Abstract
Purpose
Investigating the steadiness of the phase-coupling between the time-course of the reader's voice and brain signals of subjects with autism spectrum disorder (ASD) passively listening to connected speech using magnetoencephalography (MEG). In typically developed subjects, such coupling occurs at the right posterior temporal sulcus (pSTS) for frequencies below 1 Hz, and reflects the neural processing of sentence-level rhythmic prosody at the prelexical level.
Methods
Cortical neuromagnetic signals were recorded with MEG (Elekta Oy, Finland) while seven right-handed and native French-speaking ASD subjects (six males, one female, range: 13–20 years) listened to live (Live) or recorded (Recorded) voices continuously reading a text in French for five minutes. Coherence was computed between the reader's voice time-course and ASD subjects' MEG signals. Coherent neural sources were subsequently reconstructed using a beamformer.
Key findings
Significant coupling was found at 0.5 Hz in all ASD subjects in Live and in six subjects in Recorded. Coherent sources were located close to the right pSTS in both conditions. No significant difference was found in coherence levels between Live and Recorded, and between ASD subjects and ten typically developed subjects (right-handed, native French-speaking adults, 5 males, 5 females, age range: 21–38 years) included in a previous study.
Significance
This study discloses a preserved coupling between the reader's voice and ASD subjects' cortical activity at the right pSTS. These findings support the existence of preserved neural processing of sentence-level rhythmic prosody in ASD. The preservation of early cortical processing of prosodic elements in verbal language might be exploited in therapeutic interventions in ASD.
Introduction
Autism spectrum disorders (ASD) represent an heterogeneous group of neurodevelopmental disorders of variable severity characterized by clinical features that can be classified into two main groups: social/communication disorders and narrow interest/stereotyped-repetitive behaviors (for a review, see [1]). While other neuropsychiatric disorders can share some of these symptoms, the precocity and the pervasive characteristic of social and communication impairments are highly specific of ASD (for a review, see [2]).
Based on structural and functional neuroimaging studies, several brain areas seem to play a major pathophysiological role in ASD social and communication impairments (for a review, see [3]). Among them, the cortical structures around the posterior superior temporal sulcus (pSTS) are of particular interest as they play a key functional role in social cognition, and are structurally and functionally abnormal in ASD subjects (for a review, see [4]). Indeed, several studies pointed out the involvement of the pSTS in the visual analysis of social cues such as gaze orientation or body movements (for a review, see [5]). Accumulating evidence also reveals that the pSTS is implicated in social perception through the auditory analysis of vocal and non-vocal sounds (for reviews, see [4], [6]). Functional neuroimaging studies have actually demonstrated the existence of voice-selective regions in both pSTS [7]. Based on these data, some authors suggest that a major functional role of the pSTS is to parse auditory or visual inputs into discrete units in order to extract their meaning [6]. Finally, this brain area was also identified as being part of cortical networks supporting theory of mind (for a review, see [8]) and mirror neuron (for reviews, see [9]–[11]) processes; such processes being impaired in ASD subjects [12]–[15]. In addition, several structural and functional neuroimaging studies have shown pSTS abnormalities in ASD such as decreased gray matter concentration in the cortex around the pSTS [16]–[18], rest hypoperfusion [4], [19], and abnormal activation during social tasks or vocal sound processing [20]–[22]. Taken together, these data suggest that the pSTS plays a key role in the pathophysiology of social and communication impairments found in ASD. Several authors thus hypothesize that anomalies involving the pSTS region during early brain development could represent the first step in the cascade of neural dysfunctions underlying ASD [4].
In humans, successful speech comprehension involves segmentation of speech signals into various timescales or temporal windows (TW), so that the spectrotemporal information is concurrently extracted within separate neural streams for speech perception and then integrated in brain regions involved in subsequent lexical computations for speech comprehension (for a review, see [23]). Up to now, two timescales have been investigated in depth in the context of speech perception: the 20–50 ms TW corresponding to the duration of phonemes, and the 150–300 ms TW corresponding to the duration of syllables and some prosodic phenomena (for a review, see [24]). Longer TWs, at the level of the second(s), are involved in sentence-level information [24]. In the context of an ecological continuous listening condition, we recently identified a novel coupling phenomenon at the sentence level, referred to as cortico-vocal coherence (CVC), between the time-course of the reader's voice and the listener's brain signals [25]. Significant coherence or phase coupling below 1 Hz was observed in all subjects within right pSTS and posterior superior temporal gyrus (pSTG) for the speech conditions, and within supra-temporal auditory cortices only for the non-speech vocal condition. In addition, significant coupling at 4–6 Hz (syllable level) was observed in 40% of the subjects at the supratemporal auditory cortex bilaterally. The coupling observed below 1 Hz corresponded to the neural integration of rhythmic prosody at the sentence level within right temporal voice sensitive areas; rhythmic prosody corresponding here to natural pauses occurring in the text in relation to punctuation marks or pauses associated with reader's breathing. These data suggested that right, non dominant, temporal voice sensitive areas contain neurons preferentially integrating sentence rhythmic prosody (TW>1 second) during natural speech perception reflected in rhythmic neuronal activity fluctuations below 1 Hz. Considering the key role of the right pSTS in inter-individual communicative behaviors and the importance of pauses or silences to guide successful verbal conversation [26], the strong reader–listener coupling at the right pSTS observed in this listening setting, was also interpreted as potentially corresponding to the neural processes involved in the recognition of relevant transitions in the speaker's discourse. These neural processes might serve as cues to drive the listener to initiate speech during interactional verbal communication or turn-taking transitions. Interestingly, turn-taking ability is known to be impaired in ASD with reasonable speech development (for a review, see [27]). This impairment is considered to be associated with pragmatic language deficits such as difficulties in sustaining conversation and turn-taking transitions, or in prosody perception [27]. Prosody, which is defined by the suprasegmental features of speech (variation in pitch, intonation, stress, rate, rhythm, duration, pausing, and loudness), facilitates speech comprehension and interindividual verbal communication (for a review, see [28]). While deficits in prosody appear not to be universal in ASD, atypical use of prosody is considered as a hallmark of speakers with such disorders [29]. Furthermore, several behavioral, neurophysiological and neuroimaging data have reported impairments in the production and the perception of prosody in ASD (for reviews, see [28], [30]).
