Abstract
Neuromyelitis optica (NMO) is a debilitating autoimmune inflammatory disease of the central nervous system (CNS) that is distinct from multiple sclerosis (MS). The discovery of NMO-IgG in the serum of NMO, but not MS, patients was a breakthrough in defining diagnostic criteria for NMO. NMO-IgG is an antibody directed against the astrocytic water channel protein aquaporin-4 (AQP4). While there is evidence that NMO-IgG is also involved in mediating tissue damage in the CNS, many aspects of the pathogenic cascade in NMO remain to be determined. It is clear that antigen-specific T cells contribute to the generation of NMO-IgG in the peripheral immune compartment, as well as to the development of NMO lesions in the CNS. T helper 17 cells, equipped both in providing B cell help and inducing tissue inflammation, may be involved in NMO development and pathogenesis. Here, we review immunologic aspects of NMO, placing recent findings the biology of T–B cell cooperation in autoimmunity of the CNS into perspective.
Keywords: neuromyelitis optica, inflammation, CNS
Introduction
Neuromyelitis optica (NMO) is an inflammatory demyelinating disease of the CNS in which lesions occur predominantly in the spinal cord and optic nerves.1 For several years, it has been a matter of debate whether NMO was a distinct disease entity or a variant of multiple sclerosis (MS). Like conventional MS, NMO often shows a relapsing remitting course.2 However, NMO is distinct from MS in several aspects, including clinical, neuroimaging, cerebrospinal (CSF), and serological features.2,3 While patients with MS typically have mild attacks with good recovery, attacks of NMO produce severe disability, often with incomplete recovery. After six years, about one-third of NMO patients have permanent motor disability, one-fourth wheel chair bound, one-fifth have bilateral visual disability, and 10% will have died;4 complications such as respiratory failure have also been reported.5 However, in contrast to MS, it is uncommon for clinical disability in NMO to progress independently of relapse,6 suggesting that the pathogenic cascades in NMO and MS are different. Epidemiologic data reveal a pronounced preponderance of women over men afflicted with NMO, compared with MS patients (9:1 versus 2:1, respectively).2,7 While brain MRI scans often show no or few inflammatory lesions in NMO patients, longitudinally extensive signal abnormalities can be detected in the spinal cord during acute attacks, typically extending over three, or more vertebral, segments.2,7 Analysis of the CSF occasionally reveals a striking pleocytosis, with a polymorph nuclear predominance. Oligoclonal bands of IgG are observed in a minority of NMO patients (whereas they occur in about 85% of MS patients).8
Defining NMO as a distinct disease entity came from the identification of a highly specific serum antibody, NMO immunoglobulin G (NMO-IgG), which is absent in patients with conventional MS.9 Thus, clinical, imaging, and serological hallmarks have led to the conclusion that NMO is a distinct disease entity with specific diagnostic criteria.3
Pathologic features of NMO
Historic reports on histopathologic findings in autopsy and biopsy material from patients with NMO highlighted acute spinal cord lesions with diffuse swelling and tissue softening involving several spinal segments and, occasionally, the entire spinal cord in a patchy or continuous distribution.10,11 A comparison of lesions in patients who suffered from conventional MS and NMO revealed a unique pathological pattern in the latter: the presence of immunoglobulins located near activated complement in perivascular regions constitutes a prominent feature of NMO lesions.12 Activated complement (C3a and C5a) has chemoattractant properties that facilitate the recruitment of macrophages and eosinophils into lesion sites. Both eosinophils and macrophages have the ability to mediate complement- and/or antibody-dependent cytotoxicity via either complement or Ig/Fc receptors, respectively. In addition, activated macrophages, together with eosinophils and neutrophils, can locally generate cytokines, proteases, and either reactive oxygen or nitrogen species, resulting in non-selective bystander destruction of both grey and white matter structures, including axons and oligodendrocytes, and finally demyelination. Additional characteristics of NMO lesions are increased vascular permeability and edema that might secondarily aggravate tissue destruction via edema-induced ischemia.13,14 These observations suggested an important role for humoral immune mechanisms in the pathogenesis of NMO.
The recent identification of a specific serum autoantibody, NMO-IgG, which targets aquaporin-4 (AQP4), the most abundant water channel in the CNS, strengthened the hypothesis of a humoral mechanism in NMO pathogenesis.9,15 Interestingly, the distribution pattern of AQP4 expression at glial–fluid interfaces (e.g., perivascular foot processes of astrocytes and at the glia limitans) mirrors the sites of immunoglobulin and complement deposition detected in NMO lesions.9,12 In NMO lesions, AQP4 expression is lost, and reduced immunoreactivity to AQP4 correlates with the loss, or reduction, of glial fibrillary acidic protein (GFAP) immunostaining, while myelin basic protein (MBP) expression is relatively preserved, indicating primary structural damage of astrocytes but not oligodendrocytes. Thus, NMO might principally be an astrocyte disease, with demyelination being a secondary event in lesion development.16,17
While the expression pattern of AQP4 in the CNS and lesion topography in NMO largely support the idea that AQP4 is a target of the immune response in NMO, the correlation of AQP4 expression and lesion topography is not entirely straightforward (see below). Moreover, loss of AQP4 in NMO lesions is associated with structural damage to astrocytes, as indicated by concomitant loss of GFAP immunoreactivity particularly in the spinal cord and optic nerve;12,16–18 however, in some niches, NMO lesions appear to be non-destructive. For example NMO lesions in the area postrema at the floor of the fourth ventricle are characterized by loss of AQP4, with preserved GFAP expression in astrocytes.19 The reason for the non-destructive and reversible nature of NMO lesions in the area postrema is unclear, although specific properties of area postrema astrocytes, including expression of complement regulatory proteins and lack of co-expression of the glutamate transporter EAAT2 (Glt-1) with AQP4 have been speculated to contribute to their resistance to NMO-IgG–mediated destruction.19 In contrast to NMO lesions, in which loss of AQP4 immunoreactivity is a unifying feature, AQP4 appears to be upregulated in the periplaque white matter of early- and late-active MS lesions. Only long-standing inactive lesions are characterized by a reduced immunoreactivity for AQP4, while remyelinating lesions (shadow plaques) show a diffusely increased expression of AQP4.
