski & DeYoe, 1999; Desimone & Duncan, 1995; Gandhi, et al., 1999; Kastner, et al., 1999; Slotnick, et al., 2003; Smith, et al., 2000). The present data suggest that this modulation of visual cortex activity can have critical implications for learning and memory processes. Specifically, these data suggest that enhanced signal at the attended location coupled with suppressed signal at the opposing location supports a more robust, less noisy signal that in turn serves as input to learning and memory systems and leads to enhanced encoding efficacy. This finding is consistent with previous work relating attentional modulation of visual cortex activity to working memory performance (Rutman, Clapp, Chadick, & Gazzaley, 2010; Zanto, Rubens, Thangavel, & Gazzaley, 2011), similarly suggesting that this modulation of visual cortex signal can have meaningful implications for encoding efficacy.
We capitalized on the classic spatial cueing/IOR task to compare encoding in the context of selection via supression (i.e., IOR) vs. basic orienting (i.e., facilitation) while maintaining equivalent presentation parameters across the two conditions. However, as noted above, concurrent excitation and suppression is not unique to IOR, but rather is inherent to selective attention, as attention functions to resolve conflict among stimuli that continually compete for processing resources. We hypothesize that the mechanism by which the suppression component of IOR supports enhanced encoding should function similarly in multiple contexts that elicit concurrent excitation of attended items/locations and suppression of competing information. As such, the present results provide insight into not only IOR, but also into selective attention mechanisms more generally.
The present results also indicated that higher IQ scores predicted more accurate recognition memory. We interpret this relation between IQ and memory performance to reflect differences in learning strategy and/or ability to encode briefly presented information into memory, consistent with previous work linking IQ and learning (Fletcher, Maybery, & Bennett, 2000; van den Bos, Crone, & Güroğlu, 2012). It is unlikely that explicit rehearsal or vocabulary skills contributed to differences in recognition memory. The targets were common objects that should be easily identified, even among young children and those with lower IQ. Furthermore, the spatial cueing task moved quickly and participants were not aware that they would be asked to remember the target images.
Perhaps most interestingly, this effect of IQ varied across the two conditions, as IQ predicted memory performance for individuals in the facilitation condition but was unrelated to memory performance for those in the IOR condition. Importantly, although the mean IQ scores were high, consistent with the general observed increase in childhood IQs (Pietschnig, Voracek, & Formann, 2010), they did not differ across conditions. This result indicates that in the absence of concurrent excitation and suppression, individual differences in intelligence are the best predictor of recognition memory, consistent with previous evidence linking memory and intelligence (Engle, et al., 1999; Reber, et al., 1991). However, eliciting selection via suppression boosts encoding efficacy and essentially overrides this relationship between IQ and memory performance.
Numerous studies have shown that IQ/cognitive functioning can be affected by a host of environmental factors, including parental socioeconomic status (SES) and early childhood educational opportunities (Nisbett, et al., 2012). As a result, children from high-risk backgrounds often enter school at a disadvantage (Gutman, Sameroff, & Cole, 2003; Sameroff, Seifer, Barocas, Zax, & Greenspan, 1987). The present results offer the intriguing insight that enhancing attentional control skills may help to ameliorate this disadvantage in educational contexts that place high demand on learning and memory systems.
Previous intervention studies aimed at improving attentional control have yielded positive effects on children’s basic attention skills and more global cognitive control (Diamond, Barnett, Thomas, & Munro, 2007; Rueda, Rothbart, McCandliss, Saccomanno, & Posner, 2005). The present study offers the additional insight that online learning strategies that engage controlled selective attention, even without long-term cognitive control training, can boost basic learning and memory skills during the school years. Close inspection of Figures 3A and 3B reveals that memory performance shows improvement on the lower end of our IQ spread in the IOR condition (Figure 3B) relative to that range in the facilitation condition (Figure 3A). We take this as preliminary indication that children who are at greater risk may also be more likely to benefit from attention-based interventions. Given that the participants in the present study were highly functioning, future studies will need to replicate and extend this work by examining a broader sample of children with lower IQs. Nonetheless, having established this important relation sets the stage for development of such interventions that can be applied in classroom settings. In particular, the present data suggest that the coupling between attention and memory is critical, and interventions that drive this coupling, rather than focusing on improving these skills separately, may be especially effective in promoting achievement.