Therefore, considering the putative dysfunction of the pSTS in ASD and the impairments in prosodic perception and pragmatic language in subjects with this disorder, this study aims at investigating the steadiness of the coupling between the time-course of the reader's voice and ASD subjects' brain signals using magnetoencephalography (MEG) and the CVC approach. We predict that pSTS dysfunction in ASD might be reflected in a weaker coupling in ASD subjects, or that the location of coherent brain areas will differ from that observed in typically developed subjects. Conversely, if coupling is unaffected in ASD, it might represent a theoretical support for therapeutic interventions based on low-frequency prosodic content of language to foster social communication in this disorder.
Methods
Participants
ASD subjects' clinical details are summarized in Table 1.
Table 1. Patients' clinical details.
| Mean | ||||||||
| Subjects | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Age | 18 | 20 | 19 | 15 | 14 | 13 | 15 | 16 |
| Sex | M | M | M | M | M | F | M | |
| Diagnosis | AD | AD | AD | ASD | AD | ASD | AD | |
| Weschler Scales (mean = 10, SD = 3) | ||||||||
| Block design | 13 | 12 | 3 | 4 | 9 | 12 | 7 | 8,5 |
| Similarities | 8 | 9 | 11 | 14 | 8 | 19 | 5 | 10,5 |
| Matrix reasoning | 13 | 12 | 8 | 5 | 10 | 8 | 8 | 9,1 |
| Comprehension | 6 | 5 | 6 | 9 | 1 | 14 | 3 | 6,2 |
| TLOCC | ||||||||
| Comprehension (mean = 57,8; SD = 12,1) | 48 | 58 | 38 | 62 | 22 | 68 | 44 | 48,5 |
| Expression (mean = 46,1; SD = 14,4) | 20 | 42 | 27 | 58 | 14 | 68 | 24 | 36,1 |
| ADOS | ||||||||
| Communication (cut-off for autism = 3) | 4 | 6 | 5 | 5 | 5 | 5 | 6 | 5,1 |
| Social interaction (cut-off for autism = 6) | 11 | 14 | 9 | 11 | 9 | 7 | 12 | 10,4 |
| Total C+SI (cut-off for autism = 10) | 15 | 20 | 14 | 16 | 14 | 12 | 18 | 15,5 |
| Speech abnormalities* (0 = normal, 2 = speech clearly abnormal) | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 1,7 |
(M: male; F: female; AD: autistic disorder; ASD: Asperger syndrome; SD: standard deviation; TLOCC: test de langage oral complexe pour collégiens; ADOS: autism diagnostic observation schedule; C: communication; SI: social interaction;
*: speech abnormalities associated with autism, coded item 2 of the ADOS Language and Communication domain).
Seven right-handed and native French-speaking subjects (6 males and 1 female, age range: 13–20 years, mean age: 16, normal audition) diagnosed with Asperger Syndrome (2 patients) or autistic disorder (5 patients) were included in the study. The handedness was assessed with the Edinburgh handedness Inventory [31]. The intellectual functioning was assessed by an abbreviated version of the Wechsler Intelligence scale for Children (Fourth Edition) [32] or the Wechsler Adult Intelligence Scale (Third Edition) [33]. The ASD was diagnosed according to DSM-IV criteria [34] and subsequently confirmed by the Autism Diagnostic Observation Schedule-Generic (ADOS) [35]. Based on ADOS, all subjects had weak (2 subjects) or clear (5 subjects) expressive prosodic disorder. To complete the assessment of the expressive language already explored with the ADOS, a brief assessment of the ASD subjects' lexical level was conducted with the Test de Langage Oral Complexe pour Collégiens (TLOCC) [36]. Subjects with neurological comorbidities or taking psychotropic treatment were excluded from the study except for one subject who was taking quetiapine (200 mg) for sleep disorder.
The study had the prior approval by the ULB-Hôpital Erasme Ethics Committee. The ASD subjects and their parents gave written informed consent before participating to the study.
Data obtained in the ten right-handed and native French-speaking typically developed healthy adult subjects (range 21–38 years; mean age: 25 years; 5 females and 5 males) included in the seminal CVC study [25] were used for statistical comparisons. This study had the prior approval by the ULB-Hôpital Erasme Ethics Committee. The subjects gave written informed consent before participating to the study.
