Opinion statement
White matter disease is commonly detected on brain MRI of aging individuals as white matter hyperintensities (WMH), or ‘leukoaraiosis.” Over the years it has become increasingly clear that the presence and extent of WMH is a radiographic marker of small cerebral vessel disease and an important predictor of the life-long risk of stroke, cognitive impairment, and functional disability. A number of large population-based studies outlined the significance of WMH as a biomarker for long-term cerebrovascular disease and dementia, and in this review we describe the conceptual framework and methodology that support this association and link the existing knowledge to future lines of investigations in the field.
Ischemic stroke and leukoaraiosis – a definition for the 21st century
The definition of “stroke” has undergone a significant evolution throughout the decades of cerebrovascular studies. Most recently, the expert consensus statement from the American Heart Association/American Stroke Association underlined the importance of objective clinical (neuroimaging) or laboratory (pathological) evidence of cerebral infarction, or brain cell death attributable to ischemic injury in a defined vascular distribution, to be present for a diagnosis of ischemic stroke, whether symptomatic or silent.[1] In addition, a common condition that is found on brain MRI of asymptomatic aging adults is WMH, or “leukoaraiosis”. Defined by Hachinski in 1985"leukoaraiosis” implies “diminished density of white matter which is seen on brain computed tomography (CT),” which in turn is hyperintense on T2-weighted, proton-density, and fluid-attenuated inversion recovery (FLAIR) brain MRI sequences.[2–3] Over the years, MRI has demonstrated greater sensitivity of detecting abnormal white matter, including lesions not otherwise visible on head CT. [4
Radiographic assessment of WMH severity
There are two main radiographic approaches to assessment of WMH severity. First, the visual grade rating scales that are used to measure white matter lesion (WML) on CT or MRI are based on the site and the severity of white matter disease. MRI-based scales, such as the Fazekas scale, grades WML in both periventricular (PVH) and deep white matter locations (score 0–3). [5] The Scheltens scale added the location, size, and number of WML in PVH (score 0–6), WMH (score 0–24), basal ganglia hyperintensities (BG) (score 0–30) and infratentorial foci of hyperintensities (ITF) (score 0–24). [6] The Rotterdam Scan Study (RSS) scale rates WML in the periventricular region (score 0–9) and subcortical WML. [7] The Age-Related White Matter Changes (ARWMC) scales for rating WMH on CT and MRI included the site, size, and number of WML (score 0–3) and basal ganglia lesions (score 0–3) in 5 different regions (frontal, parieto-occipital, temporal, basal ganglia, and infratentorial) of bilateral hemispheres.[8] Second, a volumetric approach to WMH analysis has been based on the various semiautomated protocols using analytical software such as Sparc 5 (SUN, Palo Alto, CA) [9] or MRIcro (University of Nottingham School of Psychology, Nottingham, UK; www.mricro.com), which quantify WMH volume (WMHv) on axial FLAIR of the entire brain, or just supratentorially. [10
Albeit more labor-intense, volumetric methods provide more accuracy and reliability in assessing WMH severity especially when evaluating WMH progression, as compared to the visual rating scales. [11–12] Some WMH progression scales, such as the Rotterdam Progression and Schmidt Progression scales, are also sensitive, consistent, and relate to volumetric volume change. [12]. Clinical application of different scales may depend on its sensitivity for a specific functional domain. For example, in the Leukoaraiosis And DISability (LADIS) study, visual rating scale and volumetric methods demonstrated greater WMH burden in subjects with impaired physical performance and cognition as compared to normal controls.[13] However, volumetric assessment was more sensitive than visual scores in detecting memory symptoms. [9] Many studies used WMHv to evaluate the association between leukoaraiosis and risk of stroke, cognitive impairment, dementia, mortality, stroke severity, and other functional disability.
