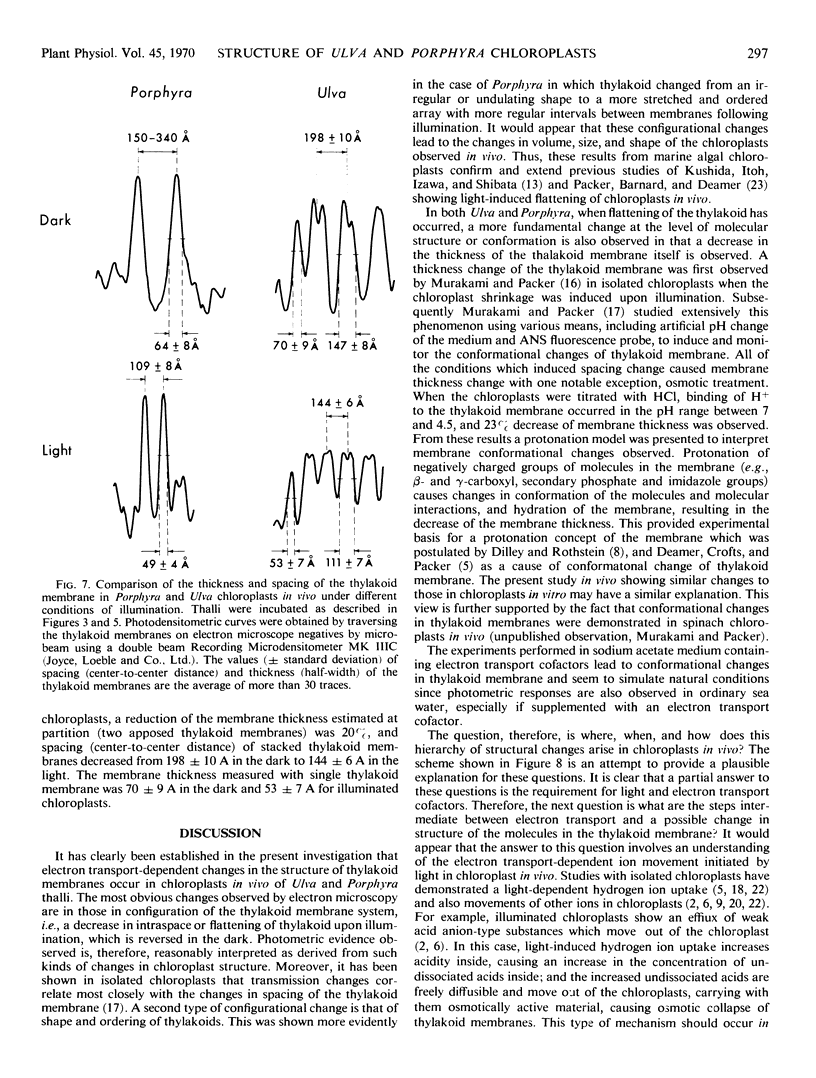
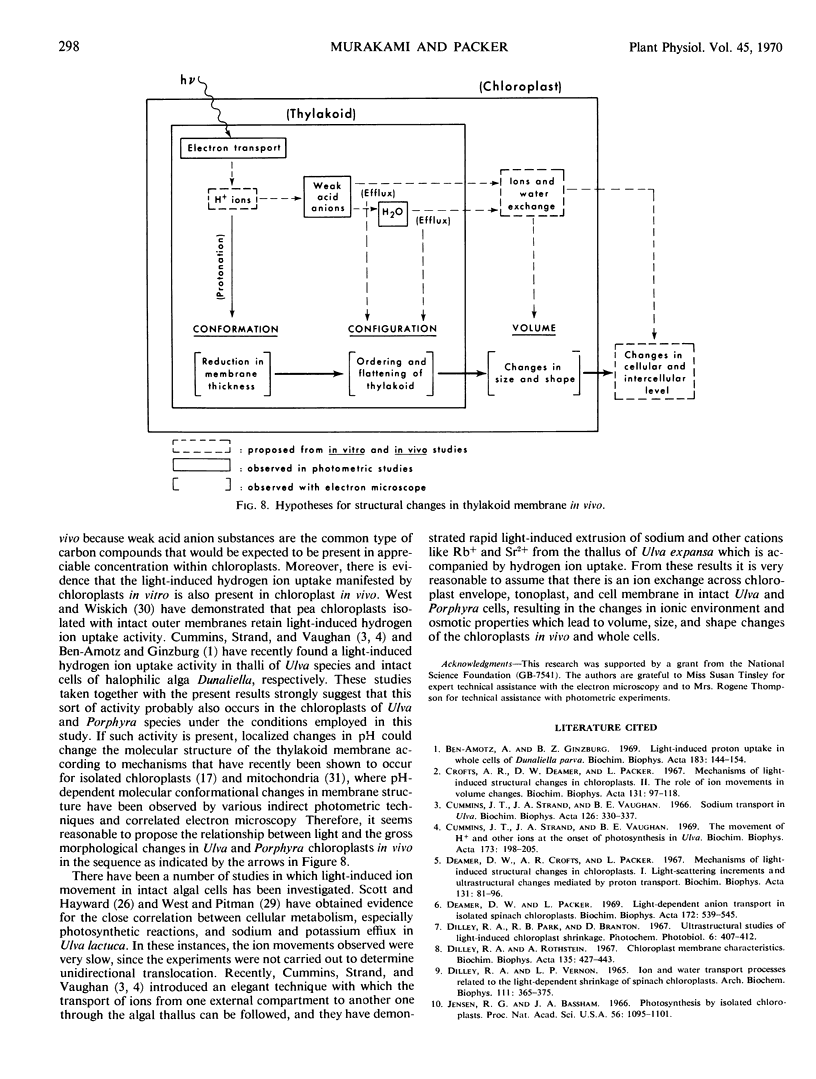
Abstract
The ultrastructural basis of light-induced transmission and light scattering changes of thalli of Ulva and Porphyra were investigated by high resolution electron microscopy and microdensitometry. The results show that upon illumination of dark thalli (a) a reduction in thickness of thylakoid membranes (conformational change), (b) a more regular ordering, and (c) flattening of the thylakoids (configurational changes) have occurred. An explanation for the observed conformational and configurational changes was proposed in terms of correlated changes in ionic environment and osmotic properties of chloroplasts in vivo which are initiated by photosynthetic reactions.
Full text
PDF

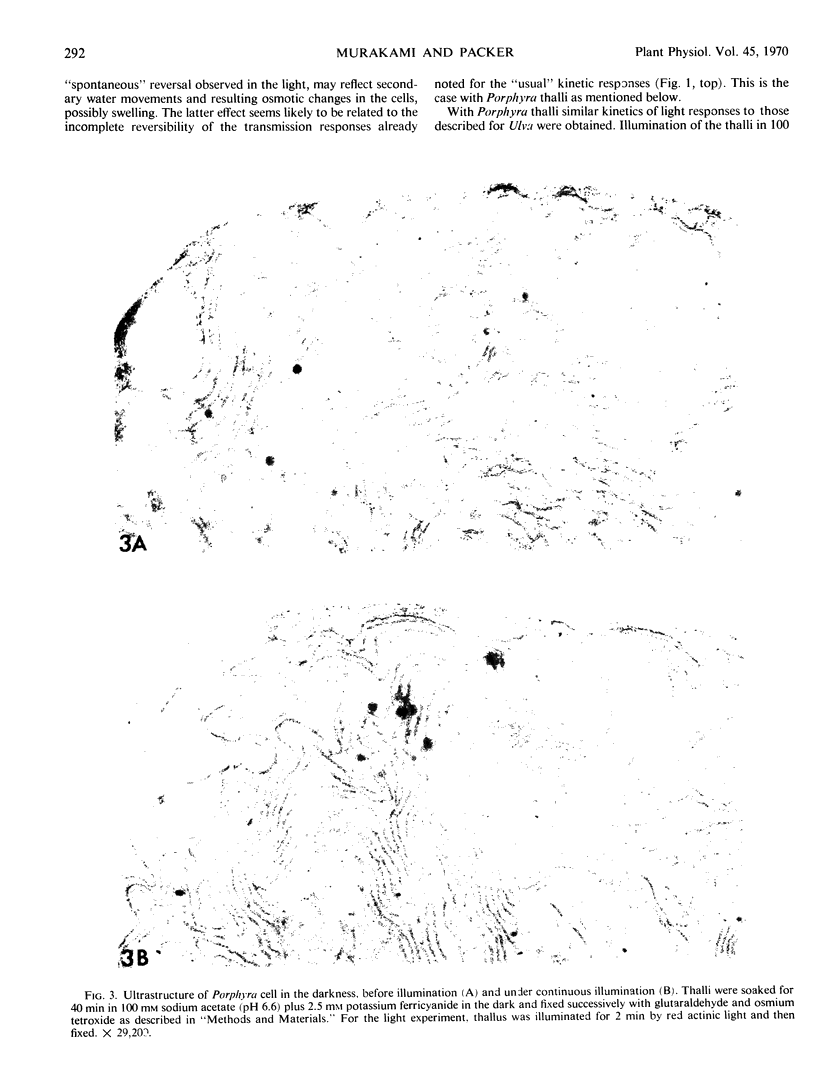

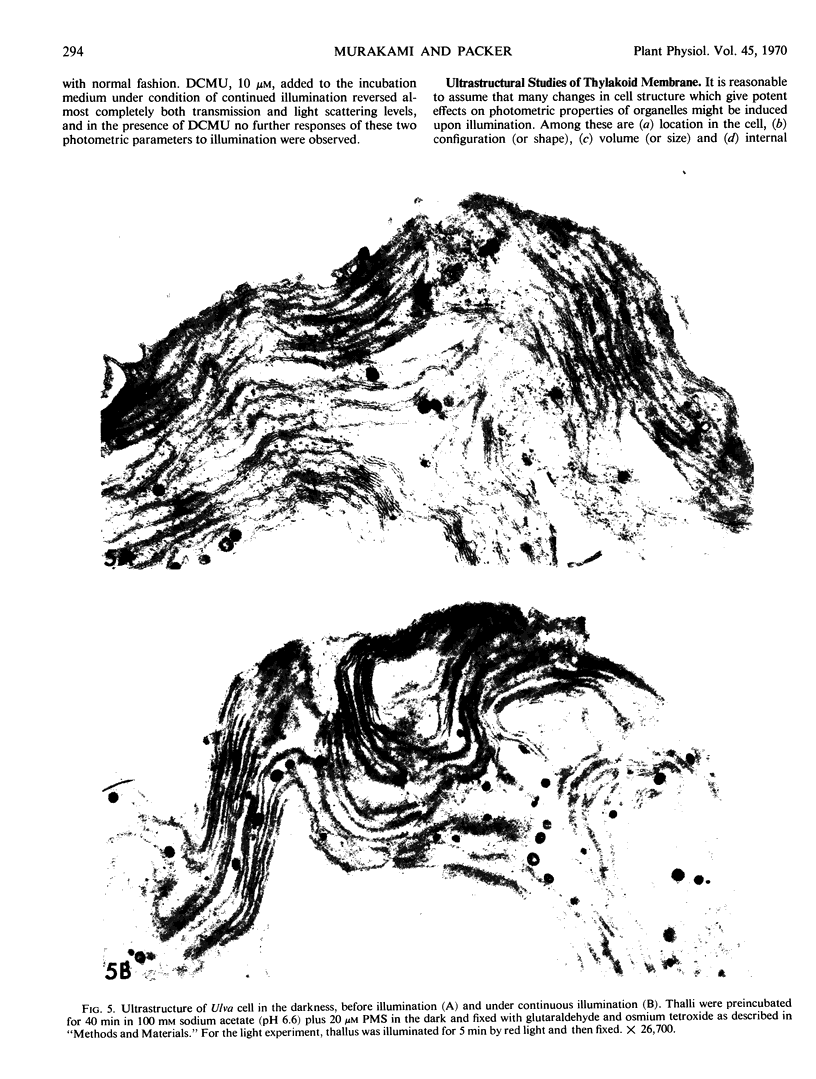
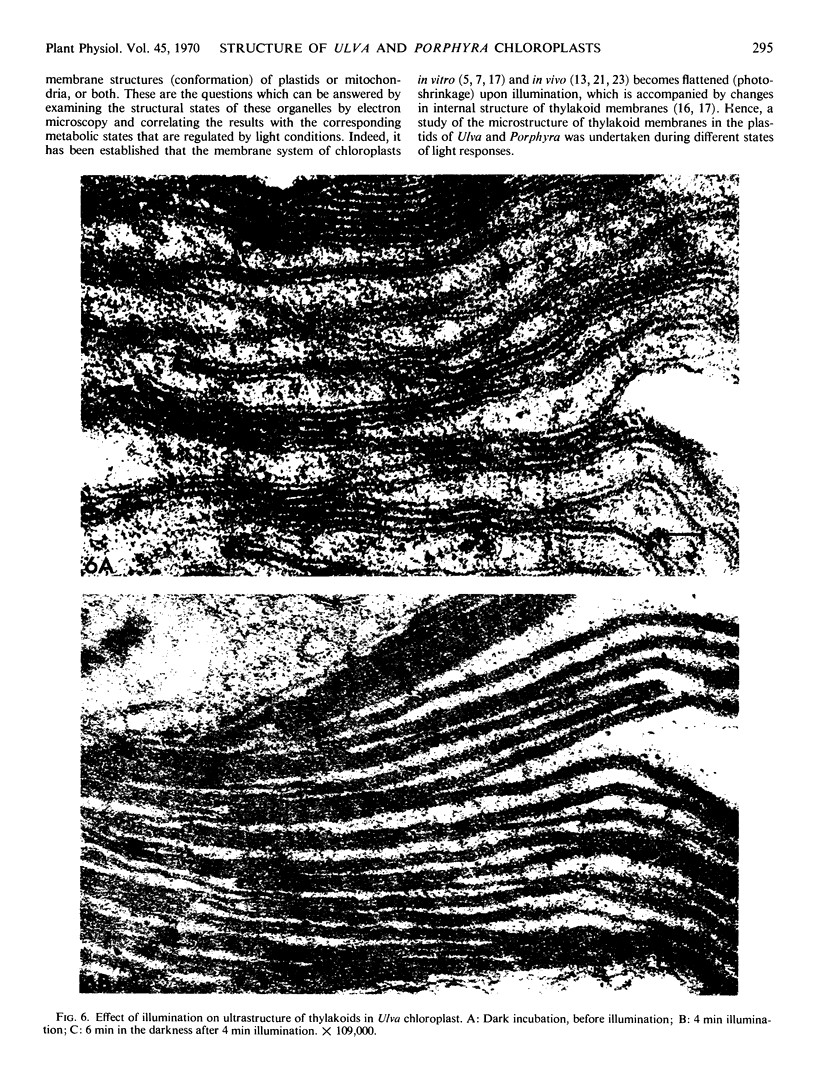
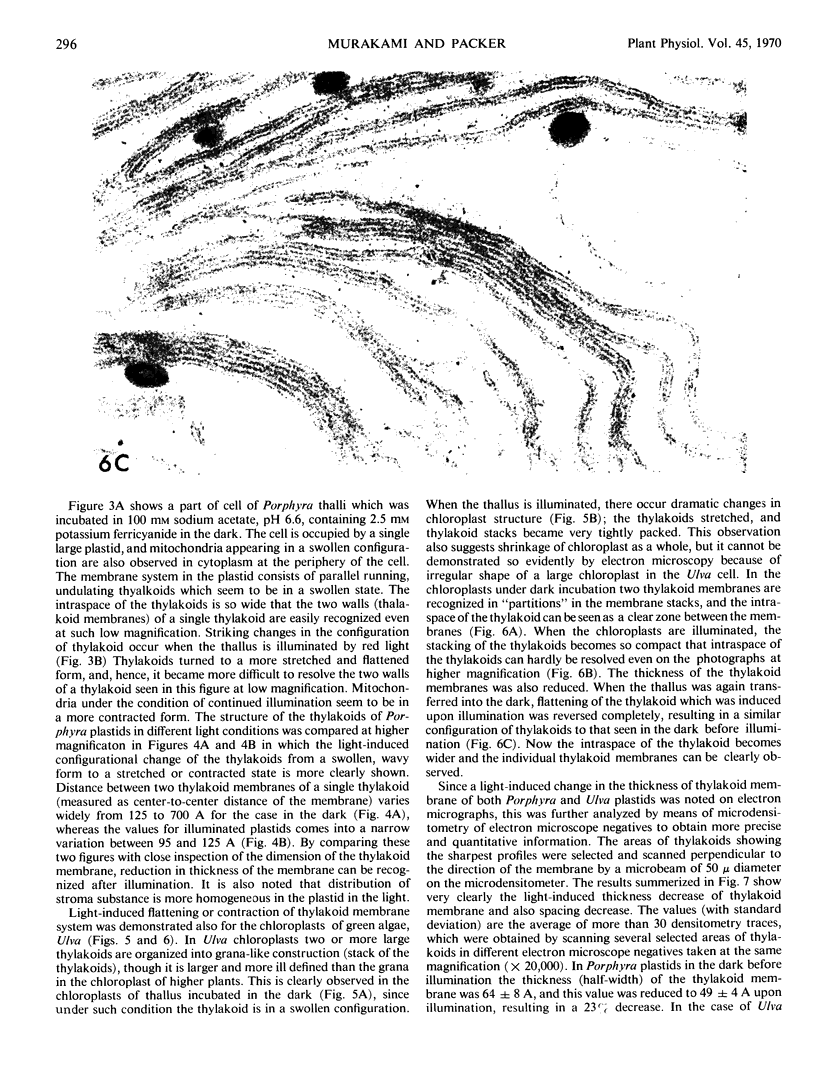