Experimental paradigm
The experimental paradigms used in this study were adapted from [25]. Subjects' cortical neuromagnetic signals were recorded while they were listening to live (Live) or recorded (Recorded) voices continuously reading a text in French during 5 min. Both texts were chosen for their emotional neutrality in order to reduce the effect of affective prosody, and for the absence of any dialog in order to minimize prosody modulations. As in the seminal study, the first condition (Live) consisted in the passive listening of a text (http://www.eodi.org/la_revolution_francaise.html) read by a native French-speaking male (XDT, same as Exp1m in [25]) sitting 2 m in front of the subject inside the magnetically shielded room (MSR). The reader's vocal activity was recorded, time-locked to MEG signals, with a three-axis accelerometer attached to the left side of the reader's throat (ADXL330 iMEMS Accelerometer, Analog Devices, Inc., Norwood, MA, USA). Subjects were asked to fixate the gaze at a point in the MSR to avoid any gaze contact with the reader. In a second condition (Recorded), subjects passively listened to a recording of a native French-speaking female reading a French text (http://www.litteratureaudio.com/livre-audio-gratuit-mp3/zola-emile-la-terre.html) during 5 min. This condition was designed to test the influence of human interactional factors (e.g., human presence) on the CVC phenomenon. A loudspeaker (Panphonics Oy, Espoo, Finland) was positioned 2 m in front of the subjects inside the MSR. Signals of the recorded voice were recorded time-locked to MEG signals. In a third 5-min session (Rest), subjects were asked, as in the two previous conditions, to relax, fixate the gaze at a point in the MSR and not to move. The three experimental conditions (Live, Recorded, and Rest) were randomized across subjects.
Data acquisition, preprocessing, and analyses
The methods used for data acquisition, preprocessing, and analyses have been extensively detailed in [25] and will therefore be briefly described below.
Data Acquisition
MEG signals were recorded at the ULB-Hôpital Erasme with a whole-scalp-covering neuromagnetometer (Vectorview & Maxshield™; Elekta Oy, Helsinki, Finland). Head position inside the MEG helmet was continuously monitored using four head-tracking coils. The locations of the coils and at least 150 head-surface (on scalp, nose and face) points with respect to anatomical fiducials were determined with an electromagnetic tracker (Fastrak, Polhemus, Colchester, VT, USA). The recording passband was 0.1–330 Hz for MEG signals and 0–330 Hz for accelerometer and recorded voice signals; all signals were sampled at 1 kHz.
Data preprocessing
Continuous MEG data were preprocessed off-line using the signal-space-separation method to suppress external interferences and to correct for head movements [37]. In one subject, MEG signals in Live were preprocessed off-line using the spatio-temporal signal-space-separation (coefficient correlation: 0.95, window length: 4 s) to subtract system artefacts. Then, for frequency and coherence analyses, 2048 ms epochs were extracted with 1638 ms overlap, leading to a frequency resolution of 0.5 Hz. Epochs where MEG signals exceeded 3 pT (magnetometers) or 0.7 pT/cm (gradiometers) were rejected to avoid contamination of the data by eye movements, muscle activity, or artefacts of the MEG sensors. This yielded more than 590 artifact-free epochs for each subject and condition.
Coherence analysis in sensor space
Subsequently, voice signals were band-passed around voice fundamental frequency (f0; 100–200 Hz, Live; 130–300 Hz, Recorded) and rectified in order to compute for each epoch the coherence between the f0 time-course and MEG signals. In both conditions, the analyses were based on the voice fundamental frequency (f0) as this parameter is a highly relevant feature for speech processing (see [25] for more details). The coherence is an extension of Pearson correlation coefficient to the frequency domain, quantifying the degree of coupling between two signals, providing a number between 0 (no linear dependency) and 1 (perfect linear dependency) for each frequency [38]. The degree of coupling between MEG signals (frequency band: 0.1–330 Hz) and the f0 time-course were first determined by computing coherence spectra in the sensor space. Frequencies that displayed significant coupling in the sensor space in most subjects were identified and used as frequencies of interest for coherent source analyses.
Coherence analysis in source space
Coherence analyses were then performed in the source space using the Montreal Neurological Institute (MNI) template. Notwithstanding the risk of decrease in spatial accuracy, the MNI template was used rather than individual structural magnetic resonance imaging (MRI) in order to facilitate ASD subjects' recruitment as some of them refused to undergo MRI. For each subject, MEG and segmented MNI coordinate systems were coregistered using the three anatomical fiducial points for initial estimation and the head-surface points to manually refine the surface coregistration. The MEG forward model was then computed using the MNE suite (Martinos Center for Biomedical Imaging, Massachusetts, USA). Cortical sources coherent with the f0 time-course were identified using dynamic imaging of coherent sources (DICS) [39] and subsequently visualized on the MNI template. Separate coherence maps were computed for each possible combination of frequencies of interest, subjects, and conditions. Both planar gradiometers and magnetometers were simultaneously used for inverse modeling.
Finally, to produce coherence maps at the group level, we computed the generalized f-mean across subjects of normalized maps, according to 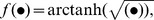 namely, the Fisher z-transform of the square root.
namely, the Fisher z-transform of the square root.
Statistical analyses
The statistical significance of sensor-space individual-level coherence was assessed using surrogate data-based statistics, based on coherence computed between surrogate f0 time-courses and original MEG data [40] (for more details, see also [41], [42]).
The statistical significance of source-space group-level coherence was assessed using a non-parametric permutation test based on coherence between vocal signals and rest condition MEG data [43] (for more details, see also [44]).
Non-parametric Wilcoxon and Mann-Whitney test were used to assess the difference between conditions and between ASD and typically developped subjects from the seminal CVC study [25]. Finally, correlations were computed to establish relations between coherence levels and age, and behavioural data reflecting autistic symptoms severity.