In MS, in contrast, the expression of AQP4 follows a stage-dependent pattern correlating with the presence of reactive astrocytes, suggesting that—as opposed to NMO—AQP4 is not the primary target of the immunopathologic process in MS.16,17 Another interesting difference between NMO and MS is the degree of cortical demyelination, which is well documented in MS but appears to be absent in NMO, in spite of cortical astrogliosis and neuronal pathology.20,21 It is unclear whether cortical demyelination is the histopathologic correlate of secondary progression in the clinical course of MS. Yet, this idea would fit well with the observation that secondary progression does not frequently occur in NMO.
Role of NMO-IgG
Tanycytes (the cells lining the third ventricle) and astrocytes express high levels of AQP4, while oligodendrocytes lack expression of this water channel. AQP4 plays an important, yet probably redundant, role in water homeostasis at the blood- and CSF-brain barriers.22 Indeed, AQP4 knockout (KO) mice do not show a spontaneous phenotype.23 Both AQP4 and AQP1 mediate water flux and seem to be involved in the development of brain edema in pathologic conditions.24–27 About 70% of all NMO patients have antibodies to AQP4,28 the majority of which recognize an extracellular determinant in the C-loop of AQP4. NMO-IgG is mainly of the IgG1 subclass and therefore capable of activating complement.29
Several clinical and histopathologic lines of evidence support the idea that NMO-IgG has a direct role in disease development. First, therapeutic plasmapheresis is an effective treatment for NMO patients.30–32 Second, AQP4 antibody titers seem to correlate with clinical severity of the disease.33–35 Third, regions of the brain and spinal cord that express AQP4 are preferential lesion sites in NMO;16,17,36 for example, AQP4 is abundantly expressed in the grey matter of the spinal cord, and the periventricular and periaqueductal areas,37,38 which are also the predominant locations of NMO lesions. Fourth, complement deposition and inflammatory infiltrates of eosinophils can be found in areas of the CNS where astrocytes highly express AQP4, suggesting that NMO-IgG supports the effector functions of complement binding and C1 activation, as well as cell-mediated cytotoxicity, both of which lead to structural damage of astrocytes16,17,39 (Fig. 1). It remains controversial whether binding of NMO-IgG also leads to functional alterations of AQP4, for example disturbed water and solute homeostasis or internalization of AQP4, with formation of cytotoxic edema as a potential consequence.40,41
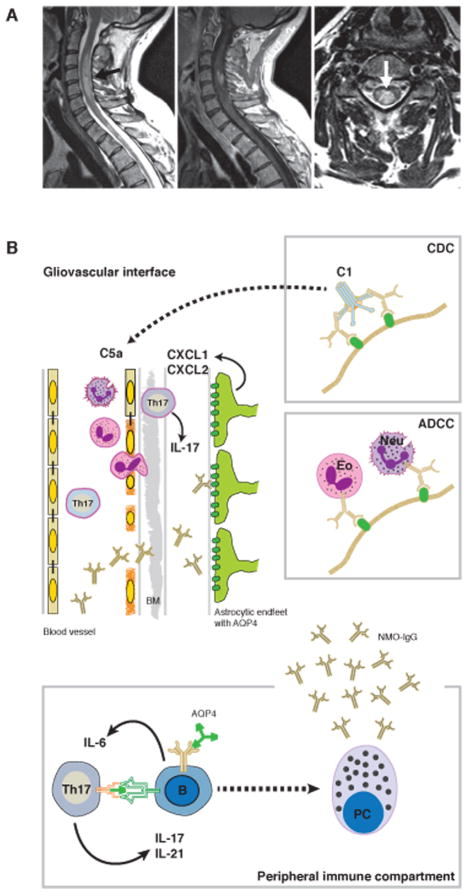
Figure 1.
Longitudinal extensive transverse myelitis is a defining feature of NMO. (A) In the left panel: T2w image of the cervicothoracic spinal cord of a patient with NMO. Note the extension of the centromedullary lesion (arrow) over more than three vertebral segments. Middle panel: Contrast-enhanced T1w image of the same lesion. Right panel: T2w image (transverse section) showing the centromedullary location of the NMO lesion (arrow). (B) Pathogenic process in NMO: in the peripheral immune compartment, B cells bearing an AQP4-specific B cell receptor might serve as antigen presenting cells to prime autoreactive T cells to develop into the Th17 lineage. In turn, Th17 cells have the ability to help B cells become plasma cells, producing antibodies to AQP4 (NMO-IgG). These serum antibodies that are not per se produced intrathecally only cross the blood–brain barrier, including the endothelial basement membrane (BM) under conditions of ongoing inflammation of the endothelium of the CNS vasculature, which might again be driven by Th17 cells. Upon binding of their target antigen (i.e., AQP4 expressed in astrocytic endfeet of the glia limitans), NMO-IgG initiate a downstream pathogenic cascade leading to tissue damage either by complement-dependent cytotoxicity (CDC, upper box) or antibody-dependent cell-mediated cytotoxicity (ADCC, lower box). Here, eosinophils (Eo) recruited into the lesion by C5a, or neutrophils (Neu) recruited into the lesion by ELR chemokines such as CXCL1, are potential effector cells.
