Abstract
Low-energy Auger and conversion electrons deposit their energy in a very small volume (a few nm3) around the site of emission. From a radiotoxicological point of view the effects of low-energy electrons on normal tissues are largely unknown, understudied, and generally assumed to be negligible. In this context, the discovery that the low-energy electron emitter, 99mTc, can induce stunning on primary thyrocytes in vitro, at low absorbed doses, is intriguing. Extrapolated in vivo, this observation suggests that a radioisotope as commonly used in nuclear medicine as 99mTc may significantly influence thyroid physiology. The aims of this study were to determine whether 99mTc pertechnetate (99mTcO4 −) is capable of inducing thyroid stunning in vivo, to evaluate the absorbed dose of 99mTcO4 − required to induce this stunning, and to analyze the biological events associated/concomitant with this effect. Our results show that 99mTcO4 −–mediated thyroid stunning can be observed in vivo in mouse thyroid. The threshold of the absorbed dose in the thyroid required to obtain a significant stunning effect is in the range of 20 Gy. This effect is associated with a reduced level of functional Na/I symporter (NIS) protein, with no significant cell death. It is reversible within a few days. At the cellular and molecular levels, a decrease in NIS mRNA, the generation of double-strand DNA breaks, and the activation of the p53 pathway are observed. Low-energy electrons emitted by 99mTc can, therefore, induce thyroid stunning in vivo in mice, if it is exposed to an absorbed dose of at least 20 Gy, a level unlikely to be encountered in clinical practice. Nevertheless this report presents an unexpected effect of low-energy electrons on a normal tissue in vivo, and provides a unique experimental setup to understand the fine molecular mechanisms involved in their biological effects.
Introduction
Thyroid stunning is a clinical problem in which exposure of a patient to diagnostic amounts of 131I has been described to alter the ability of differentiated thyroid carcinoma, or remnants of thyroid tissue after thyroidectomy, to take up therapeutic, irradiating doses of 131I [1], [2], [3], [4], [5]. Although widely documented in the literature, the reality and existence of this phenomenon are questioned by many clinicians [5]. Some experts advocate that the so-called stunning effect is in fact the consequence of an early destruction of the target tissues by an excessive amount of radiotracer used for a diagnostic purpose [5]. In this clinical context, basic scientific studies using cultured, polarized thyroid epithelial cells in vitro have recently produced clear and convincing evidence of the existence of a stunning effect and of its mechanism [6], [7], [8], [9], [10]. These studies demonstrated that irradiation of the cell culture via 131I uptake resulted in a significant reduction in basal-to-apical iodide transport, even at low absorbed doses. This effect reached a 50% reduction in iodide transport at an absorbed dose of around 1.5 Gy and was observed with no evidence of cell death [7]. Using the same experimental setup, the same group demonstrated that 131I-induced stunning could be explained by a decrease in the mRNA level of the Na/I symporter (NIS), the transmembrane protein involved in the iodide uptake by the thyroid [8]. More recently, the same group published an intriguing study in which the “stunning efficacies” of 131I, 123I, 99mTc and 211As were compared in the same cell culture system [9]. Experiments were designed to deliver a “standardized” absorbed dose of 0.5 Gy from these radioisotopes, which are all accumulated in thyroid cells via a NIS-dependent mechanism. The results showed that all of these radioisotopes were capable of reducing iodide transport by decreasing NIS mRNA levels a few days later. The “stunning power” of 131I appeared to be poorer than that of 123I or even 99mTc in this setting. Although the absorbed dose-rate resulting from the relative half-life and radiation type of these different isotopes may be partially responsible for the difference in biological activity observed, these results are puzzling. Extrapolated in vivo, this study suggests that a radioisotope as commonly used in nuclear medicine as 99mTc may significantly influence thyroid biology. In addition, SPECT/CT imaging of ectopic NIS gene expression with 99mTcO4 − has been validated at the preclinical level and is starting to be used in the clinic to determine the kinetics of gene transfer in gene therapy [11], [12], [13], [14]. Hence, the conditions required to induce a 99mTcO4 −-mediated thyroid stunning effect in vivo need to be clarified. The stunning effect of 99mTc is attributed to low-energy electrons.
The energy of low-energy electrons is deposited in a very small volume (a few nm3) around the site of de-excitation of radionuclides such as 123I, 125I, 111In, or 99mTc [15], [16]. In biology, these electrons are thought to have played a significant role as mutagenic agents in evolution [17] and today they represent the basis of attractive strategies for new, targeted cancer treatments [16]. In the latter approaches, Auger electron emitters (typically 125I or 111In) are incorporated into small molecules [18], peptides [19], proteins [20], [21] or antibodies [22] capable of targeting cancer cell nuclei, where Auger electrons will exert their irradiating effects. From a radiotoxicological point of view, however, the effects of Auger electrons on normal, nonpathological biological systems are largely unknown, understudied and generally assumed to be negligible [23].
The aims of the present study were to determine whether 99mTcO4 − was capable of inducing stunning in vivo, to evaluate the absorbed dose of 99mTcO4 − required to induce this stunning, and to analyze the biological events associated/concomitant with this effect.
Materials and Methods
Animals
Female, eight-week-old Balb/c mice were obtained from Janvier (Le Genest Saint Isle, France). Animal housing and procedures were conducted according to the French Agriculture Ministry guidelines and were approved by the local ethics committee (CIEPAL: Comité Institutionnel d'Ethique Pour l'Animal de Laboratoire) (Permit number # A06-088-14-138). Animals were fed using a standard regimen with a normal-iodide diet, except for two animals included in the protocol for dosimetric calculations that received an iodide-poor regimen for 8 days before the study (see below).
In vivo MicroSPECT/CT Studies
99mTc pertechnetate (99mTcO4 −) was obtained from a freshly eluted 99Mo/99mTc generator. Animals were administered intraperitoneally with 99mTcO4 − activities ranging from 10 MBq to 150 MBq. Thyroid tracer uptake was measured 60 minutes later using a dedicated microSPECT/CT scanner (eXplore speCZT CT120, GE) under gas anesthesia (air and 1-2% isoflurane) in an air-warmed imaging chamber (Minerve, Esternay, France) to keep body temperature constant at 37°C. The SPECT scanner uses a stationary full ring of CZT detectors and a rotating 7-pinhole (1 mm opening) collimator. A total of 350 projections were acquired over 360° in 8 minutes. Images were reconstructed using the manufacturer's 3D-OSEM algorithm (5 subsets and 11 iterations), which incorporates the system's collimator–detector response function and scatter correction. SPECT imaging system calibration for 99mTc volume sensitivity was obtained by correlation of the results with a calibrated well counter using the NEMA protocols [24]. Reconstructed images were analyzed and quantified using AMIDE software [25]. Tracer activity in the thyroid was calculated by converting the total image units measured in a 3D region of interest to MBq using the calibration factor. Thyroid uptake was expressed as percentage of the injected dose (%ID) after decay correction, except for dosimetric calculations (see Figure 1B).