Results
Coherence at the sensor level
Figure 1 and Table 2 summarize the results obtained at the sensor level.
Figure 1. Individual coherence spectra in ASD subjects.
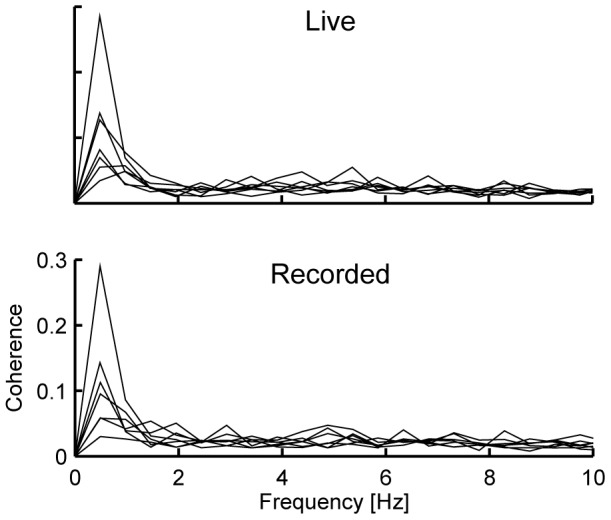
Individual coherence spectra obtained in each condition for the right-hemisphere MEG sensor showing the maximum coherence level. Significant coherence at 0.5 Hz was observed in all ASD subjects in Live and in 6 out of 7 subjects in Recorded. Y–axis values are identical on both plots.
Table 2. Sensor-space coherence levels at 0.5 Hz.
| Participants | Live | Recorded | Controls |
| 1 | 0.1266 | 0.1130 | 0.0818 |
| 2 | 0.0692 | 0.0295* | 0.0804 |
| 3 | 0.0804 | 0.0577 | 0.1302 |
| 4 | 0.0482 | 0.0953 | 0.0518 |
| 5 | 0.2846 | 0.2904 | 0.1647 |
| 6 | 0.0564 | 0.0582 | 0.1862 |
| 7 | 0.1377 | 0.1427 | 0.1255 |
| 8 | — | — | 0.0960 |
| 9 | — | — | 0.3072 |
| 10 | — | — | 0.1802 |
| min | 0.0482 | 0.0295 | 0.0518 |
| max | 0.2846 | 0.2904 | 0.3072 |
| mean | 0.1126 | 0.1121 | 0.1404 |
*p>0.05.
Live and Recorded: sensor-space coherence levels obtained in ASD patients.
Controls: sensor-space coherence levels obtained in Exp1m in the seminal CVC study (see Table 1, Bourguignon et al. 2013).
In line with the seminal CVC study carried out in a group of healthy adult subjects [25], coherence between voice and MEG signals consistently peaked at ∼0.5 Hz in all ASD subjects. Right-hemisphere-dominant coherence levels were statistically significant at ∼0.5 Hz in all subjects in Live (coherence values from 0.04 to 0.28; p<0.05; see Figure 1 and Table 2) and in six subjects in Recorded (coherence values from 0.06 to 0.29; p<0.05; see Figure 1 and Table 2). No difference in sensor-space coherence levels at 0.5 Hz was found between Live and Recorded (p = 0.87), nor between ASD subjects and the typically developed healthy adult subjects investigated in the seminal CVC study (Live, p = 0.28; Recorded, p = 0.31).
No other peak in coherence was found at the sensor level, and particularly at 4–6 Hz. The 0.5-Hz coherent frequency was therefore considered as the only frequency of interest to compute individual and group-level coherence maps.
Coherence at the source level
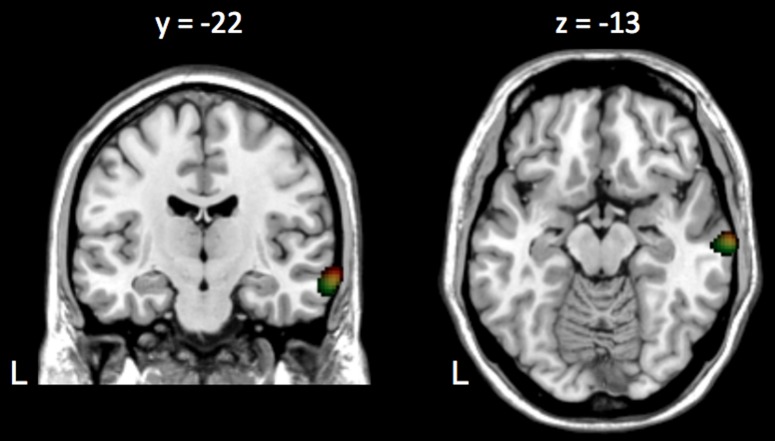
Figure 2 illustrates the location of the coherence local maxima on the MNI template in both conditions.
Figure 2. Group-level coherence maps in ASD subjects.
Group level coherence maps computed at 0.5(Live, red; Recorded, green; intersection between Live and Recorded, yellow). In Live, the maximum coherence occured at the right superior temporal sulcus and in Recorded, at the upper bank of the middle temporal gyrus. Coherence maps were thresholded at the maximum of coherence to isolate the local coherence maxima (Live, coherence threshold: 0.64–0.71; Recorded, coherence threshold: 0.60–0.67).