Recent studies have investigated potential effector functions of NMO-IgG in greater detail. AQP4 antibody and complement factors have been shown to induce necrosis of astrocytes in vitro.42 In addition, from co-culture systems of astrocytes and oligodendrocytes it was proposed that NMO-IgG might bind to astrocytes and disturb glutamate homeostasis; in support of this, downregulation of EAAT2 in astrocytes has been shown to result in impaired glutamate uptake into astrocytes and cause glutamate excitotoxicity in neighboring oligodendrocytes, and demyelination in ex vivo experiments.18,43 However, appropriate animal models of NMO are still needed to test these hypotheses in vivo.
Systemic transfer of NMO-IgG alone into experimental animals has not been shown to provoke disease activity. This might be due, in part, to limited ability of serum proteins to pass the blood–brain or blood–CSF barriers into the CNS compartment. On the other hand, lack of a tight blood–brain barrier appears not to be sufficient for NMO-IgG to exert its effector functions in the CNS, as NMO-IgG transfer into juvenile laboratory animals, which have a leaky blood–brain barrier, fail to induce pathology.44
In fact, the necessary component for induction of NMO-IgG–mediated pathology is inflammatory alterations of the blood brain–barrier. Most likely, AQP4 antibodies only become pathogenic at sites of inflammation in the presence of either activated effector cells or sufficient amounts of complement in the absence of complement inhibitory factors. Passive transfer of NMO-IgG into EAE rats—which have been immunized to develop a subclinical T cell response against a myelin antigen—not only exacerbates EAE severity but also causes striking histopathologic changes reminiscent of NMO lesions, including extensive loss of astrocytes as well as perivascular deposition of immunoglobulin and complement.44–46 Loss of AQP4 extended beyond the borders of the structurally damaged area, and some areas adjacent to the lesions showed astrocytic processes devoid of AQP4 but with preserved GFAP expression.44 Interestingly, a recent study showed that co-injection of NMO IgG and human complement directly into the cerebrum of experimental mice was sufficient to provoke NMO-like lesions, with loss of AQP4 expression and glial cell edema.47 These data indicate that T cells might be dispensable for the effector functions of NMO-IgG at the lesion site, albeit under experimental conditions in which the blood–brain barrier has been short-circuited and when a permissive (i.e., inflammatory and complement-sufficient) milieu is created by the approach.47 Thus, NMO-IgG alone, even after trafficking into the CNS, may not be sufficient to induce NMO lesions. Circumventricular organs, such as the area postrema, lack a tight blood–brain barrier even under physiological (i.e., non-inflammatory) conditions, and it is in these areras, not in other sites of the CNS, that binding of intravenously injected NMO-IgG can be detected, though without induced structural damage.48
Therefore, whereas NMO-IgG specific for AQP4 is pathognomonic for NMO, the presence of T cells, complement, and inflammation is required for the development of parenchymal tissue damage in NMO. Hence, there are still uncertainties regarding the exact effector mechanisms and the overall impact of anti-AQP4 antibodies. Although the topography of NMO lesions has been associated with the distribution of AQP4 expression in the CNS,8,38 it is incompletely understood why certain areas of the CNS, including the cortical grey matter, appear to be preserved from NMO lesions despite an abundance of AQP4 expression in cortical astrocytes. In addition, AQP4 is highly expressed in peripheral tissues, including gastric mucosa, kidney, and muscle; yet, seropositive NMO patients do not suffer from clinically apparent interstitial nephritis or myositis. One explanation might be that the AQP4 clustering in orthogonal arrays of particles leads to the generation of pathogenic epitopes only at particular sites of the CNS.49 The M23 isoform of AQP4 forms orthogonal arrays of particles and may react differentially to NMO-IgG binding, compared with the M1 isoform. Another hypothesis is that the absence of complement inhibitory or regulatory factors might favor NMO-IgG–mediated pathology only in certain anatomical niches.50
T cell specificity in NMO
Since the discovery of NMO-IgG in 2004, NMO has been regarded primarily as an antibody-mediated disease. However, a series of studies have provided evidence for an important role for effector T cells in various steps of NMO pathogenesis.44 As mentioned above, the presence of AQP4-specific antibodies alone is not sufficient to provoke inflammatory disease in the CNS; indeed, some patients show persistently high titers of anti-AQP4 antibodies despite clinical remission.51–54 Yet, transfer of in vitro–generated, AQP4 peptide–specific T cells into rats in the absence of NMO-IgG has been shown to provoke a subclinical disease, with inflammatory lesions along the entire neuroaxis. In contrast, co-transfer of AQP4-specific T cells plus NMO-IgG results in inflammatory tissue damage reminiscent of NMO.55 Thus, effector T cells seem to be required for lesion development, at least for disrupting the blood–brain or blood–CSF barriers, and perhaps for creating an inflammatory milieu in situ for antibodies to be operational. T cells are also found within the lesions of NMO patients, though their antigen specificity and function have not been characterized.