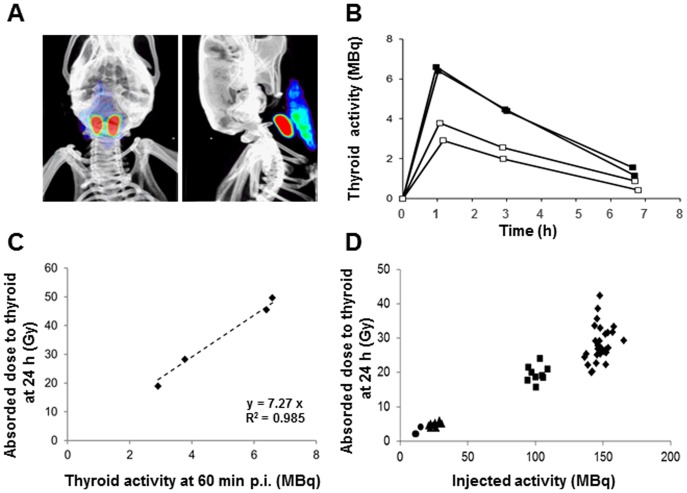
Figure 1. 99mTcO4 −-SPECT/CT imaging and absorbed dose to the thyroid.
(A) Volume-rendered, fused SPECT/CT images in anterior and lateral views demonstrating the thyroid lobes (red) and the salivary glands (blue-green), 60 minutes after 99mTcO4 − administration. (B) Kinetics of 99mTcO4 − activity in the thyroids of four Balb/c mice fed with a normal iodide diet (open squares) or with a low iodide diet (filled squares), obtained upon injection of 150 MBq of 99mTcO4 −. The values presented are not corrected for decay. (C) Correspondence between thyroid activity (MBq) at 60 minutes post injection and absorbed dose to the thyroid (Gy) at 24 hours. (D) Absorbed dose to the thyroid at 24 hours in mice injected with 10 MBq (circles, n = 4), 25 MBq (triangles, n = 9), 100 MBq (squares, n = 9) or 150 MBq (diamonds, n = 29) 99mTcO4 −.
Animal Study Protocols
For dosimetric calculations, thyroid SPECT imaging was repeated 1, 3 and 7 hours after intraperitoneal administration of 150 MBq 99mTcO4 − in four mice. Two mice had a standard-regimen diet, two had a poor-iodide-regimen diet for 8 days before the study. This dietary manipulation is known to lead to different thyroid 99mTcO4 − uptake capacity and was used to establish a correspondence between absorbed dose to the thyroid (at 24 hours) and thyroid activity at the peak.
To determine the early effect of an initial administration of 99mTcO4 − on thyroid uptake, 21 animals that received 25 MBq (n = 3), 100 MBq (n = 3) or 150 MBq (n = 15) were imaged again 24 hours after the initial SPECT, using a standard (50 MBq) 99mTcO4 − activity.
To investigate the later impact of the administration of 99mTcO4 − on the thyroid uptake, 12 animals initially received 150 MBq tracer, resulting in an absorbed dose to the thyroid of 27.5±4.1 Gy, ranging from 20.4 to 35.7 Gy. The animals were imaged again 1 (n = 3), 2 (n = 3), 4 (n = 3) or 8 days (n = 3) later, using a standard 99mTcO4 − activity (50 MBq). After the end of the second scan, the animals were culled and, when relevant, biopsies of different organs were collected.
Dosimetry
The absorbed dose in the thyroid (in Gy) was estimated based on the MIRD formalism [26]. The cumulated activity in the thyroid (Bq.s) was calculated based on quantitative imaging and multiplied by the thyroid self S-value (Gy.Bq−1.s−1) obtained from Monte Carlo simulation of a representative mouse model. The kinetics of 99mTcO4 − activity in the thyroid are presented in Figure 1B. Activities were not corrected for nuclear decay. Cumulated activity was calculated for each mouse, using a linear fitting model for the uptake phase, and a mono-exponential fitting model for the washout, both implemented in Root software (http://root.cern.ch). No activity was considered at t = 0 and the endpoint for cumulated activity calculation was taken at t = 24 hours.
S-values were calculated based on the realistic-digital-mouse (Moby) whole-body phantoms (version 2), representing a 16-week-old male C57BL/6 mouse [27]. We generated a 22 g mouse model as a 3D rectangular matrix of cubic voxels (200×200×200 μm3). The final 3D image dataset was composed of 256×550×256 voxels and saved in a raw format (16-bit; unsigned integer; little-endian; 72 MB). Thyroid mass was reduced to 5.4 mg by applying an erode mask implemented on ImageJ software. Soft tissues, lungs, bones and air density, and material composition [28] were also taken into account for radiation transport purposes.
S-values were calculated using Monte Carlo modeling of radiation transport and energy deposition in the voxel-based mouse model. Up to 106 particles were simulated using GATE (version 6.1), based on the GEANT4 toolkit (version 9.04 patch01), both of which are well-established codes for radiation transport [29], [30], [31].
The voxel-based mouse was implemented with the CompressedMatrix option, the most suited function available for dosimetric purposes in that version, and regions of interest were defined using the range option. Physics List Standard Option 3 was used to define physics processes. The deposited energy was scored at the voxel level of the phantom with the DoseActor, doseDistributionEdep. Statistical uncertainties were calculated using the associated UncertaintyEdep option. GATE was run with Mersenne Twister [32].
The thyroid self S-value was calculated for 99mTc. All detailed photon and electron emissions were based on the “MIRD radionuclide data and decay schemes” [33]. Cubic spline interpolation was applied to all continuous energy spectra used in simulations. The source was assumed to be distributed homogeneously within the thyroid and statistical uncertainties in self S-values were kept below 1%.
Membrane Vesicle Preparation And SDS-PAGE Analyses
Thyroid membrane proteins were obtained as previously described [34] and subjected to SDS-PAGE electrophoresis. Western blotting was performed with antibody 25 anti-mouse NIS, an affinity-purified rabbit immunoreactive serum fraction, as previously described [35], an anti-Ser139 phospho H2AX mouse monoclonal Ab (Millipore, France), or with an anti-β-actin antibody (Sigma).
RNA Extraction, Quantitative RT-PCR and Microarrays
Extraction of total RNA from mouse thyroid, and quantitative RT-PCR, were performed as previously described [36], [37], [38]. Relative mRNA expression levels were determined using ΔCt values obtained by subtracting Ct control (mouse actin) from Ct target gene (mouse NIS), and expressed using the comparative CT method (2−ΔCT).
For microarray analysis, RNA samples were labeled with Cy3 dye using the Low RNA Input QuickAmp Kit (Agilent), as recommended by the supplier. Labeled cRNA probes (400 ng) were hybridized on 8×60K high-density SurePrint G3 gene mouse GE 8×60K Agilent microarrays. Four biological replicates were performed for each experimental condition. The experimental data are deposited in the NCBI Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) under the record number, GSE46470. Normalization of microarray data was performed using the Limma package (http://www.bioconductor.org). Interslide normalization was performed using the quantile methods. Means of ratios from 99mTc-treated versus control tissues were calculated and B-test analysis was performed. Differentially expressed genes were selected based on an adjusted p value of 0.05. Data from expression microarrays were analyzed for enrichment in biological themes (Gene Ontology molecular function and canonical pathways) and biological networks were built using Ingenuity Pathway Analysis software (http://www.ingenuity.com/) and Mediante [39].
Histology/Immunohistochemistry
Formalin-fixed, paraffin-embedded thyroid and salivary gland tissue sections were stained with hematoxylin/eosin for morphologic evaluation. Mouse NIS and phospho-Histone H2AX immunostaining were performed using a rabbit polyclonal anti-NIS antibody (antibody 25, see [35]) and an anti-Ser139 phospho H2AX rabbit mAb (Cell Signalling, France), followed by HRP-conjugated anti-rabbit antibodies and the avidin-biotin complex immunoperoxidase method, respectively. Apoptosis (TUNEL) staining was performed using the pro-TUNEL assay kit (Euromedex, France). The sections were analyzed by a certified veterinary pathologist.