Group-level analysis revealed maximum coherence at the right STS (MNI coordinates: [64 –15 –11] mm; coherence value: 0.07, p<0.05) in Live and at the middle temporal gyrus (MTG) (MNI coordinates: [64 –18 –18] mm; coherence value: 0.07, p<0.05) in Recorded. Considering the limited spatial resolution afforded by the coregistration procedure used in this study, these coherence local maxima can be considered very close to the one reported in the seminal CVC study (Exp1m, pSTS, [66 -26 -1] mm) [25].
No local coherence maximum was observed at supratemporal auditory cortex bilaterally. At a lower threshold, a local coherence maximum was also observed at the left parietal operculum in both listening conditions, in accordance with the seminal CVC study [25].
Correlations between sensor-space coherence level and behavioural data
Correlation analyses did not disclose any association between sensor-space coherence levels and age or behavioural scores (age, IQ, ADOS and TLOCC: p>0.05).
Discussion
Despite scientific evidences of right pSTS dysfunction in ASD, this study demonstrates significant coupling in ASD subjects between neural activity at this cortical structure and the slow fluctuations of a heard voice during continuous connected speech listening, a phenomenon previously demonstrated in healthy subjects. Indeed, the coupling between the reader's voice f0 time-course and listener's cortical MEG signals was similar at 0.5 Hz in terms of coherence levels and location of coherence local maxima between ASD subjects and the typically developed healthy adult subjects investigated in the seminal CVC study [25]. This study also suggests that CVC levels at 0.5 Hz do not vary according to age or autistic symptom severity in ASD subjects. No coupling was found at 4–6 Hz between the reader's voice f0 time-course and listener's cortical MEG signals in ASD subjects, in accordance with the scarcity of such coupling in typically developed subjects using our experimental setting [25].
Preserved coupling between the reader's voice and listener's MEG signals in ASD
The preserved coupling between the reader's voice and listener's MEG signals observed in the ASD subjects investigated in this study may be explained by several factors. In line with the neural complexity hypothesis [45], [46] and the enhanced perceptual theory (for a review, see [47]), the cortico-vocal coupling might be preserved in ASD subjects because of the low-level neural processing engaged in this coupling phenomenon. Indeed, the CVC experimental paradigm consists in unimodal (auditory) stimulation. Moreover, this coupling phenomenon occurring at the right pSTS is considered to involve prelexical processing levels in speech perception as it was also observed when typically developed subjects listened to a totally incomprehensible language (native French-speaking subjects listening to Finnish) [25]. Interestingly, accumulating data suggest that ASD subjects perform normally, or even better than typically developed subjects, in tasks that require low-level processing or involve simple material [48], whereas they underperform in tasks that engage high-level neural processing such as multimodal sensory processing [49], [50] or complex cognitive or social tasks [13], [51]. Electrophysiological and neuroimaging data support this particular perceptive profile (for reviews, see [28], [46], [47]), which seems to be explained by an association of local over-connectivity and long-range under-/dys-connectivity [52], [53]. Still, some authors consider that speech perception is complex per se and numerous studies have suggested more severe impairments or cerebral dysfunctions for speech than for non-speech stimuli in ASD subjects [28], [46]. However, albeit speech signals are indeed highly complex auditory stimuli, it might be hypothesized that the prelexical speech perception processes subtending the CVC phenomenon involve low-level neural processes that would not be impaired in ASD subjects.
It could be hypothesized that the preserved coupling observed in our ASD subjects might have been favored by the slow rate at which the suprasegmental pauses or silences occur in speech (typically, for sentence rhythmic prosody, below 1 Hz). Indeed, it has been proposed that the temporality of the sensory stimulations, apart from their multimodal character, plays a key role in the pathophysiological basis of some behavioural impairments characterizing ASD [53]. Indeed, although altered temporal integration in the auditory modality as well as impaired spatio-temporal integration in the visual modality have been repeatedly observed in this population, slowing down the speed of sensory stimulations has been shown to improve imitative, verbal and cognitive performances [53]. It might therefore be of interest to determine if the coupling observed for shorter timescales (4–7 Hz, a TW corresponding to syllables and some prosodic phenomena) between neural and vocal activities during speech are preserved or not in ASD subjects [54].
Finally, a functional MRI study investigating the neural basis of prosodic speech deficits (linguistic and emotional prosody) in subjects with high functioning autism failed to find any clear deficit in pSTS recruitment compared with typically developed healthy subjects [55]. But and more interestingly, apart from an increased recruitment of the left supramarginal gyrus, ASD subjects were characterized by an absence of deactivation in key nodes of the default mode network (DMN) such as the precuneus, the anterior cingulate cortex and the left middle frontal gyrus; DMN deactivation correlating with prosodic skills in that study [55]. The DMN is a set of brain regions characterized by a high level of metabolic activity at rest and a low level of activity during goal-directed or externally focused cognitive tasks (for a review, see [56]). Several lines of evidence suggest that DMN suppression during goal-directed tasks is functionally relevant, particularly in terms of cognitive performance (i.e., the more the DMN activity is suppressed, the better is the cognitive performance) (for a review, see [57]). DMN suppression possibly reflects adaptive disengagement of “goal-irrelevant” brain functions (e.g., mind-wandering), which would be required to get properly engaged in goal-directed tasks [57]. So, the inability of deactivating DMN nodes while processing prosodic connected speech observed in ASD subjects could account for the existence of a less efficient processing of the relevant information, i.e. the prosodic dimension of speech, in ASD subjects [55]. Whether the deficits in DMN deactivation are related to altered functional integration between task-dependent networks and the DMN, or to a DMN dysfunction per se remains an open issue. Still, these fMRI data are congruent with the results of the present study suggesting preserved neural processing of suprasegmental rhythmic prosody at the right pSTS. Taken together, these data suggest that alterations in functional integration beyond the pSTS might therefore account for the rhythmic prosodic impairments described in the ASD population. This latter hypothesis is supported by MEG data obtained in subjects with Asperger syndrome that investigated another sensory modality, i.e. social visual cues processing [58]. In that study, neural activations were normal in strength and timing at the early stage of the cortical activation sequence (i.e., visual, STS and inferior parietal lobule activations) but were abnormal for the later stage (i.e., inferior frontal and primary motor cortex activations). This finding was interpreted as the absence of any significant deficit of high-level visual stimuli processing at the STS level in Asperger syndrome [58].
