Abstract
Maputaland–Pondoland–Albany, South Africa has been identified as a biodiversity hotspot and centre for endemism. Odonata make good indicators of freshwater ecosystem health. Consequently we compiled a list of Odonata species recorded to date in the iSimangaliso Wetland Park. We then detailed important species in terms of endemism, conservation status, and potential as indicator species. Finally, we compared Odonata assemblages of different sites sampled within the park to illustrate habitat importance. Species identified during two formal surveys and incidental observations made during the study period were combined with an existing database to compile an accurate and up to date species list for the iSimangaliso Wetland Park. Data from this study were then analyzed to determine which water bodies had the most similar species composition. The Dragonfly Biotic Index (DBI) value of each study area was also determined. We recorded 68 odonate species in the iSimangaliso Wetland Park, adding 13 species to the Ezemvelo KwaZulu-Natal Wildlife database for the area. This brings the total number of Odonata species for the iSimangaliso Wetland Park to 86. Eight species are red-listed, 12 are restricted in South Africa to the coastal plains of northern KwaZulu-Natal, and the remainder occurs widely across the southern African savanna. Analyses indicate that species odonate assemblages were most similar in water bodies with comparable habitats. iSimangaliso Wetland Park is identified as an important area for Odonata diversity and endemism, a trend also reflected by the DBI values. Shifts in the existing species assemblages would indicate changes within the ecosystem and thus this species account provides necessary baseline data for the area. Species Conservation efforts should thus target water bodies of varying habitat types to protect greater species diversity.
Introduction
Freshwater ecosystems contain 10% of current recorded species and comprise only 1% of Earth's surface [1]. They are considered one of the most jeopardized ecosystems [2] and their importance as a resource in undeniable. To better monitor the state and health of these ecosystems, indicator species are often used. Odonata (dragonflies) make particularly good indicators of freshwater ecosystem health as they are visible above water, but rely on the quality of the water and surrounding habitat to persist [3], [4]. Among insects Odonata have comparatively long life cycles and as a group are well defined and studied [3]–[5]. They have an aquatic larval stage that can last up to one year and a terrestrial adult phase, with males holding favourable territories in many species [6]. Consequently, they serve as indicators for changes in both water quality and surrounding vegetation [7], [8]. Their value as flagship species for freshwater conservation is further highlighted by their important role within freshwater ecosystem species assemblages and their presence on all continents, with the exception of Antarctica [3], [4]. Odonata assemblages can also be used as surrogates to determine aquatic areas for conservation prioritization [9].
iSimangaliso Wetland Park (iWP), South Africa, is known for rich diversity and unique habitats and is therefore a Ramsar wetland of global significance and a UNESCO World Heritage Site. It is located within Maputaland at a significant intersection, with the coastal lowlands bordered by the ocean to the east and an inland plateau to the west [10]. Maputaland's position lends itself to colonization by tropical biota from the north and sub-tropical and temperate biota from both the south and high altitude west [10]. Being a transition zone between these environments has resulted in great biodiversity [10]. Maputaland's conservation value as a centre of endemism is internationally recognised [11]. Today it is accepted that predominantly tropical species are found in the area, largely due to warm ocean currents flowing south from Mozambique, presenting a rich and diverse ecosystem at relatively high latitude [10]. It is unique as it is made up of several habitat types including estuaries, coastal/marine habitats, freshwater lakes and rivers, wetlands, dune and coastal and swamp forests, and mangroves. Many of the vegetation units are vulnerable or endangered outside of the protected iWP, where agricultural practices and invasive alien plants pose the biggest threats [12]. Africa's largest estuarine system, Lake St. Lucia, and southern Africa's largest natural freshwater lake, Lake Sibaya, are both found within iWP [13], [14].
Within South Africa, Maputaland–Pondoland–Albany (MPA) has been identified as a hotspot with the greatest Odonata richness, particularly for red-listed species [9]. The iWP's diverse odonate fauna is due to the subtropical climatic conditions with relatively high rainfall, and variable landscapes and wetland types within the park. Odonata assemblages are associated with different habitat types [15]. Consequently, increased habitat heterogeneity can lead to increased Odonata diversity at a particular site [16], [17]. Of South Africa's 162 taxa, one quarter are Red Listed [9]. The greatest threats to Odonata are those that alter the natural landscape [17]. These include: invasive tree species which cause excessive shading, urbanization, pollution, damming, mining, and introduced fish species [9], [17]–[19] to name a few.
Disturbance to these habitats can result in a reduction of odonate species [7]. Odonata assemblages should therefore be monitored to recognize what effect human actions have on water quality [20]. Therefore species lists for wetland areas are important as these will serve as baseline data and may indicate changes within the ecosystem. Furthermore, information on hotspots within a reserve can serve as focal points for management to direct cost-effective conservation strategies [21]. Finally, it is important that all habitat types be surveyed within an area as these can yield different species assemblages.
As the MPA is a biodiversity hotspot and centre for endemism, and Odonata are indicators of freshwater ecosystem health, the aim of this study was to determine the Odonata diversity of the iWP. In addition we compared the odonate species composition at different sites to illustrate habitat importance. From this odonate data we detailed important species in terms of endemism, conservation status, and potential as indicator species. It was predicted that odonate species assemblages would differ at sites that varied in habitat type, and so affect conservation management strategies.
Materials and Methods
Study area
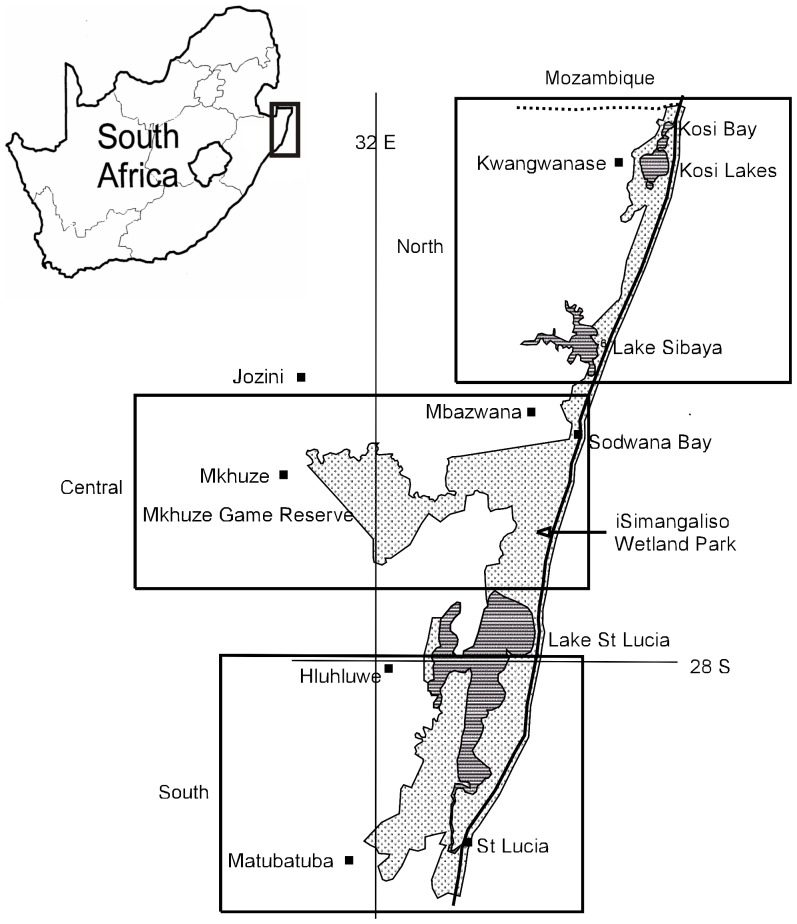
The iWP (26°51′S–28°26′S; 32°09′E–32°53′E) extends along the coastal plain of north-eastern KwaZulu-Natal (KZN) Province of South Africa and covers an area of 332000 ha. It stretches from Maphelana in the south to the Mozambique border, north of Kosi Bay, in the north and extends inland for approximately 50 km to include Mkhuze Game Reserve (Fig 1). In general most of the park is less than 40 m above sea-level, with the exception of Mkhuze Game Reserve (c. 60–100 m). Rainfall varies greatly, with the coastal area receiving 1000–1100 mm annually and decreasing to 600 mm in the west at the foot of the mountain range [22].