Burden of WMH and risk of stroke
Risk of first ever stroke in population based studies
Six large prospective, population based studies provided pivotal evidence of correlation between WMH and risk of first ever stroke. The Atherosclerosis Risk in Communities Study (ARIC) in 4 US communities followed up 1,684 persons for 4.7 years found that people with WML had a higher 5-year cumulative incidence of clinical stroke than people without WML (6.8% vs 1.4%; RR 3.4; 95%CI 1.5–7.7), independent of common stroke risk factors. [14] The Rotterdam Scan Study in the Netherlands followed 1,077 participants for an average of 4.2 years, and demonstrated that WML in both periventricular and subcortical locations significantly increased the risk of stroke. [15] The Cardiovascular Health Study (CHS) in 4 US communities included 3,293 persons followed for an average of 7 years, and showed that the risk of stroke increased proportionally to the WMH grade increase, independent of conventional stroke risk factors. [16] Based on the results of a prospective cohort study in Shimane, Japan, marked PVH and subcortical white mater lesion (SWML) burden independently increased the risk of stroke in 2,684 subjects followed for an average of 6.3 years. [17] The 3-City Dijon (France) Study of 1,643 persons followed for an average 4.9 years showed a significant increase in the risk of stroke with increasing WML. [18] And finally, the Framingham Offspring Study, which systematically evaluated 2,177 persons during 5.6 years of follow-up demonstrated that greater WMHv were associated with an increased risk of stroke (HR 2.28, 95%CI:1.02–5.13), independent of vascular risk factors. [19] The meta-analysis of these six large population based cohort studies demonstrated a significant association of WMH with risk of stroke (HR 3.1, 95%CI: 2.3–4.1, p<0.001). [20] Furthermore, a pooled population-based analysis of the ARIC and CHS, which included 4,872 stroke-free individuals followed for a median of 13 years, demonstrated that higher WMH grade on baseline MRI is a significant predictor of spontaneous intracerebral hemorrhage (ICH) (p for trend < 0.0001). [21
Risk of stroke in high risk populations
Many prospective studies evaluated WMH and risk of stroke in high risk populations. A study in 89 Japanese participants who had clinical lacunar infarction and followed up for a mean of 51 months found that extensive WMH at baseline was a significant predictor of stroke risk (RR 1.60; 95%CI 1.02–2.54; p<0.05) [22] A study in 121 American patients with lobar ICH showed that CT-based evidence of white matter damage nearly quadrupled the risk of recurrent ICH (HR 3.7, 95%CI 1.1–12.3, p= 0.02) after 2.7 years of follow-up. [10] A study of 81 Swedish patients with lacunar infarction found that severity of WML was a predictor of recurrent stroke (OR 1.7, 95%CI 1.2–2.7), when followed long-term (five years). [23] Similarly, extensive WML was associated with recurrent stroke in a study of 228 Chinese patients with stroke (p=0.0001). [24] The Amsterdam Vascular Medicine Group in the Netherlands reported that patients (n=230) with confirmed atherosclerotic disease including recent myocardial infarction (MI), ischemic stroke (IS), or peripheral arterial disease (PAD) and evidence of PVH on neuroimaging have a higher recurrent ischemic stroke rate at 3.5 years compared to those without PVH (18% versus 5%, p=0.001). [25] A study in 266 Japanese patients with ischemic stroke or ICH found that those patients with advanced WMH but no microbleeds developed had the highest recurrence rate of ischemic stroke among 3 other patient subgroups (10.5% one-year and 17.4% in two-year follow-up, HR 10.7, 95%CI 2.6–43.7). [26
Finally, combined data analyses demonstrate convincingly that WMH severity is linked to the risk of recurrent stroke: a meta-analysis from 3 studies in high risk populations reported HR 7.4, 95%CI 2.4–22.9, p=0.001, whereas pooled data from six population based studies and three high risk populations showed HR 3.5, 95%CI 2.5–4.9, p<0.001. [20
Burden of WMH and risk of vascular cognitive impairment and dementia
Risk of vascular cognitive impairment
Many population based studies evaluated the association between leukoaraiosis and the risk of cognitive impairment. Some studies used the severity of WMH at baseline but many assessed the progression of WMH longitudinally.