Importantly, the preservation of early cortical processing of prosodic elements in verbal language might potentially be exploited in therapeutic interventions in ASD. An anecdotic report already suggests in 1979 that the stress put on the melodic content of language has been helpful in the language therapy of an autistic boy [59]. The benefit of music therapy for verbal communication reported in ASD might also stem from the low frequency variations of auditory (music) stimuli carrying pertinent information potentially exploitable for social communication improvements [60], [61]. Also, it is important to consider that disruption of interactional synchrony is an important feature of ASD that has recently attracted some attention [62]. Since interpersonal synchrony is the dynamic and reciprocal adaptation of the temporal structure of behaviors between interactive partners [63], preservation of coherent activity at the pSTS suggests that a neural substrate is present in ASD for interactional synchrony based on auditory inputs during dyadic oral communication. This represents a neurophysiological support for early therapeutic interventions based on prosody, including those building on the typical prosodic characteristics of the so-called “parentese”, a special type of speech directed towards infants typically used by adults (higher pitch, slower tempo, and exaggerated intonation contours [62].
Limitations of the study
First, ASD subjects were compared with the typically developed healthy subjects investigated in the seminal CVC study [25] and were therefore not matched for age and sex. Still, as all ASD subjects (except for one subject in Recorded) obtained significant sensor-space coherence levels with values similar to those observed in the seminal CVC study, we consider that the conclusions of this study are not affected by this limitation.
Second, the use of the MNI template for the source space coherence analyses in ASD subjects limits the accuracy of the coherent source localization (as compared with the use of individual MRI) [64]. Still, in the absence of an individual MRI, using a template head model results in a fairly precise head model for source space modelling [65]. This is further supported by the fact that, even with this lower spatial resolution approach, the localization of the right STS maximum coherence was very close to the one described in the seminal CVC study. This finding highly suggests that similar brain areas are involved in the CVC phenomenon in ASD and typically developed subjects.
Finally, we did not test behaviourally the rhythmic prosody perception skills of our ASD subjects, which limits the interpretation of the functional relevance of the preserved CVC phenomenon in this population. Indeed, even if our result highly suggest a normal neural processing of the suprasegmental speech rhythmic prosody at the prelexical level, we cannot determine if this preserved coupling gives rise to normal prosody perception at the behavioural level. The ASD subjects included in this study had weak to clear expressive prosodic disorder. This highlights the importance to investigate in future studies the correlation between CVC levels and prosody perception skills.
Conclusions
This study discloses a preserved coupling between the reader's voice and the listener's cortical activity in ASD subjects at the right pSTS. These findings support the existence of preserved neural processing of sentence-level rhythmic prosody in ASD. The preservation of early cortical processing of prosodic elements in verbal language might be exploited in therapeutic interventions in ASD.
Acknowledgments
The authors would like to thank Professor Riitta Hari (Brain Research Unit, O.V. Lounasmaa Laboratory and MEG Core, Finland), Professor Véronique Delvenne (Centre de référence des troubles envahissants du développement et des troubles autistiques, HUDERF, Belgium), and Professor Paul Linkowski (Laboratoire de Recherches Psychiatriques, UNI – ULB Neuroscience Institute, Belgium) for their support and fruitful discussions.