In a different sense, it is clear that AQP4-specific T cells are required in the peripheral immune compartment to help generate production of NMO-IgG, a class-switched antibody, from B cells (Fig. 1). Some efforts have been made to define AQP4-specific T cells in NMO patients. However, the AQP4 epitopes restricted to HLA alleles that are overrepresented in NMO patients (e.g., DR17 (DRB1*0301)) have not been indentified.56,57 T cells from NMO patients have been shown to respond to an immunodominant DR-restricted AQP4 epitope (AA61-80),58 although the exact haplotype restriction was not determined. Interestingly, a study on eleven Japanese NMO patients showed a clonal expansion of T cells expressing Vβ1 and Vβ13 chains,59 although no information was given on the patients’ HLA status; in Japanese cohorts, HLA-DPB1*0501, but not DRB1*0301, is overrepresented in anti-AQP4+ NMO patients.53 Recent studies in mice identified the major immunogenic T cell epitopes of AQP4 presented by I-Ab.60,61 Thus, it might be possible to build experimental models that allow investigation of anti-AQP4 specific adaptive immune responses similar to MOG-specific responses, but utilizing the putatively autoantigen AQP4 implicated in the development of NMO. It would then be possible to ask questions about the cytokine phenotype, the timing, and the relevant compartments of adaptive immune responses against AQP4.
T cell phenotype in NMO
Upon antigen-specific activation, T helper cells become effector T cells that produce cytokines. T helper cells have been classified into various subsets on the basis of specific signature effector cytokines with distinct functions.62 The different subsets, or T helper cell lineages, are distinguished from one another on the basis of the differentiation factors (usually produced by innate immune cells or antigen presenting cells like B cells) required to begin a specific developmental program leading to activated T helper cell subtypes; the individual developmental programs require distinct transcriptional modules controlled by specific transcription factors. For example, innate immune cell–derived interleukin (IL)-12 is necessary for the development of Th1 cells, which express the transcription factor T-bet and produce interferon (IFN)-γ as a signature cytokine; Th2 cells are induced by IL-4, express Gata3, and produce IL-4, IL-5, and IL-13. Th17 cells are induced by a combination of TGF-β plus IL-6, with IL-21 and IL-23 enhancing their precursor frequency and stabilizing their phenotype, respectively; Th17 cells express the transcription factor RORC and produce IL-17, IL-21, and IL-22.63,64 The various T helper cell lineages have specific functions in host defense. While Th1 cells are required to control viruses and intracellular bacteria, Th2 cells orchestrate the immune response against parasites; Th17 cells are important for immune responses to certain extracellular bacteria and fungi.
Immunopathology in organ-specific autoimmunity—such as that in MS—is believed to be due to dysregulated Th1 and Th17 responses, as Th1 and Th17 cells and their effector molecule signatures are found in the CSF and in MS lesions.65 However, secondary inflammatory infiltrates in MS are dominated by macrophages, which are activated by IFN-γ, while neutrophils, which are attracted by IL-17–induced chemokines, are rare in MS lesions and absent in the CSF.66 Compared with MS patients, NMO patients have elevated IL-6 and IL-17 in the CSF;67,68 and consistent with this, NMO patients display higher proportions of Th17 cells and IL-17–producing CD8+ T cells in the peripheral blood, compared with controls.58,69 Thus, it is possible that NMO is actually a paradigm for a Th17-driven autoimmune disease.70
However, if NMO is a Th17-mediated autoimmune disease, how can this be reconciled with the prominent role of B cells in NMO, including the potential effector functions of NMO-IgG? In fact there is increasing evidence of extensive cross-talk (in both directions) between Th17 and B cells. And while the cellular sources of IL-6 and IL-23 for the differentiation of Th17 cells have not been defined in vivo, it is possible that B cells, which can serve as antigen presenting cells in the context of NMO and are an excellent source of IL-6, might skew T cells towards a Th17 response (Fig. 1). Also, it has been demonstrated that Th17 cells provide very effective help to B cells.71 Consistent with this idea, the presence of high frequencies of Th17 cells was shown to be sufficient to drive the generation of germinal centers and the production of autoantibodies (anti-GPI) to induce arthritis in K/BxN mice.72 Furthermore, MOG-specific Th17 cells have been shown to have the unique ability to induce the generation of ectopic lymphoid follicles in the subarachnoid space upon transfer into naive recipient mice; this was partly dependent on the expression of the cell surface molecule podoplanin and the secretion of IL-17 by MOG-specific Th17 cells.73
Detailed analyses of the interaction between T and B cells reactive against the same cognate antigen have been performed in MOG-specific models of CNS autoimmunity. Double transgenic mice with both T and B cells specific for MOG developed very severe, spontaneous CNS autoimmunity, while the presence of antigen-specific B or T cells alone provoke little or no disease74,75 Histopathologic analyses identified the spinal cord and optic nerves as major locations for lesion development in the double transgenic animals, whereas the brain was devoid of any lesions. Indeed, spontaneous CNS autoimmunity in double transgenic mice has some of the features of NMO and was proposed to be a model for opticospinal MS. However, comparisons with NMO should be made cautiously since MOG has no relevance to the disease and on the assumption that relevant antigen for induction of NMO is AQP4. On the other hand, about 30% of NMO patients, diagnosed according to the 2006 criteria,3 are seronegative for NMO-IgG.76 Interestingly, the clinical phenotype of the classic Devic’s syndrome (i.e., a sequence of monophasic, longitudinal, extensive transverse myelitis and optic neuritis1) is quite frequent among seronegative NMO patients, and it has recently been reported that a fraction (16%) of seronegative NMO patients harbor serum antibodies to MOG, which are not found in conventional adult MS patients.77 Another histopathologic hallmark of EAE in MOG T and B cell receptor double transgenic mice is the presence of lymph follicle–like structures in the meninges,74 which are germinal center reactions within the meningeal compartment. In addition, these models suggest that antigen-specific T cells also recruit antigen-specific B cells (and not only transgenic B cells) from the natural repertoire to develop into antibody-producing cells within the subarachnoid and CNS compartments, and thus contribute to lesion development by means of MOG-specific antibodies.78
Although these transgenic model systems reflect important histopathologic features of MS, it has been unclear whether the cooperation between MOG-specific T and B cells translates to the case of AQP4 being the disease-relevant target autoantigen. Importantly, in seropositive NMO patients, NMO-IgG is not produced intrathecally (i.e., antibody-producing plasmablasts or plasma cells are not recruited to the subarachnoid space);79 consistent with this, follicle-like structures in the meninges have not been reported in NMO patients. Rather, it appears that the humoral immune response against AQP4 is exclusively generated in the peripheral immune compartment; this is different from MS. Specifically, intrathecal synthesis of antibodies is frequently observed in MS, with the presence of lymph follicle like structures in the meninges in about 40 to 50% of secondary progressive patients—a was associated with cortical demyelination (which is not found in NMO).