Figure 1. Map of study area.
Grey shaded areas indicate the iSimangaliso Wetland Park.
We had permission from the local conservation authority for the odonate species surveys. The iWP was divided into three sections; north (Fig 2), central (Fig 3) and south (Fig 4). Within these sections a total of fifty sites were identified and numbered accordingly (Table 1; Fig. 2, 3, 4). For brevity, site names were abbreviated from Kosi Bay to Kosi, Mkhuze Game Reserve to Mkhuze, etc. Most sites were photographed and a description of each site was provided (Table 1). GPS co-ordinates, air temperature, and the total dissolved solids (TDS) of the water were also recorded for each site (Table 1). TDS was measured using Milwaukee Instruments CD97 Total Dissolved Salts (TDS) meter.
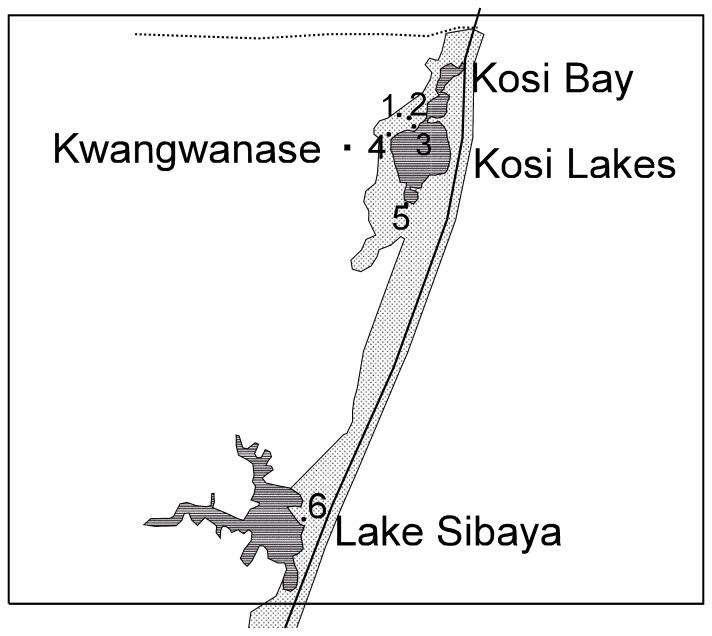
Figure 2. Map of the northern section of the iSimangaliso Wetland Park.
The numbers 1–6 represent the positions of sample sites described in Table 1.
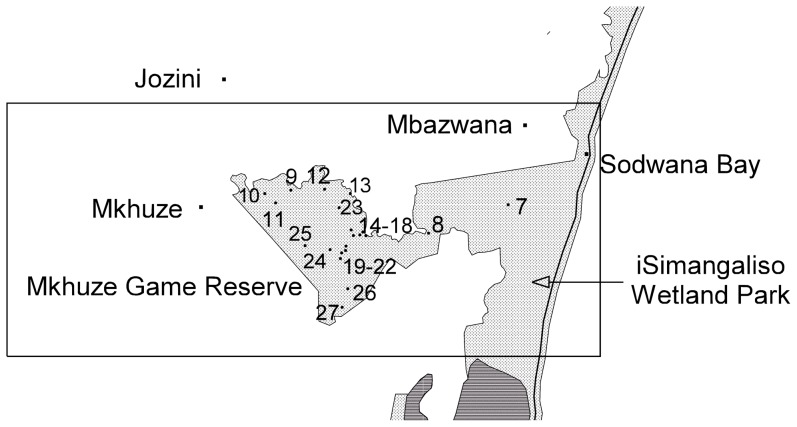
Figure 3. Map of the central section of the iSimangaliso Wetland Park.
The numbers 7–27 represent the positions of sample sites described in Table 1.
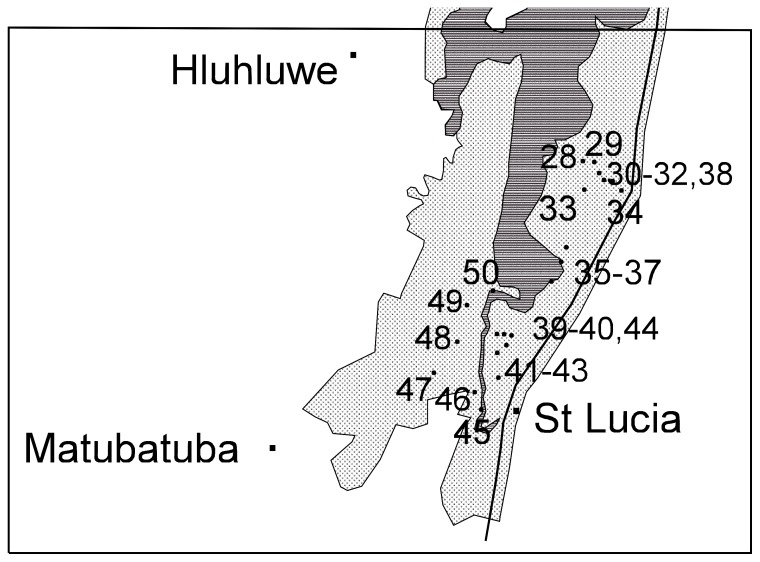
Figure 4. Map of the southern section of the iSimangaliso Wetland Park.
The numbers 28–50 represent the positions of sample sites described in Table 1.