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Amotz A., Ginzburg B. Z. Light-induced proton uptake in whole cells of Dunaliella parva. Biochim Biophys Acta. 1969 Jun 3;183(1):144–154. doi: 10.1016/0005-2736(69)90138-2. [DOI] [PubMed] [Google Scholar]
- Cummins J. T., Strand J. A., Vaughan B. E. The movement of H+ and other ions at the onser of photosynthesis in ulva. Biochim Biophys Acta. 1969 Mar 11;173(2):198–205. doi: 10.1016/0005-2736(69)90103-5. [DOI] [PubMed] [Google Scholar]
- Cummins J. T., Strand J. A., Vaughn B. E. Sodium transport in ulva. Biochim Biophys Acta. 1966 Oct 10;126(2):330–337. doi: 10.1016/0926-6585(66)90070-7. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Packer L. Light-dependent anion transport in isolated spinach chloroplasts. Biochim Biophys Acta. 1969 Apr 8;172(3):539–545. doi: 10.1016/0005-2728(69)90149-2. [DOI] [PubMed] [Google Scholar]
- Dilley R. A., Rothstein A. Chloroplast membrane characteristics. Biochim Biophys Acta. 1967 Jul 3;135(3):427–443. doi: 10.1016/0005-2736(67)90032-6. [DOI] [PubMed] [Google Scholar]
- Dilley R. A., Vernon L. P. Ion and water transport processes related to the light-dependent shrinkage of spinach chloroplasts. Arch Biochem Biophys. 1965 Aug;111(2):365–375. doi: 10.1016/0003-9861(65)90198-0. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson U. G., Porter K. R. Fine structure of cell division in Chlamydomonas reinhardi. Basal bodies and microtubules. J Cell Biol. 1968 Aug;38(2):403–425. doi: 10.1083/jcb.38.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUSHIDA H., ITOH M., IZAWA S., SHIBATA K. DEFORMATIONS OF CHLOROPLASTS ON ILLUMINATION IN INTACT SPINACH LEAVES. Biochim Biophys Acta. 1964 Jan 27;79:201–203. doi: 10.1016/0926-6577(64)90051-8. [DOI] [PubMed] [Google Scholar]