Hence, the contribution of T cells in the pathogenic cascades of MS and NMO appears to be fundamentally different, and much work remains to be done on the actual T cell contribution to the disease process in NMO-IgG seropositive NMO patients, although accumulating data suggest that T cells may have an important role not only in helping B cells but also in collaborating with antibodies in mediating tissue damage.
Therapy of NMO
Treatment regimens for NMO will probably never reach evidence level A (randomized controlled trial; meta-analysis) or even B (other evidence) simply due to the rarity of NMO: being100 times less frequent than MS (thus, patient numbers required to test treatment options in randomized trials with adequate power will not be feasible). Yet, since the pathogenic concepts in NMO are quite advanced, therapeutic strategies may be introduced based on these disease mechanisms.
Currently, the standard of care includes treatment of acute attacks, on the one hand, and prophylactic strategies to prevent future attacks, on the other. Intravenous corticosteroid therapy is usually the initial treatment for acute attacks of optic neuritis or myelitis in NMO patients. However, patients who do not respond promptly to corticosteroid treatment might benefit from plasmapheresis, which is recommended to be initiated early, particularly for patients with severe myelitis who are at high risk for neurogenic respiratory failure.80 While this strategy is broadly accepted for the treatment of attacks, there is some controversy its use as a prophylactic treatment. In line with the concept that antibodies contribute to the disease mechanism in NMO, treatment of patients with the B cell–depleting anti-CD20 monoclonal antibody rituximab appears to dampen disease activity and prevent relapses.81,82 Because reappearance of memory B cells after depletion of B cells with anti-CD20 has been observed to be a potential marker of resuming disease activity,83 efforts are being undertaken to target memory B cells even more efficiently by using a depleting antibody to CD19. In addition, general immunosuppressant agents, such as azathioprine or mycophenolate mofetil, have been used, with mixed responses.84,85 Novel and more targeted therapies are required for both acute intervention in NMO attacks and long-term immune prophylaxis.
The knowledge of potential effector functions of NMO-IgG has been used to design new targeted therapeutic approaches. For example, a recombinant anti-AQP4 antibody has been developed that binds to the C-loop of AQP4 but lacks the effector functions of NMO-IgG (i.e., it does not lead to complement fixation and antibody-dependent cell-mediated cytotoxicity that is eosinophil mediated; Fig. 1). The recombinant antibody, aquaporumab, is thought to competitively bind the AQP4 epitope and prevent pathogenic NMO-IgG from binding; it has been shown to block cell surface AQP4 binding of polyclonal NMO-IgG derived from NMO patient sera in cell culture and in ex vivo spinal cord slices, as well as in an in vivo mouse model. As a consequence of AQP4 epitope occupancy by aquaporumab (or other small molecule inhibitors), downstream cytotoxicity of NMO-IgG and formation of typical NMO lesions was suppressed.86,87 Another therapeutic strategy to treat NMO relapses made use of eculizumab, a monoclonal antibody against C5, which inhibits its cleavage by the C5 convertase and, thus, prevents complement activation.
In summary, information on NMO pathogenesis has been promptly translated into clinical treatment strategies for NMO attacks. However, regarding immune prophylactic therapies, the standard of care in NMO still largely relies on non-selective immunosuppression to inhibit the generation of NMO-IgG in the peripheral immune compartment. Unfortunately, some immune prophylactic therapies, including interferons, fingolimod, and natalizumab, which are beneficial in MS, have not been shown to be beneficial when were used in individual NMO patients or in NMO patient series.88–93 Another possible therapeutic target are molecules related to the biology of Th17 cells, as anti–IL-17 treatment appears to be safe and works very well in other Th17-related diseases like psoriasis.94 A monoclonal antibody to the IL-6 receptor (tocilizumab) was proven effective in severe cases of NMO that were refractory to standard of care treatment.95,96
It will be a major challenge in future research to improve, and then apply, the knowledge on antigen-specific adaptive immune responses against AQP4 to design well tolerated, antigen-or phenotype-specific, immune prophylactic therapies to prevent debilitating NMO relapses in the first place.