Interestingly, the reported attention and IQ effects on memory were consistent across the age range that we studied. Our primary goal was to examine the role of selective attention in promoting effective memory encoding within a school-aged population; as such, we purposely selected paradigms that was relatively simple in order to mechanistically expose the hypothesized interaction. Basic IOR is evident within the first year of life (Hood, 1993; Johnson & Tucker, 1996; Richards, 2000) and has also been observed during childhood and adolescence (Dennis, et al., 2005; Li, Chang, & Lin, 2003). Additional work, however, has shown that IOR in early childhood is sensitive to task parameters and cognitive control demands. For example, MacPherson, Klein, and Moore (2003) found that 5 – 10 year-olds showed IOR only when a second cue reoriented their attention away from the peripheral cued location and back to the central fixation. The cueing task in the present study used a central fixation that was more engaging than in typical adults tasks, which may have supported our youngest participants’ efforts to avoid overt orienting to the cue, and perhaps, as in MacPherson et al. (2003) promoted reorienting of attention away from the cued location. In addition, our use of saccade latency rather than manual reaction times provided a sensitive measure of IOR effects on attention orienting. Together, these factors likely allowed us to identify stable IOR even among the youngest participants in our sample.
Recognition memory can be subdivided into two distinct processes, recollection and familiarity (Mandler, 1980; Yonelinas, 2002). Recollection refers to memory that is accompanied by contextual details, (i.e., “source” memory), whereas familiarity refers to a sense that an item has been encountered, without remembering specific details (i.e., “item” memory). Developmental studies have shown that recollection improves throughout adolescence, whereas familiarity stabilizes between 6 and 8 years of age and shows little subsequent improvement (Billingsley, Smith, & McAndrews, 2002; Cycowicz, Freidman, Snodgrass, & Duff, 2001; Cycowicz, Friedman, & Duff, 2003; Ghetti & Angelini, 2008; Ofen, et al., 2007). In the present study we used a recognition memory task that targeted familiarity rather than recollection in order to maximize the potential to observe the hypothesized attention-memory interaction even among our youngest participants. However, as noted, previous research suggests that developmental effects may emerge in the context of more challenging tasks, such as source memory/recollection tasks.
The present study thus highlights a functional interaction between two cognitive systems that is available early during the formal education years and remains stable across development. The ultimate goal of truly developmental investigations is to identify agents of change in some process or knowledge structure (Wohlwill, 1970). Both attention and memory are building block developmental mechanisms that constrain what information guides knowledge and action. The present study shows that attention is a catalyst of change in memory performance during development and offers an important step towards understanding how selective attention mechanistically influences memory encoding during the school years.
5. Conclusions
The present results demonstrate that selection via suppression supports robust memory encoding. More broadly, these results demonstrate that attentional processes have the power to promote basic memory processes and underscore the importance of examining the development of attention and memory as integrated systems. The present tasks were chosen to be relatively stable across our age span in order to best expose the hypothesized relation with minimal noise. However, cognitive control via inhibition of competing alternatives follows a protracted developmental course. Future work can probe how developments in such higher-level attentional selection affords efficacy in more complex learning and memory situations.
Highlights.
Memory was enhanced when selection via suppression was engaged during encoding.
The extent of suppression during encoding predicted recognition accuracy at test.
In the absence of selection via suppression, IQ best predicted recognition accuracy.
Selection via suppression at encoding boosted memory among children with lower IQs.