Funding Statement
Catherine Clumeck (Research Fellow) and Xavier De Tiège (Postdoctorate Clinical Master Specialist) benefit of a research grant form the Fonds de la Recherche Scientifique (FRS-FNRS, Belgium). This study was supported by a research grant from the FRS-FNRS (research project: J.0021.13). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Baron-Cohen S, Belmonte MK (2005) Autism: a window onto the development of the social and the analytic brain. Annu Rev Neurosci 28: 109–126. [DOI] [PubMed] [Google Scholar]
- 2. Schultz RT (2005) Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci 23: 125–141. [DOI] [PubMed] [Google Scholar]
- 3. Schroeder JH, Desrocher M, Bebko JM, Cappadocia MC (2010) The neurobiology of autism: Theoretical implications. Research in Autism Spectrum Disorders 4: 555–564. [Google Scholar]
- 4. Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, et al. (2006) Autism, the superior temporal sulcus and social perception. Trends Neurosci 29: 359–366. [DOI] [PubMed] [Google Scholar]
- 5. Allison T, Puce A, McCarthy G (2000) Social perception from visual cues: role of the STS region. Trends Cogn Sci 4: 267–278. [DOI] [PubMed] [Google Scholar]
- 6. Redcay E (2008) The superior temporal sulcus performs a common function for social and speech perception: implications for the emergence of autism. Neurosci Biobehav Rev 32: 123–142. [DOI] [PubMed] [Google Scholar]
- 7. Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B (2000) Voice-selective areas in human auditory cortex. Nature 403: 309–312. [DOI] [PubMed] [Google Scholar]
- 8. Frith CD, Frith U (2006) The neural basis of mentalizing. Neuron 50: 531–534. [DOI] [PubMed] [Google Scholar]
- 9. Hari R, Kujala MV (2009) Brain basis of human social interaction: from concepts to brain imaging. Physiol Rev 89: 453–479. [DOI] [PubMed] [Google Scholar]
- 10. Kilner JM (2011) More than one pathway to action understanding. Trends Cogn Sci 15: 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rizzolatti G, Sinigaglia C (2010) The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci 11: 264–274. [DOI] [PubMed] [Google Scholar]
- 12. Happe FG (1994) An advanced test of theory of mind: understanding of story characters' thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord 24: 129–154. [DOI] [PubMed] [Google Scholar]
- 13. Kaland N, Callesen K, Moller-Nielsen A, Mortensen EL, Smith L (2008) Performance of children and adolescents with Asperger syndrome or high-functioning autism on advanced theory of mind tasks. J Autism Dev Disord 38: 1112–1123. [DOI] [PubMed] [Google Scholar]
- 14. Rizzolatti G, Fabbri-Destro M, Cattaneo L (2009) Mirror neurons and their clinical relevance. Nat Clin Pract Neurol 5: 24–34. [DOI] [PubMed] [Google Scholar]
- 15. Williams JH, Whiten A, Suddendorf T, Perrett DI (2001) Imitation, mirror neurons and autism. Neurosci Biobehav Rev 25: 287–295. [DOI] [PubMed] [Google Scholar]
- 16. Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, et al. (2004) Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage 23: 364–369. [DOI] [PubMed] [Google Scholar]
- 17. Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H (2006) Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex 16: 1276–1282. [DOI] [PubMed] [Google Scholar]
- 18. von dem Hagen EA, Nummenmaa L, Yu R, Engell AD, Ewbank MP, et al. (2011) Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cereb Cortex 21: 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, et al. (2000) Abnormal regional cerebral blood flow in childhood autism. Brain 123 (Pt 9) 1838–1844. [DOI] [PubMed] [Google Scholar]
- 20. Castelli F, Frith C, Happe F, Frith U (2002) Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 125: 1839–1849. [DOI] [PubMed] [Google Scholar]
- 21. Gervais H, Belin P, Boddaert N, Leboyer M, Coez A, et al. (2004) Abnormal cortical voice processing in autism. Nat Neurosci 7: 801–802. [DOI] [PubMed] [Google Scholar]
- 22. Wang AT, Lee SS, Sigman M, Dapretto M (2007) Reading affect in the face and voice: neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Arch Gen Psychiatry 64: 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hickok G, Poeppel D (2004) Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition 92: 67–99. [DOI] [PubMed] [Google Scholar]
- 24. Poeppel D, Idsardi WJ, van Wassenhove V (2008) Speech perception at the interface of neurobiology and linguistics. Philos Trans R Soc Lond B Biol Sci 363: 1071–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bourguignon M, De Tiège X, Op de Beeck M, Ligot N, Paquier P, et al. (2013) The pace of prosodic phrasing couples the listener's cortex to the reader's voice. Hum Brain Mapp 34: 314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilson M, Wilson TP (2005) An oscillator model of the timing of turn-taking. Psychon Bull Rev 12: 957–968. [DOI] [PubMed] [Google Scholar]
- 27. Rapin I, Dunn M (2003) Update on the language disorders of individuals on the autistic spectrum. Brain Dev 25: 166–172. [DOI] [PubMed] [Google Scholar]
- 28. O'Connor K (2012) Auditory processing in autism spectrum disorder: a review. Neurosci Biobehav Rev 36: 836–854. [DOI] [PubMed] [Google Scholar]
- 29. Jarvinen-Pasley A, Peppe S, King-Smith G, Heaton P (2008) The relationship between form and function level receptive prosodic abilities in autism. J Autism Dev Disord 38: 1328–1340. [DOI] [PubMed] [Google Scholar]
- 30. Groen WB, Zwiers MP, van der Gaag RJ, Buitelaar JK (2008) The phenotype and neural correlates of language in autism: an integrative review. Neurosci Biobehav Rev 32: 1416–1425. [DOI] [PubMed] [Google Scholar]
- 31. Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- 32.Wechsler D (2003) Wechsler Intelligence Scale for Children - Fourth Edition Administration and scoring manual. San Antonio, Texas: Harcourt Assessment, Inc. [Google Scholar]
- 33.Wechsler D (1997) Wechsler Adult Intelligence Scale - Third Edition. San Antonio, Texas: The Psychological Corporation. [Google Scholar]
- 34.American Psychiatric Association (1994) DSM-IV Diagnostic and Statistical Manual of Mental Disorders. Washington DC. [Google Scholar]
- 35. Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, et al. (2000) The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30: 205–223. [PubMed] [Google Scholar]
- 36.Maurin N (2006) Test de Langage Oral Complexe pour Collégiens (TLOCC). In: Ortho-Edition, editor editors. Isbergues.