Acknowledgments
We thank Dr. Claus Zimmer (Department of Neuroradiology, Klinikum rechts der Isar) for providing the MRI images. This work was supported by intramural funding (KKF) to MM (B02-12). V.K.K. is supported by the Guthy Jackson Charitable Foundation and by Grants from the NIH. T.K. is recipient of a Heisenberg award from the DFG (KO2964/3-2). This work was supported by the DFG (SFB TR 128/A06 and Grants within the framework of the Munich Cluster for Systems Neurology (EXC 1010 SyNergy)).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Devic E. Myélite subaiguë compliquée de névrite optique. Bull Med (Paris) 1894;8:1033–1034. [Google Scholar]
- 2.Wingerchuk DM, et al. The clinical course of neuromyelitis optica (Devic’s syndrome) Neurology. 1999;53:1107–1114. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 3.Wingerchuk DM, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 4.Kitley J, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain: a journal of neurology. 2012;135:1834–1849. doi: 10.1093/brain/aws109. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi Z, et al. Intractable hiccup caused by medulla oblongata lesions: a study of an autopsy patient with possible neuromyelitis optica. J Neurol Sci. 2009;285:241–245. doi: 10.1016/j.jns.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Wingerchuk DM, et al. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology. 2007;68:603–605. doi: 10.1212/01.wnl.0000254502.87233.9a. [DOI] [PubMed] [Google Scholar]
- 7.O’Riordan JI, et al. Clinical, CSF, and MRI findings in Devic’s neuromyelitis optica. J Neurol Neurosurg Psychiatry. 1996;60:382–387. doi: 10.1136/jnnp.60.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wingerchuk DM, et al. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 9.Lennon VA, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 10.Cloys DE, Netsky MG. Neuromyelitis optica. North Holland Publishing Co; Amsterdam: 1970. [Google Scholar]
- 11.Mandler RN, et al. Devic’s neuromyelitis optica: a clinicopathological study of 8 patients. Ann Neurol. 1993;34:162–168. doi: 10.1002/ana.410340211. [DOI] [PubMed] [Google Scholar]
- 12.Lucchinetti CF, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125:1450–1461. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prineas JW, McDonald WI. Demyelinating diseases. Arnold; London: 1997. [Google Scholar]
- 14.Aboul-Enein F, et al. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62:25–33. doi: 10.1093/jnen/62.1.25. [DOI] [PubMed] [Google Scholar]
- 15.Lennon VA, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Misu T, et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain: a journal of neurology. 2007;130:1224–1234. doi: 10.1093/brain/awm047. [DOI] [PubMed] [Google Scholar]
- 17.Roemer SF, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 18.Hinson SR, et al. Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. The Journal of experimental medicine. 2008;205:2473–2481. doi: 10.1084/jem.20081241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popescu BF, et al. Neuromyelitis optica unique area postrema lesions: nausea, vomiting, and pathogenic implications. Neurology. 2011;76:1229–1237. doi: 10.1212/WNL.0b013e318214332c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucchinetti CF, et al. Inflammatory cortical demyelination in early multiple sclerosis. The New England journal of medicine. 2011;365:2188–2197. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popescu BF, et al. Absence of cortical demyelination in neuromyelitis optica. Neurology. 2010;75:2103–2109. doi: 10.1212/WNL.0b013e318200d80c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicchia GP, et al. The role of aquaporin-4 in the blood-brain barrier development and integrity: studies in animal and cell culture models. Neuroscience. 2004;129:935–945. doi: 10.1016/j.neuroscience.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 23.Ma T, et al. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. The Journal of clinical investigation. 1997;100:957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manley GT, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 25.Saadoun S, et al. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniguchi M, et al. Induction of aquaporin-4 water channel mRNA after focal cerebral ischemia in rat. Brain Res Mol Brain Res. 2000;78:131–137. doi: 10.1016/s0169-328x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 27.Warth A, Mittelbronn M, Wolburg H. Redistribution of the water channel protein aquaporin-4 and the K+ channel protein Kir4.1 differs in low- and high-grade human brain tumors. Acta Neuropathol. 2005;109:418–426. doi: 10.1007/s00401-005-0984-x. [DOI] [PubMed] [Google Scholar]
- 28.Waters P, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol. 2008;65:913–919. doi: 10.1001/archneur.65.7.913. [DOI] [PubMed] [Google Scholar]
- 29.Hinson SR, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–2231. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]
- 30.Jarius S, et al. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. 2008;4:202–214. doi: 10.1038/ncpneuro0764. [DOI] [PubMed] [Google Scholar]
- 31.Matiello M, et al. Neuromyelitis optica. Curr Opin Neurol. 2007;20:255–260. doi: 10.1097/WCO.0b013e32814f1c6b. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe S, et al. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler. 2007;13:128–132. doi: 10.1177/1352458506071174. [DOI] [PubMed] [Google Scholar]
- 33.Jarius S, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072–3080. doi: 10.1093/brain/awn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi T, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130:1235–1243. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- 35.Weinstock-Guttman B, et al. Neuromyelitis optica immunoglobulins as a marker of disease activity and response to therapy in patients with neuromyelitis optica. Mult Scler. 2008;14:1061–1067. doi: 10.1177/1352458508092811. [DOI] [PubMed] [Google Scholar]