Table 1. Description of study sites in each area surveyed.
| Area | Site No. | Site name | Site Description | Latitude | Longitude | Temp. (°C) | Humidity (%) | TDS (ppm) | No. of Species |
| Kosi | 1 | Kosi | 10 ha rain-filled grassy pan in grassland. | 26.952050 | 32.802417 | 35.3 | 41 | 56 | 12 |
| Kosi | 2 | Kosi | Forested stream. | 26.957267 | 32.829883 | 104 | 14 | ||
| Kosi | 3 | Kosi campsite & jetty | Edge of lake. | 26.960200 | 32.826967 | 650 | 7 | ||
| Kosi | 4 | Kosi | Swamp forest stream. | 26.955033 | 32.828833 | 31.2 | 40 | 101 | 4 |
| Kosi | 5 | Inlet to 4th Lake | Narrow inlet, Nymphaea. | 27.040300 | 32.818917 | 97 | 20 | ||
| Sibaya | 6 | Sibaya | Eastern shore | 27.395650 | 32.711633 | 30.5 | 58 | 95 | 7 |
| Ozabeni | 7 | Samango Crossing | Flowing stream in forest | 27.617467 | 32.549183 | 12 | |||
| Ozabeni | 8 | Neshe Pan | Open water, Nymphaea. | 27.654700 | 32.402650 | 28.1 | 58 | 221 | 14 |
| Mkhuze | 9 | Nhlonhlela Bush Camp | Floodplain below a nearly dry reedbed | 27.597450 | 32.198167 | 97 | 3 | ||
| Mkhuze | 10 | Rhino wallow | Small 50×10 m, shallow, seasonally rain-filled wallow with emergent grass. Disturbed by game. | 27.607817 | 32.167400 | 210 | 4 | ||
| Mkhuze | 11 | Rhino wallow | Small 50×10 m, shallow, seasonally rain-filled wallow with emergent grass. Disturbed by game. | 27.621733 | 32.185733 | 120 | 2 | ||
| Mkhuze | 12 | Mbonene Pan | Small, rain-filled, grass edge. | 27.632817 | 32.262767 | 30 | 54 | 53 | 7 |
| Mkhuze | 13 | Ophansi bridge | Mkuze river bridge, fast-flowing, mud-laden River 20 m wide, fringed by degraded fig forest. | 27.598750 | 32.302083 | 24/25.9* | 75* | 297/255* | 5/10* |
| Mkhuze | 14 | Nsumo Pan | Bridge at first inlet 3 m wide open water channel fringed by flooded grass. | 27.656600 | 32.301417 | 21/32* | 49* | 405/175* | 3/12* |
| Mkhuze | 15 | Nsumo Pan | West hide open water, with reeds along edge | 27.665117 | 32.302150 | 33.5 | 51 | 139 | 7/2* |
| Mkhuze | 16 | Nsumo Pan Picnic site | Open water, with reeds along edge. | 27.668750 | 32.305400 | 324 | 5/5* | ||
| Mkhuze | 17 | Fig Forest | First bridge 10 m wide open water channel fringed by flooded grass and thick bush. | 27.668783 | 32.316717 | 21/13* | |||
| Mkhuze | 18 | Fig Forest | Second bridge strongly flowing, mud-laden river fringed by tall fig forest and shrubby understory. | 27.669517 | 32.322850 | 340/231* | 8/9* | ||
| Mkhuze | 19 | near Nxwala Camp | Shaded, stagnant residual pond on seasonal drainage line in dense bush. | 27.703150 | 32.284333 | 32.1/24* | 48 | 96/144* | 11/5* |
| Mkhuze | 20 | Nsumo Nxwala side | Lily-covered channel backfill from Nsumu Pan. Nymphaea covered water below fever trees. | 27.692050 | 32.291050 | 155 | 12/7* | ||
| Mkhuze | 21 | Nsumo Nxwala side | Nsumo western inlet, flooded grass below fever trees | 27.686550 | 32.292433 | 363 | 8 | ||
| Mkhuze | 22 | Nsumo Nxwala side | Nsumo western inlet, flooded grass below fever trees | 27.689783 | 32.293600 | 23 | 7/12* | ||
| Mkhuze | 23 | Ediza | Inlet, dense flooded grass | 27.606617 | 32.288250 | 31.8 | 44 | 4 | |
| Mkhuze | 24 | Rhino wallow | Small 50×10 m, shallow, seasonally rain-filled wallow with emergent grass. Disturbed by game. | 27.692517 | 32.278633 | 91 | 7 | ||
| Mkhuze | 25 | Mkhuze | Rain-filled quarry, open water with flooded grass edges. | 27.685833 | 32.238167 | 27 | 4 | ||
| Mkhuze | 26 | Noshoshela dam | Dam of 2 ha, open water fringed by fever trees. | 27.756983 | 32.297300 | 32.3 | 43 | 98/140* | 10/22* |
| Mkhuze | 27 | uMkhumbe dam | Dam of 1 ha, open water fringed by extensive flooded grass with narrow channel of flowing water below the dam. | 27.775117 | 32.297800 | 28 | 169/98* | 18/13* | |
| E Shores | 28 | E Shores | Grassy pan. | 28.118083 | 32.506783 | 29.7 | 57 | 225 | 11 |
| E Shores | 29 | E Shores | Small pan. | 28.118417 | 32.515467 | 28.2 | 58 | 3 | |
| E Shores | 30 | E Shores | Forested causeway and Mfabeni Swamp. | 28.131117 | 32.527167 | 34.8 | 47 | 345 | 8 |
| E Shores | 31 | E Shores | Forested causeway and Mfabeni Swamp. | 28.137000 | 32.534683 | 32.8 | 61 | 280 | 10 |
| E Shores | 32 | E Shores | Lake Bangazi road. | 28.141367 | 32.541100 | 24 | 68 | 6 | |
| E Shores | 33 | E Shores | Barbet Pan. | 28.194633 | 32.489200 | 28 | 60 | 225 | 8 |
| E Shores | 34 | Cape Vidal house | Garden and road. | 28.146333 | 32.547933 | 32 | 55 | 3 | |
| E Shores | 35 | E Shores | Forested stream and old excavations. | 28.205900 | 32.490700 | 12 | |||
| E Shores | 36 | Catalina jetty | Freshwater edge on Lake St Lucia. | 28.220650 | 32.487283 | 32.3 | 58 | 174 | 8 |
| E Shores | 37 | E Shores | Freshwater seep on Lake St Lucia edge. | 28.238867 | 32.486950 | Seep 63, Lake 974 | 20 | ||
| E Shores | 38 | E Shores | Forested causeway and Mfabeni Swamp. | 28.137550 | 32.538567 | 7 | |||
| E Shores | 39 | E Shores | Dense flooded sedge beds. | 28.296617 | 32.434383 | 10 | |||
| E Shores | 40 | E Shores | Freshwater seep on swamp-forest edge. | 28.297150 | 32.440900 | 27 | 59 | 85 | 13 |
| E Shores | 41 | E Shores | Two rhino, open grassy pans. | 28.316500 | 32.433717 | 26.9 | 67 | 85 | 13 |
| E Shores | 42 | E Shores | Papyrus choked pond. | 28.318550 | 32.427050 | 31.8 | 64 | 129 | 3 |
| E Shores | 43 | E Shores | Two grassy pans divided by causeway. | 28.318583 | 32.430400 | 32.5 | 61 | 88 | 10 |
| E Shores | 44 | E Shores | Warthog grassy pan. | 28.268267 | 32.466783 | 33.9 | 53 | 110 | 3 |
| W Shores | 45 | W Shores | St Lucia estuary bridge, reedbed. | 28.369783 | 32.409667 | 31 | 63 | 7 | |
| W Shores | 46 | Ndonyena | Small lily pond. | 28.352850 | 32.385350 | 31.6 | 57 | 101 | 3 |
| W Shores | 47 | Mpati Weir | Flowing stream under forest canopy | 28.331200 | 32.361367 | 32.4 | 54 | 97 | 5 |
| W Shores | 48 | Mpati River | Stream low, not flowing, choked with Phragmites | 28.298467 | 32.383600 | 34.4 | 51 | 536 | 10 |
| W Shores | 49 | W Shores | Hippo pan, open grassy. | 28.255017 | 32.393750 | 34.1 | 50 | 108 | 8 |
| W Shores | 50 | Makakatana Bay | Saline open water, bare edges. | 28.248900 | 32.419617 | 33.5 | 46 | 940 | 4 |
Site numbers refer to site positions marked on Fig 1. ‘*’ indicates a second reading or count for the same site.