Statistical Analysis
Dual comparisons were made using the Student's t-test and comparisons between multiple conditions were analyzed using ANOVA. Statistical significance was set at p<0.05.
Results
Dosimetric calculations
Figure 1A presents typical, volume-rendered, fused SPECT/CT images in anterior and lateral views of the thyroid region. 99mTcO4 − is accumulated in the thyroid (red) and the salivary gland (blue-green). To measure precisely the thyroid 99mTcO4 − content, regions-of-interest excluding the salivary glands were drawn around the entire thyroid. The kinetics of 99mTcO4 − uptake and release in the thyroid were measured by SPECT/CT on live, anesthetized mice, 60 minutes, three hours, and seven hours after intraperitoneal administration of 150 MBq 99mTcO4 −. Figure 1B shows that the radioisotope content in the thyroid reaches its maximal value within one hour after 99mTcO4 − injection, thereafter decreasing progressively to reach near-basal levels at an estimated time of 8 hours. To enrich the dataset, we performed a similar experiment on animals that were fed on a poor-iodide diet. Accumulation of 99mTcO4 − in the thyroid followed the same overall pattern (Figure 1B) but the value at the peak averaged 6.5 MBq with a low-iodide diet while it averaged 3.5 MBq in mice fed a normal-iodide diet. Dosimetric calculations based on these pharmacokinetic data are detailed in Material S1. They indicate that the thyroid self S-value was 4.55E−10 Gy.Bq−1.s−1. In these experimental conditions, the peak activity measured in the thyroid (ranging from 3 to 7 MBq) was strongly correlated (R2 = 0.985) with the cumulated activity, and hence with the absorbed dose to the thyroid at 24 hours (range 18.9 to 49.6 Gy) (Figure 1C). Based on these kinetics and calculations, SPECT/CT analyses of thyroid activity throughout the study were systematically performed 60 minutes after radioisotope administration and, when relevant, transformed into absorbed doses. Absorbed doses to the thyroid at 24 hours averaged 2.6±1.0, 4.8±0.7, 19.6±2.4, and 28.4±5.2 Gy, for injected activities of 10, 25, 100 and 150 MBq, respectively (Figure 1D). Thyroid activity (expressed as %ID) was 2.84±0.63, 2.6±0.35, 2.67±0.33 and 2.63±0.47 in mice injected with 10 (n = 4), 25 (n = 9), 100 (n = 9) or 150 (n = 29) MBq 99mTcO4 −, respectively. MBq, respectively (Figure 1D).
Dosimetric Requirement For 99mTcO4 −-Mediated Thyroid Stunning
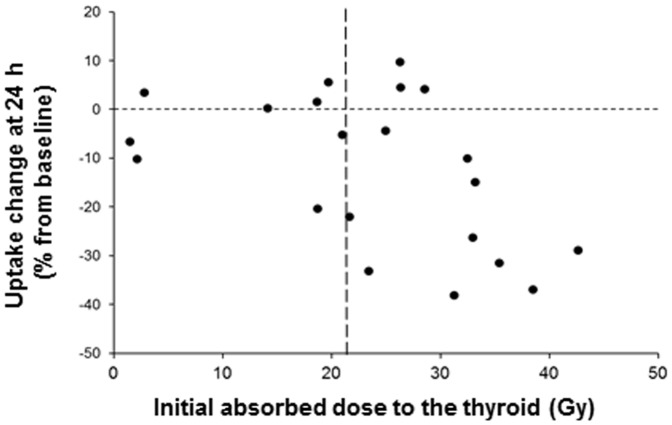
Figure 2 shows that the threshold of initial absorbed dose required to observe a significant reduction (p = 0.048) in the capacity of the thyroid to take up 99mTcO4 − one day later was 22 Gy. In animals that received less than 22 Gy, the average decrease in 99mTcO4 − uptake was 3.95%, while in animals that received more than 22 Gy it was 17.5%.
Figure 2. Changes in thyroid uptake 2499mTcO4 −.
Mice were injected with various amounts of 99mTcO4 − (25 to 150 MBq). Twenty-four hours later, a standard dose of 50 MBq 99mTcO4 − was injected to determine the capacity of the thyroid to take up the radioisotope. The figure represents the relationship between the initial absorbed dose to the thyroid (in Gy) and the percent change in 99mTcO4 − uptake 24 hours later.
Kinetics of 99mTcO4 −-mediated thyroid stunning
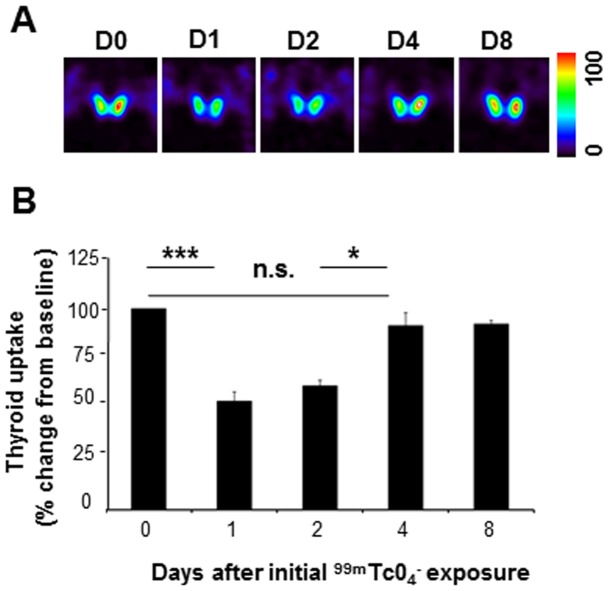
Using longitudinal follow up of individual mouse thyroids by SPECT/CT imaging, we next determined whether the 99mTcO4 −-mediated stunning was reversible. Thyroid activity was measured at day 0, upon administration of 150 MBq of 99mTcO4 −, resulting in an absorbed dose to the thyroid of 30 Gy, and reassessed after reinjection of 99mTcO4 −, at either day 1, 2, 4, or 8. Figure 3 shows that a single 30 Gy irradiation of the thyroid reduced the magnitude of 99mTcO4 − thyroid uptake by 45% at 24 hours. This impairment in thyroid uptake was observed for up to two days and the ability of the gland to take up the radiotracer was completely restored four days after the initial administration of 99mTcO4 −. These data demonstrate that the stunning is rapidly reversible.
Figure 3. Longitudinal follow up of 99mTcO4 −-mediated thyroid stunning by SPECT/CT.
99mTcO4 − uptake in the thyroid was measured by SPECT/CT in 12 mice at day 0 (D0) and again at day 1 (D1, n = 3), day 2 (D2, n = 3), day 4 (D4, n = 3), or day 8 (D8, n = 3). The second scan was performed by injecting a dose of 50 MBq 99mTcO4 −. (A) Representative SPECT/CT images of 99mTcO4 − uptake by the thyroid. The images are normalized (100%) to the voxel with the highest activity in the series. (B) Thyroid uptake presented as percentage of the value at day 0± SD. (*: p<0.05; ***: p<0.001).