Acknowledgments
The authors gratefully acknowledge the National Institutes of Health (K01 MH07793 to DA) and the James S. McDonnell Foundation (Scholar Award in Understanding Human Cognition to DA) for their generous support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astle DE, Nobre AC, Scerif G. Attentional control constrains visual short-term memory: Insights from developmental and individual differences. The Quarterly Journal of Experimental Psychology. 2012;65(2):277–294. doi: 10.1080/17470218.2010.492622. doi: 10.1080/17470218.2010.492622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. TRENDS in Cognitive Sciences. 2001;5(3):119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Billingsley RL, Smith ML, McAndrews MP. Developmental patterns in priming and familiarity in explicit recollection. Journal of Experimental Child Psychology. 2002;82:251–277. doi: 10.1016/s0022-0965(02)00007-3. [DOI] [PubMed] [Google Scholar]
- Brefczynski JA, DeYoe EA. A physiological correlated of the ‘spotlight’ of visual attention. Nature Neuroscience. 1999;2(4):370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- Chun MM. Visual working memory as visual attention sustained internally over time. Neuropsychologia. 2011;49:1407–1409. doi: 10.1016/j.neuropsychologia.2011.01.029. doi: 10.1016/j.neuropsychologia.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: Functional anatomy by positron emission tomography. Journal of Neuroscience. 1991;11(8):2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N, Elliott EM, Saults JS, Morey CC, Mattox S, Hismjatullina A, et al. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology. 2005;51:42–100. doi: 10.1016/j.cogpsych.2004.12.001. doi: 10.1016/j.cogpsych.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N, Fristoe NM, Elliott EM, Brunner RP, Saults JS. Scope of attention, control of attention, and intelligence in children and adults. Memory & Cognition. 2006;34(8):1754–1768. doi: 10.3758/bf03195936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N, Nugent LD, Elliott EM, Ponomarev I, Saults JS. The role of attention in the development of short-term memory: Age differences in the verbal span of apprehension. Child Development. 1999;70(5):1082–1097. doi: 10.1111/1467-8624.00080. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Govoni R, Naveh-Benjamin M, Anderson ND. The effects of divided attention on encoding and retrieval processes in human memory. Journal of Experimental Psychology: General. 1996;125(2):159–180. doi: 10.1037//0096-3445.125.2.159. [DOI] [PubMed] [Google Scholar]
- Cycowicz Y, Freidman D, Snodgrass JG, Duff M. Recognition and source memory for pictures in children and adults. Neuropsychologia. 2001;39:255–267. doi: 10.1016/s0028-3932(00)00108-1. [DOI] [PubMed] [Google Scholar]
- Cycowicz Y, Friedman D, Duff M. Pictures and their colors: What do children remember? Journal of Cognitive Neuroscience. 2003;15(5):759–768. doi: 10.1162/089892903322307465. [DOI] [PubMed] [Google Scholar]
- Dennis M, Edelstein K, Copeland K, Frederick JA, Francis DJ, Hetherington R, et al. Space-based inhibition of return in children with spina bifida. Neuropsychology. 2005;19(4):456–465. doi: 10.1037/0894-4105.19.4.456. doi: 10.1037/0894-4105.19.4.456. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General. 1999;128(3):309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Fernandes MA, Moscovitch M. Divided attention and memory: Evidence of substantial interference effects at retrieval and encoding. Journal of Experimental Psychology: General. 2000;129(2):155–176. doi: 10.1037//0096-3445.129.2.155. doi: 10.1D37//0096-3445.129.2.155. [DOI] [PubMed] [Google Scholar]
- Fletcher J, Maybery MT, Bennett S. Implicit learning differences: A questions of developmental level? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(1):246–252. doi: 10.1037//0278-7393.26.1.246. doi: 10.1037//0278-7393.26.1.246. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Executive frontal functions. Experimental Brain Research. 2000;133:66–70. doi: 10.1007/s002210000401. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proceedings of the National Academy of Sciences. 1999;96(6):3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, Angelini L. The development of recollection and familiarity in childhood and adolescence: Evidence from the dual-process signal detection model. Child Development. 2008;79(2):339–358. doi: 10.1111/j.1467-8624.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- Gutman LM, Sameroff AJ, Cole R. Academic growth curve trajectories from 1st grade to 12th grade: Effects of multiple social risk factors and preschool child factors. Developmental Psychology. 2003;39(4):777–790. doi: 10.1037/0012-1649.39.4.777. doi: 10.1037/0012-1649.39.4.777. [DOI] [PubMed] [Google Scholar]