Odonata identification and analyses
A checklist for possible species in the area was compiled using the database compiled by Ezemvelo KwaZulu-Natal Wildlife (EKZNW), which listed 486 records of 70 odonate species for the iWP (Table 2). Nearly 52% of these records were collected from 1997–2001 and are accredited to Samways and the University of KwaZulu-Natal. Records and a dragonfly collection for St. Lucia and elsewhere in the iWP held at the National Museum in Pretoria (Ditsong) from Balinsky [23], [24] were also consulted. Finally, three species were added from literature searches.
Table 2. Species presence in each region and the number of sites at which they were recorded.
| Family | Species | Common name | Samango Crossing | Mkhuze | Neshe Pan | E & W Shores | Lake Sibaya | Kosi Bay | EKZNW database | Other records | No. of our sites |
| Calopterygidae | Phaon iridipennis | Glistening Demoiselle | yes | yes | yes | 4 | |||||
| Chlorocyphidae | Platycypha caligata | Dancing Jewel | yes | yes | yes | yes | 6 | ||||
| Coenagrionidae | Aciagrion dondoense | Opal Slim | yes | ||||||||
| Coenagrionidae | Africallagma glaucum | Swamp Bluet | yes | yes | |||||||
| Coenagrionidae | Agriocnemis exilis | Little Whisp | yes | yes | yes | 3 | |||||
| Coenagrionidae | Agriocnemis falcifera | White-masked Whisp | yes | yes | |||||||
| Coenagrionidae | Agriocnemis gratiosa | Gracious Whisp | yes | ||||||||
| Coenagrionidae | Agriocnemis ruberrima | Orange Whisp | yes | yes | yes | 2 | |||||
| Coenagrionidae | Azuragrion nigridorsum | Black-tailed Bluet | yes | yes | yes | yes | 5 | ||||
| Coenagrionidae | Ceriagrion glabrum | Common Citril | yes | yes | yes | yes | yes | 36 | |||
| Coenagrionidae | Ischnura senegalensis | African Bluetail | yes | yes | yes | yes | yes | 16 | |||
| Coenagrionidae | Pseudagrion acaciae | Acacia Sprite | yes | yes | 1 | ||||||
| Coenagrionidae | Pseudagrion coeleste | Umsingazi Sprite | yes | yes | yes | 9 | |||||
| Coenagrionidae | Pseudagrion commoniae | Black Sprite | yes | yes | 3 | ||||||
| Coenagrionidae | Pseudagrion hageni | Hagen's Sprite | yes | yes | yes | yes | 5 | ||||
| Coenagrionidae | Pseudagrion hamoni | Hamon's Sprite | yes | yes | yes | 8 | |||||
| Coenagrionidae | Pseudagrion kersteni | Kersten's Sprite | yes | yes | 3 | ||||||
| Coenagrionidae | Pseudagrion massaicum | Masai Sprite | yes | yes | yes | yes | 17 | ||||
| Coenagrionidae | Pseudagrion sublacteum | Cherry-eye Sprite | yes | yes | 4 | ||||||
| Platycnemididae | Elattoneura glauca | Common Threadtail | yes | yes | yes | 3 | |||||
| Lestidae | Lestes pallidus | Pallid Spreadwing | yes | 4 | |||||||
| Lestidae | Lestes tridens | Spotted Spreadwing | yes | yes | yes | 2 | |||||
| Lestidae | Lestes uncifer | Sickle Spreadwing | yes | ||||||||
| Gomphidae | Ictinogomphus ferox | Common Tigertail | yes | yes | yes | yes | yes | yes | 14 | ||
| Gomphidae | Paragomphus cognatus | Rock Hooktail | yes | yes | 2 | ||||||
| Gomphidae | Paragomphus genei | Green Hooktail | yes | yes | yes | yes | 3 | ||||
| Aeshnidae | Zosteraeschna minuscula | Friendly Hawker | yes | ||||||||
| Aeshnidae | Anaciaeschna triangulifera | Evening Hawker | yes | yes | 1 | ||||||
| Aeshnidae | Anax ephippiger | Vagrant Emperor | yes | yes | yes | yes | 9 | ||||
| Aeshnidae | Anax imperator | Blue Emperor | yes | yes | yes | yes | yes | 10 | |||
| Aeshnidae | Anax speratus | Orange Emperor | yes | 1 | |||||||
| Aeshnidae | Anax tristis | Black Emperor | yes | ||||||||
| Aeshnidae | Gynacantha manderica | Little Dusk-Hawker | yes | 2 | |||||||
| Aeshnidae | Gynacantha usambarica | Usambara Dusk-Hawker | yes | yes | 2 | ||||||
| Aeshnidae | Gynacantha villosa | Hairy Dusk-Hawker | yes | ||||||||
| Corduliidae | Hemicordulia africana | African Emerald | yes | yes | 1 | ||||||
| Corduliidae | Phyllomacromia contumax | Two-banded Cruiser | yes | yes | yes | yes | 3 | ||||
| Corduliidae | Phyllomacromia picta | Darting Cruiser | yes | 2 | |||||||
| Libellulidae | Acisoma panorpoides | Pintail | yes | yes | yes | yes | 10 | ||||
| Libellulidae | Aethriamanta rezia | Pygmy Basker | yes | yes | yes | 2 | |||||
| Libellulidae | Brachythemis leucosticta | Banded Groundling | yes | yes | yes | yes | yes | 34 | |||
| Libellulidae | Bradinopyga cornuta | Don-Dwala | yes | 1 | |||||||
| Libellulidae | Chalcostephia flavifrons | Inspector | yes | yes | yes | yes | 8 | ||||
| Libellulidae | Crocothemis erythraea | Broad Scarlet | yes | yes | yes | yes | yes | 34 | |||
| Libellulidae | Crocothemis sanguinolenta | Little Scarlet | yes | ||||||||
| Libellulidae | Diplacodes lefebvrii | Black Percher | yes | yes | yes | yes | 19 | ||||
| Libellulidae | Diplacodes luminans | Barbet | yes | yes | yes | yes | yes | 14 | |||
| Libellulidae | Diplacodes pumila | Dwarf Percher | yes | yes | 1 | ||||||
| Libellulidae | Hemistigma al bipunctum | Pied-Spot | yes | yes | yes | yes | 23 | ||||
| Libellulidae | Macrodiplax cora | Cora's Pennant | yes | yes | |||||||
| Libellulidae | Nesciothemis farinosa | Black-tailed Skimmer | yes | yes | yes | yes | yes | yes | yes | 20 | |