- 36.Pittock SJ, et al. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006;63:964–968. doi: 10.1001/archneur.63.7.964. [DOI] [PubMed] [Google Scholar]
- 37.Oshio K, et al. Expression of aquaporin water channels in mouse spinal cord. Neuroscience. 2004;127:685–693. doi: 10.1016/j.neuroscience.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Saini H, et al. Differential expression of aquaporin-4 isoforms localizes with neuromyelitis optica disease activity. J Neuroimmunol. 2010;221:68–72. doi: 10.1016/j.jneuroim.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Matsuoka T, et al. Heterogeneity of aquaporin-4 autoimmunity and spinal cord lesions in multiple sclerosis in Japanese. Brain: a journal of neurology. 2007;130:1206–1223. doi: 10.1093/brain/awm027. [DOI] [PubMed] [Google Scholar]
- 40.Hinson SR, et al. Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proc Natl Acad Sci U S A. 2012;109:1245–1250. doi: 10.1073/pnas.1109980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ratelade J, Bennett JL, Verkman AS. Evidence against cellular internalization in vivo of NMO-IgG, aquaporin-4, and excitatory amino acid transporter 2 in neuromyelitis optica. The Journal of biological chemistry. 2011;286:45156–45164. doi: 10.1074/jbc.M111.297275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinoshita M, et al. Astrocytic necrosis is induced by anti-aquaporin-4 antibody-positive serum. Neuroreport. 2009;20:508–512. doi: 10.1097/wnr.0b013e32832776f4. [DOI] [PubMed] [Google Scholar]
- 43.Marignier R, et al. Oligodendrocytes are damaged by neuromyelitis optica immunoglobulin G via astrocyte injury. Brain: a journal of neurology. 2010;133:2578–2591. doi: 10.1093/brain/awq177. [DOI] [PubMed] [Google Scholar]
- 44.Bradl M, et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol. 2009;66:630–643. doi: 10.1002/ana.21837. [DOI] [PubMed] [Google Scholar]
- 45.Bennett JL, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66:617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinoshita M, et al. Neuromyelitis optica: Passive transfer to rats by human immunoglobulin. Biochem Biophys Res Commun. 2009;386:623–627. doi: 10.1016/j.bbrc.2009.06.085. [DOI] [PubMed] [Google Scholar]
- 47.Saadoun S, et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain: a journal of neurology. 2010;133:349–361. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratelade J, Bennett JL, Verkman AS. Intravenous neuromyelitis optica autoantibody in mice targets aquaporin-4 in peripheral organs and area postrema. PLoS One. 2011;6:e27412. doi: 10.1371/journal.pone.0027412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicchia GP, et al. Expression of multiple AQP4 pools in the plasma membrane and their association with the dystrophin complex. J Neurochem. 2008;105:2156–2165. doi: 10.1111/j.1471-4159.2008.05302.x. [DOI] [PubMed] [Google Scholar]
- 50.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 51.Nishiyama S, et al. A case of NMO seropositive for aquaporin-4 antibody more than 10 years before onset. Neurology. 2009;72:1960–1961. doi: 10.1212/WNL.0b013e3181a82621. [DOI] [PubMed] [Google Scholar]
- 52.Pittock SJ, et al. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol. 2008;65:78–83. doi: 10.1001/archneurol.2007.17. [DOI] [PubMed] [Google Scholar]
- 53.Matsushita T, et al. Association of the HLA-DPB1*0501 allele with anti-aquaporin-4 antibody positivity in Japanese patients with idiopathic central nervous system demyelinating disorders. Tissue Antigens. 2009;73:171–176. doi: 10.1111/j.1399-0039.2008.01172.x. [DOI] [PubMed] [Google Scholar]
- 54.Leite MI, et al. Myasthenia gravis and neuromyelitis optica spectrum disorder: a multicenter study of 16 patients. Neurology. 2012;78:1601–1607. doi: 10.1212/WNL.0b013e31825644ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pohl M, et al. Pathogenic T cell responses against aquaporin 4. Acta Neuropathol (Berl) 2011;122:21–34. doi: 10.1007/s00401-011-0824-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brum DG, et al. HLA-DRB association in neuromyelitis optica is different from that observed in multiple sclerosis. Mult Scler. 2010;16:21–29. doi: 10.1177/1352458509350741. [DOI] [PubMed] [Google Scholar]
- 57.Deschamps R, et al. Different HLA class II (DRB1 and DQB1) alleles determine either susceptibility or resistance to NMO and multiple sclerosis among the French Afro-Caribbean population. Mult Scler. 2011;17:24–31. doi: 10.1177/1352458510382810. [DOI] [PubMed] [Google Scholar]
- 58.Varrin-Doyer M, et al. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann Neurol. 2012;72:53–64. doi: 10.1002/ana.23651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warabi Y, et al. Characterization of the T cell receptor repertoire in the Japanese neuromyelitis optica: T cell activity is up-regulated compared to multiple sclerosis. J Neurol Sci. 2006;249:145–152. doi: 10.1016/j.jns.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Kalluri SR, et al. Functional characterization of aquaporin-4 specific T cells: towards a model for neuromyelitis optica. PLoS One. 2011;6:e16083. doi: 10.1371/journal.pone.0016083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nelson PA, et al. Immunodominant T cell determinants of aquaporin-4, the autoantigen associated with neuromyelitis optica. PLoS One. 2010;5:e15050. doi: 10.1371/journal.pone.0015050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 63.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 64.Korn T, et al. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 65.Korn T, Mitsdoerffer M, Kuchroo VK. Immunological basis for the development of tissue inflammation and organ-specific autoimmunity in animal models of multiple sclerosis. Results Probl Cell Differ. 2010;51:43–74. doi: 10.1007/400_2008_17. [DOI] [PubMed] [Google Scholar]