- Hauer BJA, MacLeod CM. Endogenous versus exogenous attentional cuing effects on memory. Acta Psychologica. 2005;122:305–320. doi: 10.1016/j.actpsy.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Hood BM. Inhibition of return produced by covert shifts of visual attention in 6-month-old infants. Infant Behavior & Development. 1993;16:245–254. [Google Scholar]
- Ikkai A, Curtis CE. Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia. 2011;49:1428–1434. doi: 10.1016/j.neuropsychologia.2010.12.020. doi: 10.1016/j.neuropsychologia.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Tucker LA. The development and temporal dynamics of spatial orienting in infants. Journal of Experimental Child Psychology. 1996;63:171–188. doi: 10.1006/jecp.1996.0046. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity, proactive interference, and divided attention: Limits on long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(2):336–358. doi: 10.1037//0278-7393.26.2.336. doi: 10.10371/0278-7393.26.2.336. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. TRENDS in Cognitive Sciences. 2000;4(4):138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Lahaderne H. Attitudinal and intellectual correlates of attention: A study of four sixth-grade classrooms. Journal of Educational Psychology. 1968;59(5):320–324. doi: 10.1037/h0026223. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Chang H-L, Lin S-C. Inhibition of return in children with attention deficit hyperactivity disorder. Experimental Brain Research. 2003;149:125–130. doi: 10.1007/s00221-002-1362-8. doi: 10.1007/s00221-002-1362-8. [DOI] [PubMed] [Google Scholar]
- MacPherson AC, Klein RM, Moore C. Inhibition of return in children and adolescents. Journal of Experimental Child Psychology. 2003;85:337–351. doi: 10.1016/s0022-0965(03)00104-8. doi: 10.1016/S0022-0965(03)00104-8. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87(3):252–271. [Google Scholar]
- Markant J, Amso D. Selective memories: Infants’ encoding is enhanced in selection via suppression. Developmental Science. 2013:1–15. doi: 10.1111/desc.12084. doi: 10.1111/desc.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JJ, Ward LM, Kiehl KA. An event-related brain potential study of inhibition of return. Perception and Psychophysics. 1999;61(7):1411–1423. doi: 10.3758/bf03206190. [DOI] [PubMed] [Google Scholar]
- Nisbett RE, Aronson J, Blair C, Dickens W, Flynn J, Halpern DF, et al. Intelligence: New findings and theoretical developments. American Psychologist. 2012;67(2):130–159. doi: 10.1037/a0026699. doi: 10.1037/a0026699. [DOI] [PubMed] [Google Scholar]
- Ofen N, Kao Y-C, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JD. Development of the declarative memory system in the human brain. Nature Neuroscience. 2007;10(9):1198–1205. doi: 10.1038/nn1950. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Pestilli F, Carrasco M. Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vision Research. 2005;45:1867–1875. doi: 10.1016/j.visres.2005.01.019. doi: 10.1016/j.visres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Pietschnig J, Voracek M, Formann AK. Pervasiveness of the IQ rise: A cross-temporal meta-analysis. PLoS ONE. 2010;5(12):e14406. doi: 10.1371/journal.pone.0014406. doi: 10.1371/journal.pone.0014406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis D, editors. Attention and Performance. X. Erlbaum Lawrence Associates; Hillsdale, NJ: 1984. pp. 531–556. [Google Scholar]
- Posner MI, Rafal RD, Choate LS, Vaughan J. Inhibition of return: Neural basis and function. Cognitive Neuropsychology. 1985;2(3):211–228. [Google Scholar]
- Reber AS, Walkenfeld FF, Hernstadt R. Implicit and explicit learning: Individual differences and IQ. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1991;17(5):888–896. doi: 10.1037//0278-7393.17.5.888. [DOI] [PubMed] [Google Scholar]