| Libellulidae | Notiothemis jonesi | Forest-Watcher | yes | 1 | |||||||
| Libellulidae | Orthetrum abbotti | Abbott's Skimmer | yes | yes | 2 | ||||||
| Libellulidae | Orthetrum chrysostigma | Epaulet Skimmer | yes | ||||||||
| Libellulidae | Orthetrum hintzi | Hintz's Skimmer | yes | yes | yes | 3 | |||||
| Libellulidae | Orthetrum icteromelas | Spectacled Skimmer | yes | yes | yes | 4 | |||||
| Libellulidae | Orthetrum julia | Julia Skimmer | yes | yes | yes | yes | yes | 15 | |||
| Libellulidae | Orthetrum machadoi | Machado's Skimmer | yes | ||||||||
| Libellulidae | Orthetrum robustum | Robust Skimmer | yes | yes | yes | 4 | |||||
| Libellulidae | Orthetrum stemmale | Strong Skimmer | yes | yes | yes | 7 | |||||
| Libellulidae | Orthetrum trinacria | Long Skimmer | yes | yes | yes | yes | yes | yes | 17 | ||
| Libellulidae | Palpopleura jucunda | Yellow-veined Widow | yes | 1 | |||||||
| Libellulidae | Palpopleura lucia | Lucia Widow | yes | yes | yes | yes | 16 | ||||
| Libellulidae | Palpopleura portia | Portia Widow | yes | 2 | |||||||
| Libellulidae | Pantala flavescens | Pantala | yes | yes | yes | yes | yes | 27 | |||
| Libellulidae | Parazyxomma flavicans | Banded Dusk-Darter | yes | 2 | |||||||
| Libellulidae | Rhyothemis semihyalina | Phantom Flutterer | yes | yes | yes | yes | yes | 20 | |||
| Libellulidae | Sympetrum fonscolombii | Nomad | yes | ||||||||
| Libellulidae | Tetrathemis polleni | Black-Splash | yes | yes | yes | yes | 9 | ||||
| Libellulidae | Tholymis tillarga | Twister | yes | yes | 2 | ||||||
| Libellulidae | Tramea basilaris | Keyhole Glider | yes | yes | yes | yes | yes | 28 | |||
| Libellulidae | Tramea limbata | Ferruginous Glider | yes | yes | 2 | ||||||
| Libellulidae | Trithemis aconita | Monkshood Dropwing | yes | 1 | |||||||
| Libellulidae | Trithemis annulata | Violet Dropwing | yes | yes | yes | yes | yes | 15 | |||
| Libellulidae | Trithemis arteriosa | Red-veined Dropwing | yes | yes | yes | yes | yes | 16 | |||
| Libellulidae | Trithemis dorsalis | Dorsal Dropwing | yes | ||||||||
| Libellulidae | Trithemis furva | Navy Dropwing | yes | yes | 1 | ||||||
| Libellulidae | Trithemis hecate | Hecate Dropwing | yes | yes | 1 | ||||||
| Libellulidae | Trithemis kirbyi | Kirby's Dropwing | yes | yes | 3 | ||||||
| Libellulidae | Trithemis pluvialis | River Dropwing | yes | ||||||||
| Libellulidae | Trithemis stictica | Jaunty Dropwing | yes | yes | yes | 2 | |||||
| Libellulidae | Urothemis assignata | Red Basker | yes | yes | yes | yes | yes | 14 | |||
| Libellulidae | Urothemis edwardsii | Blue Basker | yes | yes | yes | yes | yes | 9 | |||
| Libellulidae | Urothemis luciana | St Lucia Basker | yes | ||||||||
| Libellulidae | Zygonyx torridus | Ringed Cascader | yes | ||||||||
| Libellulidae | Zyxomma atlanticum | Little Dusk-Darter | yes | yes | 1 | ||||||
| Total species recorded | 12 | 43 | 14 | 39 | 7 | 49 | 70 | 8 | - | ||
| Total number counted | 86 | 799 | 150 | 1437 | 16 | 288 | - | - | - | ||
| Dragonfly Biotic Index/Site | 1.67 | 2.05 | 1.57 | 2.38 | 1.86 | 2.59 | - | - | - | ||
| Dragonfly Biotic Index | 20 | 88 | 22 | 93 | 13 | 127 | |||||
Previous records from other sources are also presented.
Species names are based on Samways [27], where full names including authors are given.
At each site odonate species were identified and counted. Identification of species was predominantly done using close-focusing binoculars. In many instances at least one individual of each species was caught and examined using a hand-lens to confirm identification and subsequently released. In addition most species were also photographed to provide a permanent record of identification and occurrence. In addition to formal surveys incidental observations were also recorded. Odonata were surveyed in Mkhuze Game Reserve and surrounds for six days in December 2009, and Eastern and Western Shores of Lake St. Lucia, Mkhuze Game Reserve, Lake Sibaya and Kosi Bay were covered over a 10 day period in February 2011. Identifications were made using the two field-guides of Tarboton and Tarboton [25], [26] and Samways [27], and from literature extracts accumulated by Tarboton.
A map of the study sites was created using ESRI ArcView GIS version 3.1. A detailed species list was compiled for iWP. Using this list, the Dragonfly Biotic Index (DBI) for each study area was determined. The DBI assigns a value ranging from 0–9 to each odonate species in South Africa [27]. This value incorporates the geographical distribution, conservation status and sensitivity to habitat change of a species, where a species scoring ‘0’ would be widespread, common and tolerant to human disturbance [27], [28]. To determine the DBI/site, the total DBI for each study area was divided by the number of species recorded at each of these and thus yielded a DBI/site value between 0–9 for each area [29], [30]. To test which study areas were most similar in species composition Non-metric Multidimensional Scaling (NMDS) was run with a Jaccard similarity coefficient (Primer E, ver. 6, UK). For Mkhuze and Kosi Bay, where there was more than one sampling trip, species composition was totalled.