- Kalberer P. P., Buchanan B. B., Arnon D. I. Rates of photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1542–1549. doi: 10.1073/pnas.57.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACROBBIE E. A. THE NATURE OF THE COUPLING BETWEEN LIGHT ENERGY AND ACTIVE ION TRANSPORT IN NITELLA TRANSLUCENS. Biochim Biophys Acta. 1965 Jan 25;94:64–73. doi: 10.1016/0926-6585(65)90008-7. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Murakami S., Packer L. Reversible changes in the conformation of thylakoid membranes accompanying chloroplast contraction or expansion. Biochim Biophys Acta. 1969 Jun 24;180(2):420–423. doi: 10.1016/0005-2728(69)90128-5. [DOI] [PubMed] [Google Scholar]
- NEUMANN J., JAGENDORF A. T. LIGHT-INDUCED PH CHANGES RELATED PHOSPHORYLATION BY CHLOROPLASTS. Arch Biochem Biophys. 1964 Jul;107:109–119. doi: 10.1016/0003-9861(64)90276-0. [DOI] [PubMed] [Google Scholar]
- Nobel P. S., Chang D. T., Wang C. T., Smith S. S., Barcus D. E. Initial ATP Formation, NADP Reduction, CO(2) Fixation, and Chloroplast Flattening Upon Illuminating Pea Leaves. Plant Physiol. 