Effect of 99mTc exposure on NIS protein- and mRNA expression
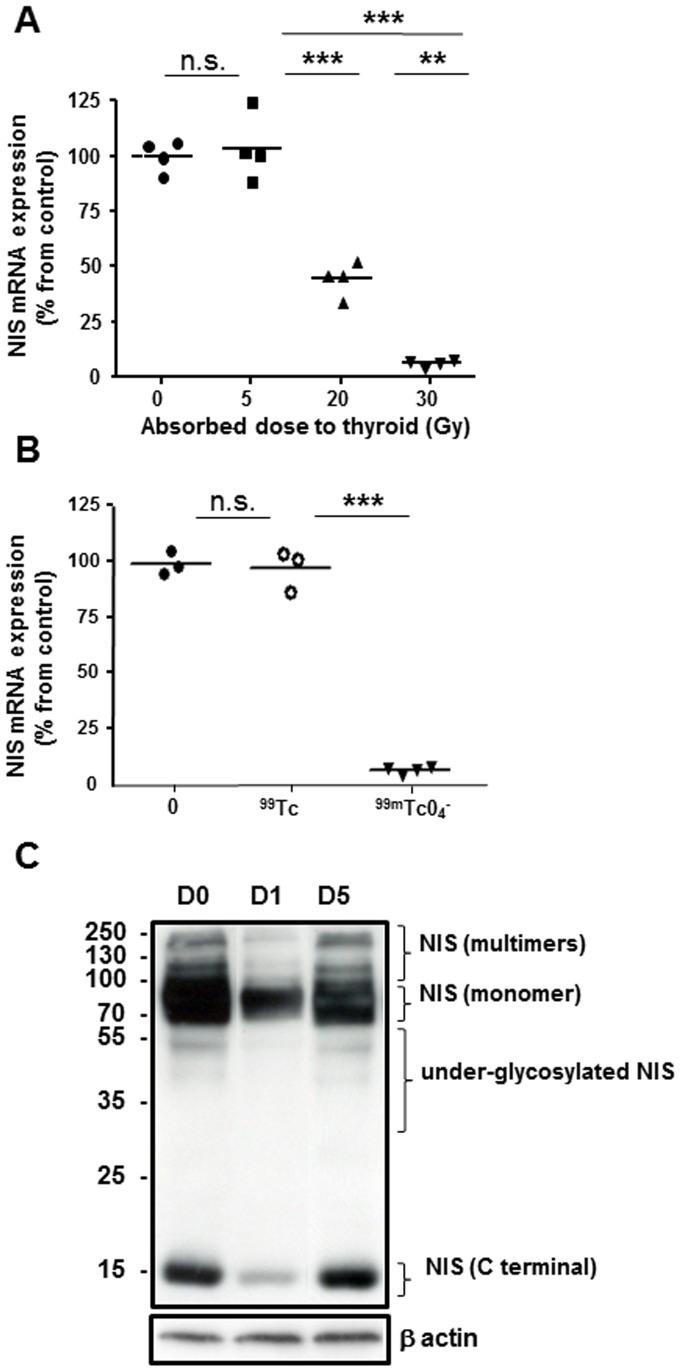
NIS mRNA levels in mouse thyroid glands were measured 24 hours after a single exposure to 99mTcO4 − (Figure 4A). Expression was high in untreated thyroids but was downregulated by 2.5-fold (P = 0.0007) and 12-fold (P = 0.003) after exposure to 20 Gy and 30 Gy, respectively. In contrast, absorbed doses of 5 Gy failed to induce any significant reduction in the messenger levels. In addition, no marked variation was measured in the mRNA levels of the housekeeping gene, β-actin, measured in the same RNA preparation. To assess whether the reduction in NIS mRNA was associated with the irradiation induced by 99mTcO4 − or to a pharmacological effect of 99Tc, we injected mice with a solution of 150 MBq 99mTcO4 − that had been left to decay for three days (about 12 periods). In these conditions, we estimate that the activity of 99mTcO4 − injected is 0.04 MBq, leading to an 8mGy irradiation of the thyroid. In these conditions, most of the technetium is in the form of 99Tc. Although 99Tc is radioactive, its half-life is very long (211000) years and we can consider that during the course of the experiment (1 day) the irradiation from 99Tc is negligible. As expected, injection of a three-days decayed solution of 99mTcO4 − failed to affect the level of NIS mRNA (Figure 4B), suggesting that the stunning induced by 99mTcO4 − is dependent on irradiation.
Figure 4. Expression of NIS mRNA and protein in thyroids of mice 2499mTcO4 −.
(A) Quantitative RT-PCR analyses of NIS expression in control thyroids or thyroids exposed to 5 Gy, 20 Gy or 30 Gy. The means (n = 4 mice/group) are presented (horizontal bars) as well as each individual point. (**: p<0.01; ***: p<0.001). (B) Quantitative RT-PCR analyses of NIS expression in control thyroids or thyroids exposed to decayed 99mTcO4 − (99Tc on the figure) or 30 Gy of 99mTcO4 − (99mTcO4 − on the figure). The means (n = 3 to 4 mice/group) are presented (horizontal bars) as well as each individual point. (C) Western blot analyses of NIS expression levels assessed on membrane preparations from thyroids of control mice or from thyroids extracted 1 and 5 days after exposure to 30 Gy 99mTcO4 −. The immunoblot shown is representative of three independent experiments.
Western blot analyses of NIS protein expression were performed using membrane preparations from mouse thyroids, 24 hours after a single exposure to 30 Gy 99mTcO4 −. Figure 4C shows a marked reduction in the amount of all functional NIS forms, including the higher molecular weight species likely to correspond to multimers and dimers as well as the monomers (90 kDa), compared with control tissues. Although not functional by itself, the 15 kDa band corresponding to the small C-terminal fragment of mNIS [35] was also markedly reduced. Re-expression of all functional NIS isoforms was observed within 5 days following the initial administration of 99mTcO4 −, thus demonstrating that the stunning is rapidly reversible (Figure 4C).
Histologic analysis of 99mTcO4 −-exposed thyroids
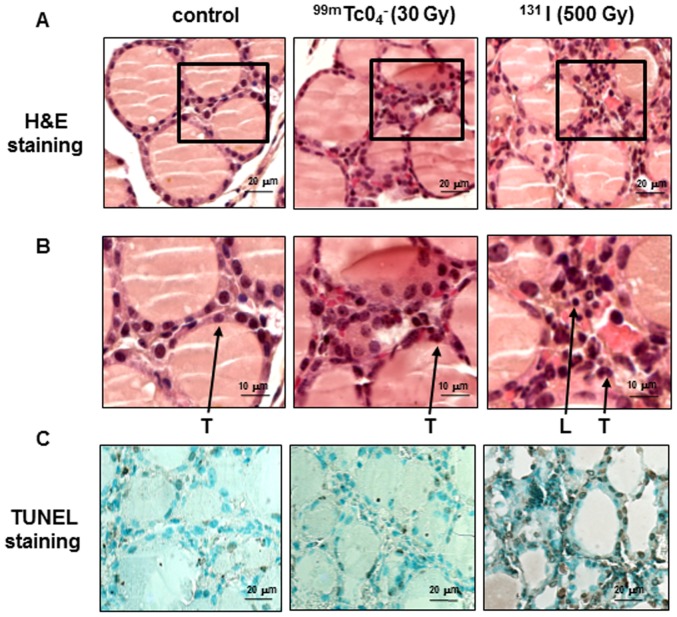
Hematoxylin-eosin–stained sections of 99mTcO4 −-exposed thyroids (absorbed dose of 30 Gy) showed normal thyroid histology, with intact colloid-containing follicles comparable with those of control mice, with no microscopic signs of cytotoxicity (Figure 5A). As a positive control, administration of a therapeutic dose of 131I (500 Gy to the thyroid for 24 hours) was used. In this latter condition, the interstitium appears to be expanded by the infiltration of inflammatory cells not present in control or 99mTcO4 −-exposed thyroids (Figures 5B). To detect whether these exposures were associated with apoptosis, TUNEL staining was performed. Figure 5C shows a very similar situation in control thyroids and thyroids from 99mTcO4 −-exposed animals. By contrast, a strong nuclear TUNEL staining is observed on thyroid sections obtained from mice injected with 500 Gy 131I (Figure 5C). More specifically, analysis of 10 randomly-selected fields showed 8 TUNEL-positive nuclei in control thyroids, 9 TUNEL-positive nuclei in 99mTcO4 −-exposed thyroids and more than 100 TUNEL-positive nuclei in131I-exposed thyroids. These results, combined with the observation that 99mTcO4 −-mediated thyroid stunning is recovered within five days of the initial exposure to the radioelement, strongly suggest an absence of significant destruction of the thyroid in response to 99mTcO4 − exposure.