Results
In total 68 species and 3734 individual Odonata were recorded at the study sites. The summation of these data provides evidence for 86 species of odonates occurring in the iWP. From the compiled checklist, two species that are recorded in the EKZNW database were rejected based on our observations. Surveys from this study provide an additional 13 species to the iWP checklist. Based on results from this study, the EKZNW database, and published records [23], [24], [27], [31]–[33] an annotated checklist for the iWP has been compiled and the DBI for each study area calculated (Table 2). An indication of relative abundance and known occurrence of each species in the iWP is provided in Appendix S1. Family and species nomenclature are revised to the currently accepted position as listed in Samways [27]. Species showed a range of DBI scores, ranging from 0–8. Based on the checklist of 86 species for the iWP the total possible DBI/site is 2.80 (Total DBI = 241; Table 2). In this study 68 species were observed with a total DBI/site of 2.57 (Total DBI = 175; Table 2). When considering the six study areas, the highest DBI/site of 2.59 was at Kosi Bay, while the lowest value of 1.57 was at Neshe Pan (Table 2).
Of the species identified, eight appear in the National Red List of South African Odonata [33], namely: Aciagrion dondoense, Agriocnemis gratiosa, Agriocnemis ruberrima subspecies ruberrima, Pseudagrion coeleste subspecies umsingaziense, Gynacantha villosa, Diplacodes pumila and Urothemis luciana. These species ranges extend north, into Mozambique, with some widely ranging into tropical Africa. Within South Africa, 12 of the identified Odonata have restricted distributions in the coastal plains of northern KwaZulu-Natal. The remaining species occur broadly across the southern African savanna. The 10 most abundant species from this study are largely similar to those in the EKZNW database. These include: Brachythemis leucosticta 920/24/25 (our count/records in EKZNW database/sites present); Hemistigma albipunctum 280/19/21; Pantala flavescens 276/8/26; Crocothemis erythraea 204/12/28; Ceriagrion glabrum 190/25/31; Diplacodes luminans 147/4/12; Tramea basilaris 143/12/27; Diplacodes lefebvrii 114/22/18; Palpopleura lucia 126/20/12; and Ischnura senegalensis 107/16/16. Ceriagrion glabrum, Crocothemis erythraea and Tramea basilaris were present at the most sites surveyed. Two doubtful species we suggest be removed from the checklist are Phyllogomphus brunneus and Ceriagrion suave. Reasons for this are discussed in Appendix S1.
The NMDS plot illustrating the similarity in Odonata species composition between sites, showed that Kosi Bay, Eastern and Western Shores, and Mkhuze were most similar (Fig. 5). Samango Crossing, Neshe Pan and Lake Sibaya were least similar in composition to any of the other study sites (Fig. 5).
Figure 5. Study areas' similarity in species composition.
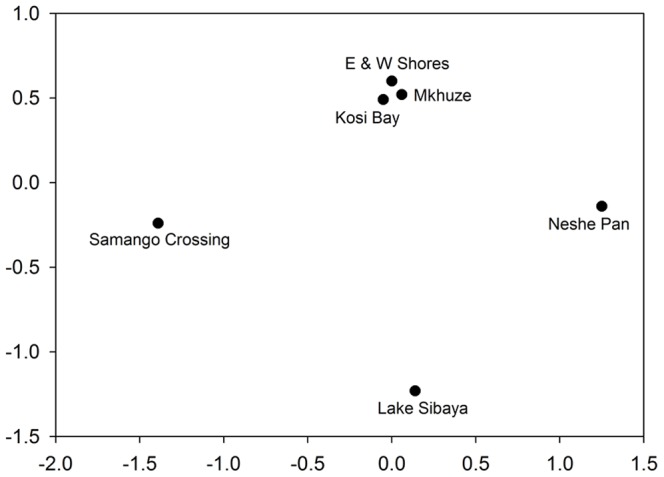
Multidimensional scaling of study areas namely: Kosi Bay, Eastern and Western Shores, Mkuze, Neshe Pan, Samango Crossing, and Lake Sibaya, based on their Jaccard index similarity matrices using presence/absence data for all Odonata species observed.
Discussion
Based on data from both the formal surveys and incidental observations, 68 odonate species were observed in this study in iWP. Thirteen species not previously recorded for this park were identified. To date 86 species have now been recorded for iWP. This total is just over 50% of the total recorded for South Africa [27]. It also exceeds Kruger National Park (n = 81), an area approximately six fold larger [34], [35]. In Africa more than 80% of odonate species, and over 70% of globally threatened species, occur within protected areas, which are largely fragmented and isolated [36]. The greater Odonata diversity at iWP is largely due to the diverse habitat types present; in particular coastal swamp forest (with Barringtonia), which supports several elusive odonates (e.g. Gynacantha, Hemicordulia and others) that do not range inland. The remaining odonate diversity generally resembles assemblages typical of the (southern) African savanna. Some common savanna species (e.g. Africallagma glaucum, Sympetrum fonscolombii, Pseudagrion kersteni, Paragomphus cognatus, Trithemis kirbyi) are however rare, or absent from the park. This could be due to climatic or other factors, for example pH, which has been shown to be strongly correlated with dragonfly diversity [18] and prevents their range from extending to the coast. Species which favour lentic wetlands dominate iWP assemblages and savanna species dependent on lotic wetlands, especially perennial streams and rivers, are least represented. This reflects the paucity of such biotopes in the park.
DBI's can be used to identify areas of conservation importance [9]. The DBI of the area under which iWP falls, has previously been identified as relatively high and therefore of conservation significance [37]. DBI's provide a useful tool for monitoring changes in odonate assemblages, for example those resulting from invasive alien plant disturbances [28], [38], [39] or changes due to human alteration of ecosystems [40]. The total DBI's observed for Kosi Bay, Eastern and Western Shores, and Mkhuze were considerably higher than values for sites in the Tsitsikamma region, Western and Eastern Cape Provinces in South Africa, although DBI/site values were lower [41]. Higher DBI/site scores can be explained by the presence of fewer, rare species and therefore higher individual DBI scores in an area. Although study areas in iWP had high species numbers, most DBI scores for species were four or less.
Of the Red Listed odonate species the iWP likely plays a significant conservation role for Urothemis luciana and Pseudagrion coeleste subspecies. Gynacantha villosa and usambarica, Hemicordulia africana, Aethriamantra rezia, Chalcostephia flavifrons and Macrodiplax cora, are also species of local interest as their South African ranges are confined to coastal Zululand. The records of Macrodiplax cora in iWP warrant further investigation, as these are the only known occurrences in Africa south of Somalia, of this fundamentally Asian species.