- 37. Taulu S, Simola J, Kajola M (2005) Applications of the Signal Space Separation Method. IEEE Trans Sign Proc 53: 3359–3372. [Google Scholar]
- 38. Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, et al. (1995) A framework for the analysis of mixed time series/point process data–theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64: 237–278. [DOI] [PubMed] [Google Scholar]
- 39. Gross J, Kujala J, Hämäläinen M, Timmermann L, Schnitzler A, et al. (2001) Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci U S A 98: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Faes L, Pinna GD, Porta A, Maestri R, Nollo G (2004) Surrogate data analysis for assessing the significance of the coherence function. IEEE Trans Biomed Eng 51: 1156–1166. [DOI] [PubMed] [Google Scholar]
- 41. Bourguignon M, Jousmäki V, Op de Beeck M, Van Bogaert P, Goldman S, et al. (2012) Neuronal network coherent with hand kinematics during fast repetitive hand movements. Neuroimage 59: 1684–1691. [DOI] [PubMed] [Google Scholar]
- 42. Piitulainen H, Bourguignon M, De Tiège X, Hari R, Jousmäki V (2013) Coherence between magnetoencephalography and hand-action-related acceleration, force, pressure, and electromyogram. Neuroimage 72: 83–90. [DOI] [PubMed] [Google Scholar]
- 43. Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bourguignon M, De Tiège X, Op de Beeck M, Van Bogaert P, Goldman S, et al. (2012) Primary motor cortex and cerebellum are coupled with the kinematics of observed hand movements. Neuroimage 66C: 500–507. [DOI] [PubMed] [Google Scholar]
- 45. Bertone A, Mottron L, Jelenic P, Faubert J (2005) Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain 128: 2430–2441. [DOI] [PubMed] [Google Scholar]
- 46. Samson F, Mottron L, Jemel B, Belin P, Ciocca V (2006) Can spectro-temporal complexity explain the autistic pattern of performance on auditory tasks? J Autism Dev Disord 36: 65–76. [DOI] [PubMed] [Google Scholar]
- 47. Mottron L, Dawson M, Soulieres I, Hubert B, Burack J (2006) Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord 36: 27–43. [DOI] [PubMed] [Google Scholar]
- 48. Bonnel A, Mottron L, Peretz I, Trudel M, Gallun E, et al. (2003) Enhanced pitch sensitivity in individuals with autism: a signal detection analysis. J Cogn Neurosci 15: 226–235. [DOI] [PubMed] [Google Scholar]
- 49. Brandwein AB, Foxe JJ, Butler JS, Russo NN, Altschuler TS, et al. (2013) The development of multisensory integration in high-functioning autism: high-density electrical mapping and psychophysical measures reveal impairments in the processing of audiovisual inputs. Cereb Cortex 23: 1329–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Collignon O, Charbonneau G, Peters F, Nassim M, Lassonde M, et al. (2013) Reduced multisensory facilitation in persons with autism. Cortex 49: 1704–1710. [DOI] [PubMed] [Google Scholar]
- 51. Golan O, Baron-Cohen S, Golan Y (2008) The ‘Reading the Mind in Films’ Task [child version]: complex emotion and mental state recognition in children with and without autism spectrum conditions. J Autism Dev Disord 38: 1534–1541. [DOI] [PubMed] [Google Scholar]
- 52. Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, et al. (2004) Autism and abnormal development of brain connectivity. J Neurosci 24: 9228–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gepner B, Feron F (2009) Autism: a world changing too fast for a mis-wired brain? Neurosci Biobehav Rev 33: 1227–1242. [DOI] [PubMed] [Google Scholar]
- 54. Peelle JE, Gross J, Davis MH (2013) Phase-locked responses to speech in human auditory cortex are enhanced during comprehension. Cereb Cortex 23: 1378–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hesling I, Dilharreguy B, Peppe S, Amirault M, Bouvard M, et al. (2010) The integration of prosodic speech in high functioning autism: a preliminary FMRI study. PLoS One 5: e11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gusnard DA, Raichle ME (2001) Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- 57. Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, et al. (2012) The role of default network deactivation in cognition and disease. Trends Cogn Sci 16: 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nishitani N, Avikainen S, Hari R (2004) Abnormal imitation-related cortical activation sequences in Asperger's syndrome. Ann Neurol 55: 558–562. [DOI] [PubMed] [Google Scholar]
- 59. Miller SB, Toca JM (1979) Adapted melodic intonation therapy: a case study of an experimental language program for an autistic child. J Clin Psychiatry 40: 201–203. [PubMed] [Google Scholar]
- 60. Lim HA, Draper E (2011) The effects of music therapy incorporated with applied behavior analysis verbal behavior approach for children with autism spectrum disorders. J Music Ther 48: 532–550. [DOI] [PubMed] [Google Scholar]
- 61. Lim HA (2010) Effect of “developmental speech and language training through music” on speech production in children with autism spectrum disorders. J Music Ther 47: 2–26. [DOI] [PubMed] [Google Scholar]
- 62. Cohen D, Cassel RS, Saint-Georges C, Mahdhaoui A, Laznik MC, et al. (2013) Do parentese prosody and fathers' involvement in interacting facilitate social interaction in infants who later develop autism? PLoS One 8: e61402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Delaherche E, Chetouani M, Mahdhaoui A, Saint-Georges C, Viaux S, et al. (2012) Interpersonal Synchrony: A Survey of Evaluation Methods across Disciplines. IEEE Trans Affect Comput 3: 349–365. [Google Scholar]
- 64. Henson RN, Mattout J, Phillips C, Friston KJ (2009) Selecting forward models for MEG source-reconstruction using model-evidence. Neuroimage 46: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Litvak V, Mattout J, Kiebel S, Phillips C, Henson R, et al. (2011) EEG and MEG data analysis in SPM8. Comput Intell Neurosci 2011: 852961. [DOI] [PMC free article] [PubMed] [Google Scholar]