Figure 5. Histology of mouse thyroids 2499mTcO4 − or 131I-iodide.
(A) Hematoxylin/eosin-stained sections of thyroids show intact morphology after exposure to 99mTcO4 − (30 Gy), but microscopic histological alterations in 131I (500 Gy)–exposed thyroids. The squares in the different sections represent the magnified areas presented in (B). (B) Close-up views of the H&E sections presented in (A). L: nuclei of leukocytes, T: nuclei of thyrocytes. (C) Representative apoptosis TUNEL staining of thyroid sections shows rare immunoreactive cells in control and 99mTc-injected mice, but numerous TUNEL-positive nuclei within the thyroids of 131I-injected mice.
Induction of double-strand DNA break repair in 99mTcO4 −-exposed thyroids
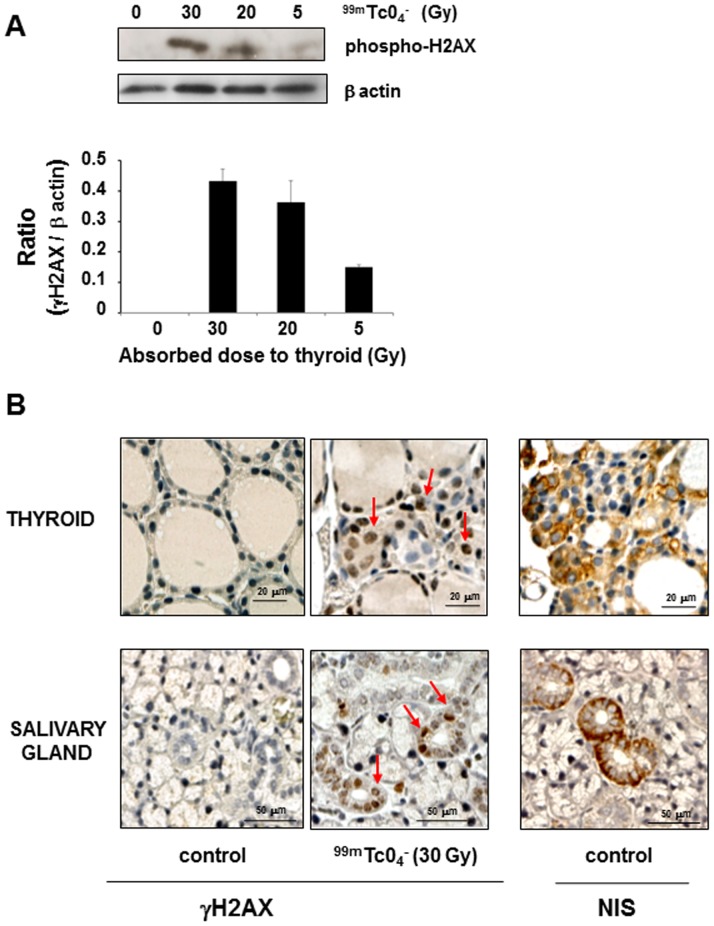
We next assessed whether exposure to 99mTcO4 − could lead to the induction of double-strand DNA break repair within the thyroid by following the level of phosphorylation of histone-2AX (γH2AX) [40]. A dose-dependent histone-2AX phosphorylation was detected, by Western blot, on extracts obtained from mouse thyroids subjected to 5, 20 and 30 Gy (Figure 6A). This observation was confirmed by immunohistochemistry (Figure 6B). Foci of nuclear γH2AX immunostaining were visible in sections generated from thyroid sections exposed to 30 Gy. To determine whether the presence of double-strand breaks is dependent on the uptake of the radioisotope by the cells, we next looked at the foci of nuclear γH2AX immunostaining in the salivary glands. In these glands, NIS is selectively expressed in the epithelial cells lining the ducts (Figure 6B) and γH2AX immunostaining was only detected in the nuclei of these ductal cells. This observation strongly suggests that the presence of double-strand breaks is dependent on the uptake of 99mTcO4 − by the cells.
Figure 6. γH2AX foci in thyroids and salivary glands of mice 24 hours after administration of 99mTcO4 −.
Mice were injected with various activities of 99mTcO4 − and culled 24 hours later for biopsy collection. (A) Western blotting of protein homogenates obtained from mouse thyroids subjected to different levels of irradiation. Results represent the normalized increase in H2AX phosphorylation level compared with the control condition. Protein quantification was performed with image J software. (B) Representative sections of thyroids or salivary glands of control mice or mice exposed to 30 Gy 99mTcO4 − were stained with a γH2AX- or NIS-specific antibody. Arrows represent foci of DNA double-strand break repair (γH2AX-positive nuclei). The figures presented are representative of 2 and 6 independent experiments for Western blot (A) and immunohistochemistry (B), respectively.
Global pattern of gene expression in thyroids of 99mTcO4 −-exposed versus control mice
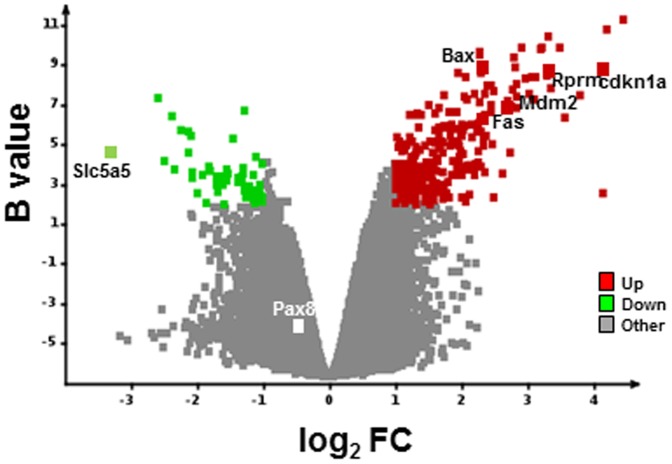
To determine the impact of 99mTcO4 − exposure on the thyroid, total RNA, extracted from four untreated thyroids and four thyroids exposed to 30 Gy with 99mTcO4 −, was analyzed using whole genome mouse microarrays. Total RNA was collected 24 hours after radioisotope administration. The comparison between these two conditions showed a significant modulation of 306 transcripts annotated in the RefSeq database, corresponding to 191 up- and 115 downregulated genes in the 99mTcO4 −-exposed thyroids (Table S1 and Figure 7). These transcripts efficiently discriminated 99mTcO4 −-exposed from control thyroids. As expected from the data presented in Figure 4, NIS (Slc5a5) mRNA appeared to be among the most significantly downregulated transcripts in 99mTcO4 −-exposed cells, while Pax8, a transcription factor regulating NIS, appeared to be unaffected (Figure 7). Gene ontology analysis using the Mediante information system [39] and Ingenuity Pathway™ showed a very strong association of these modulated transcripts with “p53 signaling” (P = 1.4×10−10) (Table S2). Several genes regulated by p53 were strongly upregulated in 99mTcO4−-exposed thyroids, including cyclin-dependent kinase inhibitor, p21 (p21/Cdkn1a/Waf1/Cip1), reprimo/p53-dependent G2 arrest mediator candidate (Rprm), BCL2-associated X protein (Bax), p53 E3 ubiquitin protein ligase (Mdm2) and members of the TNF receptor family, Fas (Apo-1, Apt1, CD95, Fas1, Fastm, Tnfrsf6) (Table S2).