Many sections of iWP remain to be surveyed as some odonate species listed are based on a single known occurrence. Further surveys are required as we believe that Platycypha fitzsimonsi, Lestes plagiatus, Lestes virgatus, Pseudagrion gamblesi, Pseudagrion salisburyense, Pseudagrion sudanicum, Lestinogomphus angustus, Crenigomphus hartmanni, Ceratogomphus pictus, Orthetrum caffrum, Orthetrum guineense, Palpopleura deceptor, Brachythemis lacustris, Trithemis donaldsoni and Zygonoides fuelleborni could be present in this region. Additionally, sites surveyed in Mkhuze indicated seasonal variability (e.g. in numbers of Phaon iridipennis, Gynacantha manderica and Brachythemis leucosticta). The EKZNW database and published records also indicate that several species temporarily extend their ranges into this area from the tropics during high rainfall years. This is not uncommon for these vagile organisms and is a trait which also contributes to their re-colonization of recovering habitats [42]. Such events would contribute to additional Odonata species. Finally, it is also important to cover water bodies of varying sizes as these can also yield different species assemblages [43].
Species composition for Eastern and Western Shores, Mkhuze and Kosi Bay shows a strong similarity. These three zones all include a range of habitat types, including permanent and temporary pans, flowing water, riverine vegetation and some forest. Kosi Bay and Eastern and Western Shores are both on the coastal plain and share a very similar geography.
Species composition for Neshe Pan was dissimilar to the other sites even though geographically it is close (12 km) to the Mkhuze sites. Neshe Pan is very different from the other pans that were sampled. It is a temporary pan on the Mkuze River, is not tree-lined, and is outside of any conservation area. In dry periods the area is cultivated, and these lands are then flooded when the river flows strongly. The vegetation of Neshe Pan is particularly suitable for odonate breeding and survival. The pan is shallow and has thick beds of reeds and large areas of Nymphaea, offering ideal breeding and feeding habitats for the larval stages. It also offers large feeding areas and many territories for adults.
Samango Crossing odonate species composition was least similar to the other water bodies surveyed in this study. This can be explained by the unique habitat there. It has a greater variation of habitat types within a small area when compared to the other sites. It has the typical vegetation of the coastal plain, but also has the fresh water Manzibomvu stream flowing through it. The stream flows beneath a canopy of swamp forest trees and there are inlets of stationary water.
The species composition for Lake Sibaya is also dissimilar to all other sites. This can be ascribed to the nature of this lake. It is positioned just behind the first dune, and is a large, clear lentic system. It is lined with dune forest on its eastern edge, and supports very little aquatic vegetation or reed beds.
Based on results from this study, it is clear that within South Africa in particular, iWP is an important area for the conservation of Odonate diversity. This is largely due to the diverse habitats found within iWP and the potential to be colonized by both tropical and temperate species [10]. Furthermore, iWP is a protected area thereby reducing the direct negative impacts to its water bodies and benefiting from monitoring and management practices. As a population, Odonata fulfil many ecosystem services either directly or indirectly [26]. These are broadly grouped into: provisioning, cultural, supporting, and regulating services [26]. Odonata vary in their sensitivity to environmental change, and while some individual species can indicate change (e.g. [25]); it is recommended that changes in odonate assemblages as a whole be considered as indicators of environmental disturbance [26]. Thus surveys of Odonata diversity, particularly within ecologically important areas such as iWP, are invaluable.
Odonata respond to climatic and environmental changes [42]. In light of global climate change understanding shifts in species assemblages and the associated implications of such changes becomes increasingly important. Logistic constraints highlight the need for an indicator species group to facilitate rapid and continued surveys in a changing environment [30], [44]. The traits of Odonata lend them to fulfill this essential role [7], [8], [30]. Maputaland was recognized for its unique habitat and as an area of significant biodiversity, thereby motivating for the establishment of a large protected area, today known as iWP [45]. The diverse habitat types within the iWP support a great diversity of Odonata, reiterating its role particularly in the conservation of aquatic diversity.
Supporting Information
Odonata species account for iSimangaliso Wetland Park. Nine families are arranged in taxonomic order, with species accounts appearing alphabetically.
(DOC)
Acknowledgments
We thank EKZNW for making their data available, for accommodation and for the assistance of game guards in their reserves; the Greater St Lucia Wetland Park Authority for access to the iWP; Ricky Taylor, Rob Taylor and Sbu Mfeka for guidance in the iWP; Scotty and Di Kyle for their hospitality and guidance at Kosi Bay; Dave Robertson, Dennis Kelly and Chris Kelly for assistance in the Mkhuze Game Reserve; Michèle Tarboton for her input in the field with spotting and identification; Kerin Bowker for assistance on field trips.
Funding Statement
The authors have no support or funding to report.
References
- 1. Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation: recent progress and future challenges. Journal of the North American Benthological Society 29: 344–358. [Google Scholar]
- 2. Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, et al. (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182. [DOI] [PubMed] [Google Scholar]
- 3. Clausnitzer V, Kalkman VJ, Dijkstra K-DB, Ram M, Collen B, et al. (2009) Odonata enter the biodiversity crisis debate: the first global assessment of an insect group. Biological Conservation 142: 1864–1869. [Google Scholar]
- 4. Kalkman VJ, Clausnitzer V, Dijkstra K-DB, Orr AG, Paulson DR, et al. (2008) Global diversity of dragonflies (Odonata) in freshwater. Hydrobiologia 363: 595–351. [Google Scholar]
- 5. Wissinger SA (1988) Life history and size structure of larval dragonfly populations. Journal of the North American Benthological Society 7: 13–28. [Google Scholar]
- 6.Picker M, Griffiths C, Weaving A (2002) Field Guide to Insects of South Africa. Cape Town: Struik Publishers.
- 7. Dolny A, Bárta D, Lhota S, Rusdianto, Drozd P (2011) Dragonflies (Odonata) in the Bornean rain forest as indicators of changes in biodiversity resulting from forest modification and destruction. Tropical Zoology 24: 63–86. [Google Scholar]
- 8. Stewart DAB, Samways MJ (1998) Conserving dragonfly (Odonata) assemblages relative to river dynamics in an African savanna game reserve. Conservation Biology 12: 683–692. [Google Scholar]
- 9. Simaika JP, Samways MJ (2009) Reserve selection using Red Listed taxa in three global biodiversity hotspots: Dragonflies in South Africa. Biological Conservation 142: 638–661. [Google Scholar]
- 10.Bruton MN (1980a) Introduction. In: Bruton MN, Cooper KH, editors. Studies on the Ecology of Maputaland: Rhodes University and Natal Branch of the Wildlife Society of Southern Africa, Durban. pp. xvii-xix.
- 11.Steenkamp Y, Van Wyk B, Victor J, Hoare D, Smith G, et al. (2004) Maputaland–Pondoland–Albany. In: Mittermeier RA, Robles-Gil P, Hoffmann M, Pilgrim J, Brooks T, et al., editors. Hotspots Revisited: Earth's Biologically Richest and Most Endangered Ecoregions. Mexico City, Mexico: Cemex. pp. 219–228.
- 12.Mucina L, Scott-Shaw CR, Rutherford MC, Camp KGT, Matthews WS, et al. (2006) Indian Ocean Coastal Belt. In: Mucina L, Rutherford MC, editors. The vegetation of South Africa, Lesotho and Swaziland South African National Biodiversity Institute, Pretoria. pp. 584–615.