- Reid V, Striano T. Adult gaze influences infant attention and object processing: implications for cognitive neuroscience. European Journal of Neuroscience. 2005;21:1763–1766. doi: 10.1111/j.1460-9568.2005.03986.x. [DOI] [PubMed] [Google Scholar]
- Reid V, Striano T, Kaufman J, Johnson MH. Eye gaze cueing facilitates neural processing of objects in 4-month-old infants. NeuroReport. 2004;15:2553–2555. doi: 10.1097/00001756-200411150-00025. [DOI] [PubMed] [Google Scholar]
- Richards JE. Localizing the development of covert attention in infants with scalp event-related potentials. Developmental Psychology. 2000;36(1):91–108. [PubMed] [Google Scholar]
- Richards JE. The development of attention to simple and complex visual stimuli in infants: Behavioral and psychophysiological measures. Developmental Review. 2010;30:203–219. doi: 10.1016/j.dr.2010.03.005. doi: 10.1016/j.dr.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter FR, Yeung N. Memory and cognitive control in task switching. Psychological Science. 2012;23:1256–1263. doi: 10.1177/0956797612444613. doi: 10.1177/0956797612444613. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ, Van Rossem R. Information processing from infancy to 11 years: Continuities and prediction of IQ. Intelligence. 2012;40:445–457. doi: 10.1016/j.intell.2012.05.007. doi: 10.1016.j.intell.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Sheehy S, Oakes LM, Luck SJ. Exogenous attention influences visual short-term memory in infants. Developmental Science. 2011;14(3):490–501. doi: 10.1111/j.1467-7687.2010.00992.x. doi: 10.1111/j.1467-7687.2010.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proceedings of the Nataional Academy of Sciences. 2005;102(41):14931–14936. doi: 10.1073/pnas.0506897102. doi: 10.1073.pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutman A, Clapp W, Chadick JZ, Gazzaley A. Early top-down control of visual processing predicts working memory performance. Journal of Cognitive Neuroscience. 2010;22(6):1224–1234. doi: 10.1162/jocn.2009.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameroff AJ, Seifer R, Barocas R, Zax M, Greenspan Intelligence quotient scores of 4-year-old children: Social-environmental risk factors. Pediatrics. 1987;79:343–350. [PubMed] [Google Scholar]
- Schmidt BK, Vogel EK, Woodman GF, Luck SJ. Voluntary and automatic attentional control of visual working memory. Perception and Psychophysics. 2002;64(5):754–763. doi: 10.3758/bf03194742. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schwarzbach J, Yantis S. Attentional inhibition of visual processing in human striate and extrastriate cortex. Neuroimage. 2003;19(4):1602–1611. doi: 10.1016/s1053-8119(03)00187-3. [DOI] [PubMed] [Google Scholar]
- Smith AT, Singh KD, Greenlee MW. Attentional suppression of activity in the human visual cortex. Neuroreport. 2000;11(2):271–277. doi: 10.1097/00001756-200002070-00010. [DOI] [PubMed] [Google Scholar]
- Szekely A, Jacobsen T, D’Amico S, Devescovi A, Andonova E, Herron D, et al. A new on-line resource for psycholinguistic studies. Journal of Memory and Language. 2004;51(2):247–250. doi: 10.1016/j.jml.2004.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper SP. The negative priming effect: Inhibitory priming by ignored objects. Quarterly Journal of Experimental Psychology. 1985;37A:571–590. doi: 10.1080/14640748508400920. [DOI] [PubMed] [Google Scholar]
- van den Bos W, Crone EA, Güroğlu B. Brain function during probablistic learning in relation to IQ and level of education. Developmental Cognitive Neuroscience. 2012;2S:S78–S89. doi: 10.1016/j.dcn.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais PE, Rubens M, Boccanfuso J, Gazzaley A. Neural mechanisms underlying the impact of visual distraction on retrieval of long-term memory. The Journal of Neuroscience. 2010;30(25):8541–8550. doi: 10.1523/JNEUROSCI.1478-10.2010. doi: 10.1523/JNEUROSCI.1478-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]
- Wohlwill JF. The age variable in psychological research. Psychological Review. 1970;77(1):49–64. [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. doi: 10.1006/jmla.2002.2864. [Google Scholar]
- Zanto TP, Rubens M, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nature Neuroscience. 2011;14(5):656–661. doi: 10.1038/nn.2773. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]