Figure 7. Global pattern of gene expression in thyroids of 99mTcO4 −-exposed versus control mice.
RNA samples from thyroids of control or 99mTcO4 −-exposed mice were harvested (n = 4 per group) 24 hours after radioisotope or saline administration and expression profiles were determined with Agilent arrays. Volcano plot showing the distribution of differentially expressed transcripts between 99mTcO4 −-exposed versus control thyroids. Log(base2) of the fold-change (LogFC) is plotted against the B-statistic value for each transcript. Several transcripts – NIS/Slc5a5, Pax8, and a subset of p53-regulated genes (p21/cdkn1a, Rprm, Bax, Mdm2, Fas) – are highlighted. Significantly down- and upregulated genes are shown in green and red, respectively.
Discussion
The first aim of this study was to determine whether 99mTcO4 − was capable of inducing stunning, in vivo, in mouse thyroid glands. Our data show that an irradiation above 22 Gy via 99mTcO4 − accumulation in the thyroid leads, in the vast majority of cases, to a marked reduction (up to 40%) in the ability of the thyroid to take up the same radioisotope 24 hours later. This stunning effect, induced by a single injection of 99mTcO4 −, could be observed for up to two days, and the ability of the thyroid to accumulate the radiotracer was completely recovered four days after the initial administration of 99mTcO4 −. At the molecular level, the initial accumulation of 99mTcO4 − in the thyroid gland led, 24 hours later, to a reduction of NIS mRNA as well as a diminution of all forms of the NIS protein present at the membrane. Histological analysis of thyroid sections did not demonstrate any significant areas of cell death/necrosis in 99mTcO4 −-exposed compared with control animals. Altogether, these results are consistent with a stunning effect, defined as a marked diminution of 99mTcO4 − uptake by the thyroid, independent of any significant tissue destruction. The reversibility of this stunning within a few days confirms this hypothesis.
The kinetics of residence of the radioisotope were determined by analysis of SPECT/CT images. Pharmacokinetic data showed that the peak 99mTcO4 − content in the thyroid was reached within 1 hour after intraperitoneal injection and strongly correlated with the cumulated dose at 24 hours. These data are in agreement with the previously published biodistribution of 99mTcO4 − measured by direct counting of radioactivity in biopsies collected at different times from different animals [41]. Our dataset highlights the fact that small-animal SPECT imaging is a very powerful tool, capable of generating reliable data, whilst minimizing the number of experimental animals. Dosimetric calculations revealed that the 99mTcO4 −-mediated thyroid stunning effect requires an absorbed dose in the thyroid in the range of 20 Gy, observed in animals injected with an activity of 150 MBq. Activities from 74 to 370 MBq 99mTcO4 − are administered to patients during routine thyroid examination, leading to an absorbed dose to the thyroid below 20 mGy (Society of Nuclear Medicine Procedure Guideline for Thyroid Scintigraphy, http://interactive.snm.org/docs/Thyroid_Scintigraphy_V3.pdf). This large difference in absorbed dose to the thyroid between humans and mice can be explained by the larger volume of distribution of the radioisotope in humans. In addition, all mouse thyrocytes are reported to express NIS, while its expression in human thyrocytes is only patchy [42]. Thus, although 99mTcO4 −-mediated thyroid stunning is a real phenomenon in mice, it is very unlikely to be encountered in clinical practice.
The biological events associated/concomitant with 99mTcO4 −-mediated stunning were studied by comparing the global pattern of gene expression in the thyroid glands of 99mTcO4 −-exposed versus control mice. Several genes were found to be up- or downregulated (see Table S1). Amongst these, the NIS gene (slc5a5) was the most highly downregulated. This result is intriguing as the most downregulated gene encodes the protein responsible for the radioisotope uptake, and therefore for the irradiation of the cell. This phenomenon bears the hallmark of an adaptive response of the thyroid to irradiation. Analysis of the whole dataset indicates, with a very high statistical significance (P value in the range of 10−16), that the variation in gene expression observed in the thyroids of 99mTcO4 −-exposed animals is the result of activation of the p53 pathway. This pathway is typically activated when cells are subjected to stress [43]. In our case, the stress is irradiation of the cells with an absorbed dose in the range of 20 Gy. A similar stress response was reported in thyroids subjected to 11 Gy 211At [44]. However, in this latter study NIS gene expression was not reported to vary, suggesting a differential regulation of NIS expression in thyroid cells subjected to α particles and to low-energy electrons.
The 99mTcO4 −-mediated irradiation led to the induction of double-strand DNA break repair. Foci of nuclear γH2AX staining were already visible in sections generated from thyroids exposed to 5 Gy, their numbers increased in sections from thyroids exposed to 30 Gy. In the salivary glands, NIS is expressed in ductal epithelial cells, and γH2AX nuclear foci are only detectable in these structures. The salivary gland dataset demonstrates that the biological events triggered by 99mTcO4 − exposure happen selectively in NIS-expressing cells capable of accumulating the radiotracer. The requirement for 99mTcO4 − to enter the cell in order to provoke maximal DNA damage and, if the dose is high enough, cell death, has been demonstrated in vitro using the NIS-expressing PCCl3 thyroid cell line [45], [46], [47], [48]. In these experiments, 99mTcO4 −-mediated toxicity was clearly magnified by 99mTcO4 − entry into the cells [45], [46], [47] and was further enhanced by 99mTcO4 − retention [48]. This increased radiotoxicity from intracellular 99mTc is likely to be the result of an increased dose deposition in cellular structures due to Auger and conversion electrons, with low range and high local energy deposition properties. The biological effects observed upon internal irradiation of the thyrocytes (double-strand DNA breaks, p53 pathway activation, decrease in NIS protein and mRNA content) may be triggered by a direct or indirect action of low-energy electrons. These mechanisms may not be mutually exclusive and could be involved to different degrees in the different biological events triggered by low-energy electrons. A direct mechanism implies that 99mTcO4 − enters the nucleus and is in direct contact with DNA. Alternatively, following the de-excitation of 99mTcO4 − nuclides in the cell, low-energy electrons may trigger the production of reactive oxygen species (ROS), which could induce the observed biological effects. These ROS could induce DNA damage and activate the p53 pathway. Considering that ROS have already been shown in vitro to decrease NIS mRNA levels and reduce iodide uptake [49], ROS may have a preponderant role in 99mTcO4 −-mediated thyroid stunning.