- 66.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 67.Ishizu T, et al. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain. 2005;128:988–1002. doi: 10.1093/brain/awh453. [DOI] [PubMed] [Google Scholar]
- 68.Uzawa A, et al. Markedly increased CSF interleukin-6 levels in neuromyelitis optica, but not in multiple sclerosis. J Neurol. 2009;256:2082–2084. doi: 10.1007/s00415-009-5274-4. [DOI] [PubMed] [Google Scholar]
- 69.Wang HH, et al. Interleukin-17-secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci. 2011;18:1313–1317. doi: 10.1016/j.jocn.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 70.Kira J. Neuromyelitis optica and opticospinal multiple sclerosis: Mechanisms and pathogenesis. Pathophysiology. 2011;18:69–79. doi: 10.1016/j.pathophys.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 71.Mitsdoerffer M, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu HJ, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peters A, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35:986–996. doi: 10.1016/j.immuni.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116:2393–2402. doi: 10.1172/JCI28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krishnamoorthy G, et al. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest. 2006;116:2385–2392. doi: 10.1172/JCI28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mealy MA, et al. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol. 2012;69:1176–1180. doi: 10.1001/archneurol.2012.314. [DOI] [PubMed] [Google Scholar]
- 77.Kitley J, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79:1273–1277. doi: 10.1212/WNL.0b013e31826aac4e. [DOI] [PubMed] [Google Scholar]
- 78.Pollinger B, et al. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J Exp Med. 2009;206:1303–1316. doi: 10.1084/jem.20090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalluri SR, et al. Quantification and functional characterization of antibodies to native aquaporin 4 in neuromyelitis optica. Arch Neurol. 2010;67:1201–1208. doi: 10.1001/archneurol.2010.269. [DOI] [PubMed] [Google Scholar]
- 80.Keegan M, et al. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58:143–146. doi: 10.1212/wnl.58.1.143. [DOI] [PubMed] [Google Scholar]
- 81.Cree BA, et al. An open label study of the effects of rituximab in neuromyelitis optica. Neurology. 2005;64:1270–1272. doi: 10.1212/01.WNL.0000159399.81861.D5. [DOI] [PubMed] [Google Scholar]
- 82.Jacob A, et al. Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients. Arch Neurol. 2008;65:1443–1448. doi: 10.1001/archneur.65.11.noc80069. [DOI] [PubMed] [Google Scholar]
- 83.Kim SH, et al. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol. 2011;68:1412–1420. doi: 10.1001/archneurol.2011.154. [DOI] [PubMed] [Google Scholar]
- 84.Bichuetti DB, et al. Neuromyelitis optica treatment: analysis of 36 patients. Arch Neurol. 2010;67:1131–1136. doi: 10.1001/archneurol.2010.203. [DOI] [PubMed] [Google Scholar]
- 85.Jacob A, et al. Treatment of neuromyelitis optica with mycophenolate mofetil: retrospective analysis of 24 patients. Arch Neurol. 2009;66:1128–1133. doi: 10.1001/archneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 86.Tradtrantip L, et al. Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann Neurol. 2012;71:314–322. doi: 10.1002/ana.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phuan PW, et al. A small-molecule screen yields idiotype-specific blockers of neuromyelitis optica immunoglobulin G binding to aquaporin-4. The Journal of biological chemistry. 2012;287:36837–36844. doi: 10.1074/jbc.M112.408716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papeix C, et al. Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler. 2007;13:256–259. doi: 10.1177/1352458506070732. [DOI] [PubMed] [Google Scholar]
- 89.Uzawa A, et al. Different responses to interferon beta-1b treatment in patients with neuromyelitis optica and multiple sclerosis. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2010;17:672–676. doi: 10.1111/j.1468-1331.2009.02897.x. [DOI] [PubMed] [Google Scholar]
- 90.Tanaka M, Tanaka K, Komori M. Interferon-beta(1b) treatment in neuromyelitis optica. Eur Neurol. 2009;62:167–170. doi: 10.1159/000227277. [DOI] [PubMed] [Google Scholar]
- 91.Kim SH, et al. Does interferon beta treatment exacerbate neuromyelitis optica spectrum disorder? Mult Scler. 2012 doi: 10.1177/1352458512439439. [DOI] [PubMed] [Google Scholar]
- 92.Min JH, Kim BJ, Lee KH. Development of extensive brain lesions following fingolimod (FTY720) treatment in a patient with neuromyelitis optica spectrum disorder. Mult Scler. 2012;18:113–115. doi: 10.1177/1352458511431973. [DOI] [PubMed] [Google Scholar]
- 93.Kleiter I, et al. Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol. 2012;69:239–245. doi: 10.1001/archneurol.2011.216. [DOI] [PubMed] [Google Scholar]
- 94.Leonardi C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. The New England journal of medicine. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 95.Ayzenberg I, et al. Interleukin 6 Receptor Blockade in Patients With Neuromyelitis Optica Nonresponsive to Anti-CD20 Therapy. JAMA Neurol. 2013:1–4. doi: 10.1001/jamaneurol.2013.1246. [DOI] [PubMed] [Google Scholar]
- 96.Kieseier BC, et al. Disease Amelioration With Tocilizumab in a Treatment-Resistant Patient With Neuromyelitis Optica: Implication for Cellular Immune Responses. Arch Neurol. 2012:1–4. doi: 10.1001/jamaneurol.2013.668. [DOI] [PubMed] [Google Scholar]