1969 May;44(5):655–661. doi: 10.1104/pp.44.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel P. S. Chloroplast shrinkage and increased photophosphorylation in vitro upon illuminating intact plants of Pisum sativum. Biochim Biophys Acta. 1968 Jan 15;153(1):170–182. doi: 10.1016/0005-2728(68)90157-6. [DOI] [PubMed] [Google Scholar]
- Nobel P. S. Light-induced changes in the ionic content of chloroplasts in Pisum sativum. Biochim Biophys Acta. 1969 Jan 14;172(1):134–143. doi: 10.1016/0005-2728(69)90098-x. [DOI] [PubMed] [Google Scholar]
- Packer L., Allen J. M., Starks M. Light-induced ion tranpsort in glutaraldehyde-fixed chloroplasts: studies with nigericin. Arch Biochem Biophys. 1968 Oct;128(1):142–152. doi: 10.1016/0003-9861(68)90017-9. [DOI] [PubMed] [Google Scholar]
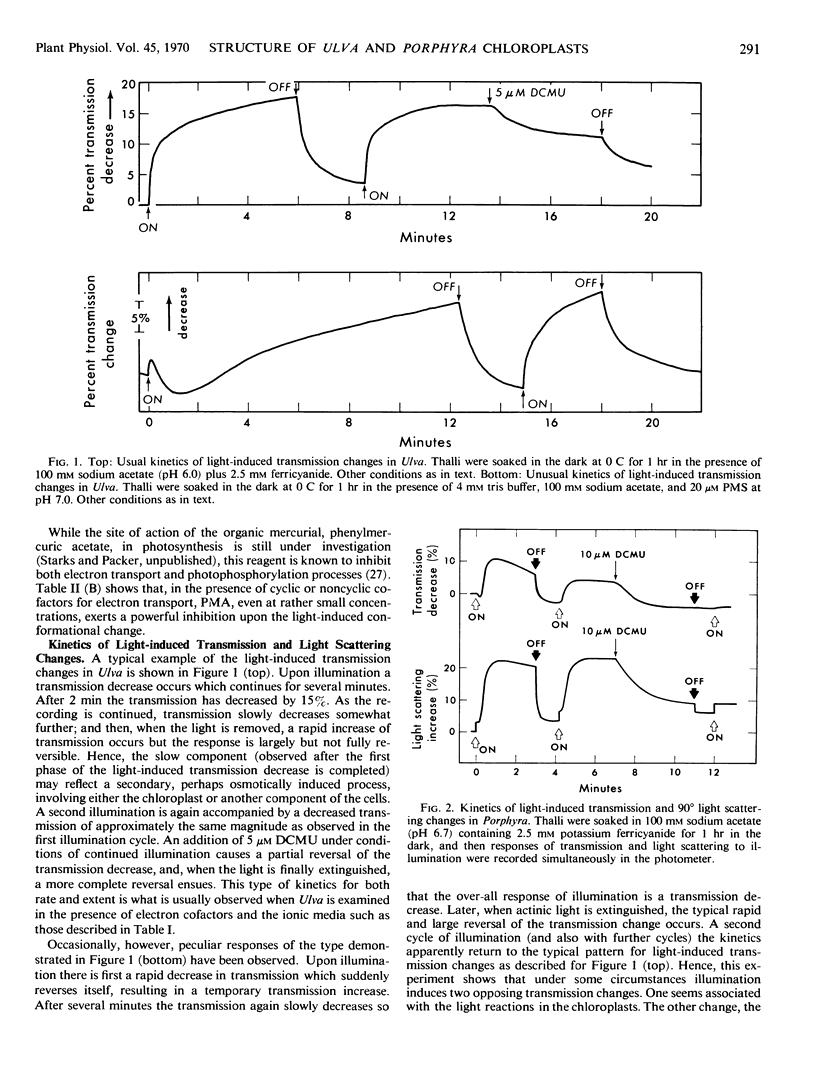
- Packer L., Barnard A. C., Deamer D. W. Ultrastructural and Photometric Evidence for Light-Induced Changes in Chloroplast Structure in vivo. Plant Physiol. 1967 Feb;42(2):283–293. doi: 10.1104/pp.42.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L., Deamer D. W., Crofts A. R. Conformational changes in chloroplasts. Brookhaven Symp Biol. 1966;19:281–302. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT G. T., HAYWARD H. R. Evidence for the presence of separate mechanisms regulating potassium and sodium distribution in Ulva lactuca. J Gen Physiol. 1954 May 20;37(5):601–620. doi: 10.1085/jgp.37.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A. Correlation between Photosynthetic Activity and Membrane Integrity in Isolated Pea Chloroplasts. Plant Physiol. 1965 Nov;40(6):1157–1161. doi: 10.1104/pp.40.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West K. R., Wiskich J. T. Photosynthetic control by isolated pea chloroplasts. Biochem J. 1968 Oct;109(4):527–532. doi: 10.1042/bj1090527. [DOI] [PMC free article] [PubMed] [Google Scholar]