In conclusion, we demonstrate in this report that 99mTcO4 −-mediated thyroid stunning can be observed in vivo in mouse thyroid. This effect is not associated with significant cell death and is reversible within a few days. At the cellular level, decreased NIS mRNA, p53 pathway activation, and the generation of double-strand DNA breaks can be observed. However, considering that the thyroid needs to be exposed to an absorbed dose of at least 20 Gy for a significant stunning, this effect is unlikely to be encountered in clinical practice. Nevertheless, from a biological point of view our system provides a unique experimental setup to compare the sensitivities of different NIS-expressing organs to low-energy electrons and to understand the fine molecular mechanisms involved in cellular stunning.
Supporting Information
List of the 306 RefSeq annotated transcripts significantly modulated in 99mTcO4−-exposed thyroids. Agilent Probe and NCBI RefSeq IDs give access to transcript annotations. Logarithm (base 2) of the average intensity and logarithm (base 2) of the ratio 99mTcO4 −/control are represented.
(DOCX)
Ingenuity Pathway Analysis of microarray data to highlight selectively affected pathways in 99mTcO4−-exposed thyroids. Affected canonical pathways ordered by p-value are presented. The probability of obtaining the number of genes in a certain pathway in the list of differentially expressed genes was compared with the representation of the same pathway among all the genes on the microarray; –log10 of the Fisher's exact probability is indicated.
(DOCX)
Dosimetric calculations.
(DOCX)
Acknowledgments
The authors would like to thank the radiopharmaceutical team of the Centre Antoine Lacassagne (Didier Alberato, Stéphane Espitallier, Guy Martinico, Isabelle Puy, Alison Richard, Nadine Sapin) for their help with radioisotope production and handling. The authors acknowledge the excellent support of the Nice-Sophia Antipolis Functional Genomics Platform in which the microarray experiments were carried out
Funding Statement
This work is supported by grants from the “Agence Nationale de la Recherche” (ANR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Medvedec M (2001) Thyroid stunning. J Nucl Med 42: 1129–1131. [PubMed] [Google Scholar]
- 2. Brenner W (2002) Is thyroid stunning a real phenomenon or just fiction? J Nucl Med 43: 835–836. [PubMed] [Google Scholar]
- 3. Allman KC (2003) Thyroid stunning revisited. J Nucl Med 44: 1194. [PubMed] [Google Scholar]
- 4. Sisson JC, Avram AM, Lawson SA, Gauger PG, Doherty GM (2006) The so-called stunning of thyroid tissue. J Nucl Med 47: 1406–1412. [PubMed] [Google Scholar]
- 5. McDougall IR, Iagaru A (2011) Thyroid stunning: fact or fiction? Semin Nucl Med 41: 105–112. [DOI] [PubMed] [Google Scholar]
- 6. Postgard P, Himmelman J, Lindencrona U, Bhogal N, Wiberg D, et al. (2002) Stunning of iodide transport by (131)I irradiation in cultured thyroid epithelial cells. J Nucl Med 43: 828–834. [PubMed] [Google Scholar]
- 7. Lundh C, Norden MM, Nilsson M, Forssell-Aronsson E (2007) Reduced iodide transport (stunning) and DNA synthesis in thyrocytes exposed to low absorbed doses from 131I in vitro. J Nucl Med 48: 481–486. [PubMed] [Google Scholar]
- 8. Norden MM, Larsson F, Tedelind S, Carlsson T, Lundh C, et al. (2007) Down-regulation of the sodium/iodide symporter explains 131I-induced thyroid stunning. Cancer Res 67: 7512–7517. [DOI] [PubMed] [Google Scholar]
- 9. Lundh C, Lindencrona U, Postgard P, Carlsson T, Nilsson M, et al. (2009) Radiation-induced thyroid stunning: differential effects of (123)I, (131)I, (99m)Tc, and (211)At on iodide transport and NIS mRNA expression in cultured thyroid cells. J Nucl Med 50: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 10. Meller B, Gaspar E, Deisting W, Czarnocka B, Baehre M, et al. (2008) Decreased radioiodine uptake of FRTL-5 cells after (131)I incubation in vitro: molecular biological investigations indicate a cell cycle-dependent pathway. Eur J Nucl Med Mol Imaging 35: 1204–1212. [DOI] [PubMed] [Google Scholar]
- 11. Barton KN, Stricker H, Brown SL, Elshaikh M, Aref I, et al. (2008) Phase I study of noninvasive imaging of adenovirus-mediated gene expression in the human prostate. Mol Ther 16: 1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barton KN, Stricker H, Elshaikh MA, Pegg J, Cheng J, et al. (2011) Feasibility of adenovirus-mediated hNIS gene transfer and 131I radioiodine therapy as a definitive treatment for localized prostate cancer. Mol Ther 19: 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Richard-Fiardo P, Franken PR, Harrington KJ, Vassaux G, Cambien B (2011) The use of molecular imaging of gene expression by radiotracers in gene therapy. Expert Opin Biol Ther 11: 1273–1285. [DOI] [PubMed] [Google Scholar]
- 14. Peerlinck I, Merron A, Baril P, Conchon S, Martin-Duque P, et al. (2009) Targeted radionuclide therapy using a Wnt-targeted replicating adenovirus encoding the Na/I symporter. Clin Cancer Res 15: 6595–6601. [DOI] [PubMed] [Google Scholar]
- 15. Kassis AI (2011) Molecular and cellular radiobiological effects of Auger emitting radionuclides. Radiat Prot Dosimetry 143: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cornelissen B, Vallis KA (2010) Targeting the nucleus: an overview of Auger-electron radionuclide therapy. Curr Drug Discov Technol 7: 263–279. [DOI] [PubMed] [Google Scholar]
- 17. Moore FD, Sastry KS (1982) Intracellular potassium: 40K as a primordial gene irradiator. Proc Natl Acad Sci U S A 79: 3556–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bloomer WD, Adelstein SJ (1977) 5-125I-iododeoxyuridine as prototype for radionuclide therapy with Auger emitters. Nature 265: 620–621. [DOI] [PubMed] [Google Scholar]
- 19. Ginj M, Chen J, Walter MA, Eltschinger V, Reubi JC, et al. (2005) Preclinical evaluation of new and highly potent analogues of octreotide for predictive imaging and targeted radiotherapy. Clin Cancer Res 11: 1136–1145. [PubMed] [Google Scholar]
- 20. Reilly RM, Kiarash R, Cameron RG, Porlier N, Sandhu J, et al. (2000) 111In-labeled EGF is selectively radiotoxic to human breast cancer cells overexpressing EGFR. J Nucl Med 41: 429–438. [PubMed] [Google Scholar]
- 21. Cornelissen B, Darbar S, Hernandez R, Kersemans V, Tullis I, et al. (2011) ErbB-2 blockade and prenyltransferase inhibition alter epidermal growth factor and epidermal growth factor receptor trafficking and enhance (111)In-DTPA-hEGF Auger electron radiation therapy. J Nucl Med 52: 776–783. [DOI] [PubMed] [Google Scholar]
- 22. Costantini DL, Chan C, Cai Z, Vallis KA, Reilly RM (2007) (111)In-labeled trastuzumab (Herceptin) modified with nuclear localization sequences (NLS): an Auger electron-emitting radiotherapeutic agent for HER2/neu-amplified breast cancer. J Nucl Med 48: 1357–1368. [DOI] [PubMed] [Google Scholar]
- 23. Paquet F, Barbey P, Bardies M, Biau A, Blanchardon E, et al. (2013) The assessment and management of risks associated with exposures to short-range Auger- and beta-emitting radionuclides. State of the art and proposals for lines of research. J Radiol Prot 33: R1–R16. [DOI] [PubMed] [Google Scholar]
- 24.Association NEM (2007) Performance measurement of gamma cameras.