- 13. Humphries MS (2013) DDT residue contamination in sediments from Lake Sibaya in northern KwaZulu-Natal, South Africa: Implications for conservation in a World Heritage Site. Chemosphere 93: 1494–1499. [DOI] [PubMed] [Google Scholar]
- 14.Taylor RH (1995) St-Lucia Wetland Park. Cape Town, South Africa: Struik Publishers.
- 15. Bried JT, Ervin GN (2005) Distribution of adult Odonata among localized wetlands in east-central Mississippi. Southeastern Naturalist 4: 731–744. [Google Scholar]
- 16. Dolny A, Harabiš F (2012) Underground mining can contribute to freshwater biodiversity conservation: Allogenic succession forms suitable habitats for dragonflies. Biological Conservation 145: 109–117. [Google Scholar]
- 17. Clausnitzer V, Dijkstra K-DB, Koch R, Boudot J-P, Darwall WRT, et al. (2012) Focus on African freshwaters: hotspots of dragonfly diversity and conservation concern. Frontiers in Ecology and the Environment 10: 129–134. [Google Scholar]
- 18. Kinvig R, Samways MJ (2000) Conserving dragonflies (Odonata) along streams running through commercial forestry. Odonatologica 29: 195–208. [Google Scholar]
- 19. Samways MJ, Taylor S (2004) Impacts of invasive alien plants on Red-Listed South African dragonflies (Odonata). South African Journal of Science 100: 78–80. [Google Scholar]
- 20. Suhling F, Sahlen G, Martens A, Marais E, Schutte C (2006) Dragonfly assemblages in arid tropical environments: a case study from western Namibia. Biodiversity and Conservation 15: 311–332. [Google Scholar]
- 21. Grant PBC, Samways MJ (2011) Micro-hotspot determination and buffer zone value for Odonata in a globally significant biosphere reserve. Biological Conservation 144: 772–781. [Google Scholar]
- 22.Maud RR (1980) The climate and geology of Maputaland. In: Bruton MN, Cooper KH, editors. Studies on the Ecology of Maputaland: Rhodes University and Natal Branch of the Wildlife Society of Southern Africa, Durban. pp. 1–7.
- 23. Balinsky BI (1967) On some intrinsic and environmental factors controlling the distribution of dragonflies (Odonata) with redescription and a new name for a little-known species. Journal of the Entomological Society of Southern Africa 29: 3–22. [Google Scholar]
- 24. Balinsky BI (1961) Observations of the dragonfly fauna of the coastal region of Zululand, with the description of three new species. Journal of the Entomological Society of Southern Africa 24: 72–91. [Google Scholar]
- 25. Smith J, Samways MJ, Taylor S (2007) Assessing riparian quality using two complementary sets as bioindicators. Biodiversity Conservation 16: 2695–2713. [Google Scholar]
- 26.Simaika JP, Samways MJ (2008) Valuing dragonflies as service providers. In: Córdoba-Aguilar A, editor. Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research. Oxfor University Pres, Oxford, UK. pp. 109–123.
- 27.Samways MJ (2008) Dragonflies and damselflies of South Africa. Bulgaria: Pensoft.
- 28.Simaika JP, Samways MJ (2008) Valuing dragonflies as service providers. In A. Cordoba-Aguilar (ed.). Dragonflies: Model Organisms for Ecological and Evolutionary Research, pp. 109–123, Oxford University Press, Oxford.
- 29.Simaika JP (2008) Conservation Biogeography of Southern African Dragonflies (Odonata), MSc Thesis, Stellendosch University.
- 30. Simaika JP, Samways MJ (2012) Using dragonflies to monitor and prioritize lotic systems: a South African perspective. Organisms, Diveristy & Evolution 12: 251–259. [Google Scholar]
- 31. Pinhey E (1985) A survey of the dragonflies (Odonata) of South Africa, 2. Journal of the Entomological Society of Southern Africa 48: 1–48. [Google Scholar]
- 32. Pinhey E (1984) A survey of the dragonflies (Odonata) of South Africa, 1. Journal of the Entomological Society of Southern Africa 47: 147–199. [Google Scholar]
- 33. Samways MJ (2006) National Red List of South African Odonata. Odonatologica 35: 341–368. [Google Scholar]
- 34. Balinsky BI (1965) A preliminary list of dragonflies (Odonata) of the Kruger National Park. Koedoe 8: 95–96. [Google Scholar]
- 35. Clark TE, Samways MJ (1994) An inventory of the damselflies and dragonflies (Odonata) of the Kruger National Park, with three new South African records. African Entomology 2: 61–64. [Google Scholar]
- 36. Simaika JP, Samways MJ, Kipping J, Suhling F, Dijkstra K-DB, et al. (2013) Continental-scale conservation prioritization of African dragonflies. Biological Conservation 157: 245–254. [Google Scholar]
- 37. Simaika JP, Samways MJ (2009) An easy-to-use index of ecological integrity for prioritizing freshwater sites and for assessing habitat quality. Biodiversity and Conservation 18: 1171–1185. [Google Scholar]
- 38. Samways MJ, Grant PBC (2007) Honing red list assessments of lesser-known taxa in biodiversity hotspots. Biodiversity and Conservation 16: 2575–2586. [Google Scholar]
- 39. Magoba RN, Samways MJ (2010) Recovery of benthic macroinvertebrate and adult dragonfly assemblages in response to large scale removal of riparian invasive alien trees. Journal of Insect Conservation 14: 627–636. [Google Scholar]
- 40. Harabiš F, Dolný A (2012) Human altered ecosystems: suitable habitats as well as ecological traps for dragonflies (Odonata): the matter of scale. Journal of Insect Conservation 16: 121–130. [Google Scholar]
- 41. Simaika JP, Samways MJ (2011) Comparative assessment of indices of freshwater habitat conditions using different invertebrate taxon sets. Ecological Indicators 11: 370–378. [Google Scholar]
- 42. Samways MJ (2010) Impacts of extreme weather and climate change on South African dragonflies. BioRisk 5: 73–84. [Google Scholar]
- 43. Oertli B, Joye DA, Castella E, Juge R, Cambin D, et al. (2002) Does size matter? The relationship between pond area and biodiversity. Biological Conservation 104: 59–70. [Google Scholar]
- 44. Kati V, Devillers P, Dufrene M, Legakis A, Vokou D, et al. (2004) Testing the value of six taxonomic groups as biodiversity indicators at a local scale. Conservation Biology 18: 667–675. [Google Scholar]
- 45.Bruton MN (1980b) Conservation and development in Maputaland. In: Bruton MN, Cooper KH, editors. Studies on the Ecology of Maputaland: Rhodes University and Natal Branch of the Wildlife Society of Southern Africa, Durban. pp. xvii-xix.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Odonata species account for iSimangaliso Wetland Park. Nine families are arranged in taxonomic order, with species accounts appearing alphabetically.
(DOC)