- 25. Loening AM, Gambhir SS (2003) AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2: 131–137. [DOI] [PubMed] [Google Scholar]
- 26.Loevinger R BT, Watson EE (1991) MIRD primer for absorbed dose calculations, Revised. New York: The Society of Nuclear Medicine.
- 27. Segars WP, Tsui BM, Frey EC, Johnson GA, Berr SS (2004) Development of a 4-D digital mouse phantom for molecular imaging research. Mol Imaging Biol 6: 149–159. [DOI] [PubMed] [Google Scholar]
- 28.Cristy M EK Specific absorbed fractions of energy at various ages for internal photon sources. Oak Ridge, TN: Oak Ridge National Lab 1987(ORNL/TM-8381).
- 29. Jan S, Santin G, Strul D, Staelens S, Assie K, et al. (2004) GATE: a simulation toolkit for PET and SPECT. Phys Med Biol 49: 4543–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jan S, Benoit D, Becheva E, Carlier T, Cassol F, et al. (2011) GATE V6: a major enhancement of the GATE simulation platform enabling modelling of CT and radiotherapy. Phys Med Biol 56: 881–901. [DOI] [PubMed] [Google Scholar]
- 31. Agostinelli S AJ, Amako K (2003) GEANT4-a simulation toolkit. Nucl Inst Meth Phys Res A506: 250–303. [Google Scholar]
- 32. Matsumoto M NT (1998) Mersenne twister: a 623-dimensionally equidistributed uniform pseudo-random number generator. ACM Transactions on Modeling and Computer Simulation 8: 1. [Google Scholar]
- 33.Eckerman KF EA (2008) MIRD:Radionuclide Data and Decay Schemes Society for Nuclear Medicine.
- 34. Peyrottes I, Navarro V, Ondo-Mendez A, Marcellin D, Bellanger L, et al. (2009) Immunoanalysis indicates that the sodium iodide symporter is not overexpressed in intracellular compartments in thyroid and breast cancers. Eur J Endocrinol 160: 215–225. [DOI] [PubMed] [Google Scholar]
- 35. Huc-Brandt S, Marcellin D, Graslin F, Averseng O, Bellanger L, et al. (2011) Characterisation of the purified human sodium/iodide symporter reveals that the protein is mainly present in a dimeric form and permits the detailed study of a native C-terminal fragment. Biochim Biophys Acta 1808: 65–77. [DOI] [PubMed] [Google Scholar]
- 36. Richard-Fiardo P, Franken PR, Lamit A, Marsault R, Guglielmi J, et al. (2012) Normalisation to blood activity is required for the accurate quantification of Na/I symporter ectopic expression by SPECT/CT in individual subjects. PLoS One 7: e34086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Merron A, Baril P, Martin-Duque P, de la Vieja A, Tran L, et al. (2010) Assessment of the Na/I symporter as a reporter gene to visualize oncolytic adenovirus propagation in peritoneal tumours. Eur J Nucl Med Mol Imaging 37: 1377–1385. [DOI] [PubMed] [Google Scholar]
- 38. Tran L, Ouisse LH, Richard-Fiardo P, Franken PR, Darcourt J, et al. (2013) Adrenal gland infection by serotype 5 adenovirus requires coagulation factors. PLoS One 8: e62191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Le Brigand K, Barbry P (2007) Mediante: a web-based microarray data manager. Bioinformatics 23: 1304–1306. [DOI] [PubMed] [Google Scholar]
- 40. Mariotti LG, Pirovano G, Savage KI, Ghita M, Ottolenghi A, et al. (2013) Use of the gamma-H2AX Assay to Investigate DNA Repair Dynamics Following Multiple Radiation Exposures. PLoS One 8: e79541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zuckier LS, Dohan O, Li Y, Chang CJ, Carrasco N, et al. (2004) Kinetics of perrhenate uptake and comparative biodistribution of perrhenate, pertechnetate, and iodide by NaI symporter-expressing tissues in vivo. J Nucl Med 45: 500–507. [PubMed] [Google Scholar]
- 42. Josefsson M, Grunditz T, Ohlsson T, Ekblad E (2002) Sodium/iodide-symporter: distribution in different mammals and role in entero-thyroid circulation of iodide. Acta Physiol Scand 175: 129–137. [DOI] [PubMed] [Google Scholar]
- 43. Reinhardt HC, Schumacher B (2012) The p53 network: cellular and systemic DNA damage responses in aging and cancer. Trends Genet 28: 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rudqvist N, Parris TZ, Schuler E, Helou K, Forssell-Aronsson E (2012) Transcriptional response of BALB/c mouse thyroids following in vivo astatine-211 exposure reveals distinct gene expression profiles. EJNMMI Res 2: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kotzerke J, Wendisch M, Freudenberg R, Runge R, Oehme L, et al. (2012) Sodium-iodide symporter positive cells after intracellular uptake of (99m)Tc versus alpha-emitter 211At. Reduction of clonogenic survival and characterization of DNA damage. Nuklearmedizin 51: 170–178. [DOI] [PubMed] [Google Scholar]
- 46. Freudenberg R, Wendisch M, Runge R, Wunderlich G, Kotzerke J (2012) Reduction in clonogenic survival of sodium-iodide symporter (NIS)-positive cells following intracellular uptake of (99m)Tc versus (188)Re. Int J Radiat Biol 88: 991–997. [DOI] [PubMed] [Google Scholar]
- 47. Wendisch M, Freudenberg R, Drechsel J, Runge R, Wunderlich G, et al. (2010) [99mTc reduces clonogenic survival after intracellular uptake in NIS-positive cells in vitro more than 131I]. Nuklearmedizin 49: 154–160. [DOI] [PubMed] [Google Scholar]
- 48. Wunderlich G, Wendisch M, Aurich D, Runge R, Freudenberg R, et al. (2012) Preincubation with Sn-complexes causes intensive intracellular retention of (99m)Tc in thyroid cells in vitro. Nuklearmedizin 51: 179–185. [DOI] [PubMed] [Google Scholar]
- 49. Leoni SG, Kimura ET, Santisteban P, De la Vieja A (2011) Regulation of thyroid oxidative state by thioredoxin reductase has a crucial role in thyroid responses to iodide excess. Mol Endocrinol 25: 1924–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of the 306 RefSeq annotated transcripts significantly modulated in 99mTcO4−-exposed thyroids. Agilent Probe and NCBI RefSeq IDs give access to transcript annotations. Logarithm (base 2) of the average intensity and logarithm (base 2) of the ratio 99mTcO4 −/control are represented.
(DOCX)
Ingenuity Pathway Analysis of microarray data to highlight selectively affected pathways in 99mTcO4−-exposed thyroids. Affected canonical pathways ordered by p-value are presented. The probability of obtaining the number of genes in a certain pathway in the list of differentially expressed genes was compared with the representation of the same pathway among all the genes on the microarray; –log10 of the Fisher's exact probability is indicated.
(DOCX)
Dosimetric calculations.
(DOCX)