A study in 67 American participants with normal cognition found that high baseline WMHv was related to the risk of progression to mild cognitive impairment (MCI) (HR 3.3; 95%CI 1.33–8.2, p= 0.01). [27] However, the Framingham Offspring Study which followed up 1,694 persons for a mean duration of 6.2 years showed that the severity of WMHv did not correlate with the risk of all MCI or amnestic MCI. [19] Similarly, the Austrian Stroke Prevention Study followed 329 participants for 6 years and demonstrated that WMHv progression was associated with cognitive decline in some domains, including memory, conceptualization, and visuopractical skills. However, changes in WMHV were not related to cognitive decline after adjustment for brain volume. [28
Risk of dementia
The link between severity of leukoaraiosis and risk of dementia has been examined in a number of large prospective, population based studies, including the Cardiovascular Health Cognitive Study, in which 480 of 3,608 persons developed dementia in up to 8 years of follow-up. This study found that extensive WMH grade at study baseline was a significant risk factor for dementia (HR 1.8) and Alzheimer’s disease (AD). [29] The Rotterdam Scan Study demonstrated that WML, predominantly in the periventricular region, independently increased the risk of dementia in 1,077 cognitively intact participants at 5.2 years (HR 1.67; 95%CI 1.25–2.24). [30] Similarly, the Osaki-Tajiri Project in Japan followed 204 healthy adults and 335 participants with questionable dementia for 5 years and showed that WML was a predictor for progression to vascular dementia (VaD). [31] The Framingham Offspring Study (n=2,013) reported that severity of WMHv was significantly associated with an increased risk of dementia (HR 2.22; 95% CI 1.32–3.72 for WMHv, HR 3.97; 95% CI 1.10–14.3 for extensive WMHv) in a mean follow-up of 5.9 years. [19] Finally, a meta-analysis of three population based studies confirmed a significant association between WMH and the risk of all types dementia (HR 2.9; 95%CI 1.3–6.3, p=0.008). [20
WMH as biomarker of total burden of cerebrovascular disease
Association with mortality
Several large prospective population based studies evaluated the association between WMH and mortality. In a cohort of 2,684 neurologically normal and stroke-free Japanese subjects, the risk of death at 6.3 years was significantly greater if the subjects had obvious PVH on brain imaging (OR 4.01; 95%CI 1.91–8.45). [17] Similarly, the Rotterdam Scan Study reported increased fatality rates among 430 subjects with WML, [32] as well as the Framingham Offspring Study of 2,208 persons which showed that the severity of WMHv was associated with an increased risk of death (HR 1.38, 95%CI 1.13–1.69 for WMHv; HR 2.27, 95%CI 1.41–3.65 for extensive WMHv) in a long-term follow-up. [19] On the other hand, in the Cardiovascular Health Study, 3,245 participants with low WMH grade demonstrated improved longevity over 10–12 years of follow up. [33] Meta-analysis in these four population based studies showed a significant correlation of WMH with risk of death (HR 2.3, 95%CI 1.9–2.8, p<0.001). [20
Some studies evaluated the association of leukoaraiosis with mortality in high risk populations. A study in Southern Norway in 134 persons with first ever ICH showed that severe WMH was independently associated with both short and long term fatality in 30 day survivors. [34] Similarly, survival was reduced in 228 Chinese subjects with WML with first ever ischemic stroke, when followed for a median of 23 months (p=0.007). [24
Association with stroke severity and post-stroke outcomes
Severity of leukoaraiosis has been linked to poor functional post-stroke outcome in both short- (90 days) and long-term follow-up studies. In patients with acute ischemic stroke, severity of WMH is significantly associated with poor functional outcome at 3 months [35–39] and beyond. [39–40] When the topography of WML is considered, PVH burden, but not subcortical or deep WMH, appears to be linked to unfavorable clinical outcome in both short- and long-term (beyond 90 days) studies. [35–36, 39] A study in patients with spontaneous ICH also showed that higher leukoaraiosis burden was an independent marker of worse functional outcome. [38
Besides being a predictor of functional outcome after stroke, severity of leukoaraiosis was independently associated with larger infarct cores [41], greater infarct volume growth [42], and increased risk of hemorrhagic transformation and parenchymal hematoma following intra-arterial thrombectomy for treatment of acute ischemic stroke, especially leukoaraiosis in deep white matter region. [43
Association with other functional disability
The Leukoaraiosis and Disability (LADIS) is a multicenter collaboration evaluating the independent role of white matter change by neuroimaging in determining disability in many clinical aspects, such as functional status, cognition, mood, motor performances, and urinary problems. [44] In the LADIS study’s assessment of 639 nondisabled participants, moderate and severe white matter changes were independently associated with worsening of gait and balance, [45] whereas progression of leukoaraiosis was associated with a gradual decline in executive function test performance. [46] One-year reassessment of 619 elderly LADIS subjects with baseline functional independence demonstrated that severe WMH placed them at risk of dependency from motor and cognitive decline in a short time period.[47] Finally, the LADIS participants with severe baseline WMH and WMH progression over the course of a 3-year follow-up had a greater risk of gait and stance abnormalities, upper motor signs and finger tap slowing (fine motor movement), independently of other vascular lesions. [48
Similarly, the Oregon Brain Aging Study, which followed 104 cognitively intact participants for up to 13 years, demonstrated that increased total and periventricular WMHv at baseline and progression of PVH correlated with gradual gait worsening, whereas progression of subcortical WMH was associated with memory decline. [49
Finally, a number of studies reported the association between urinary incontinence and white matter changes. [50–52] Furthermore, urinary urgency was linked to the initial severity of WMH among the LADIS participants, independent of other confounders and vascular brain lesion. [53
Future directions
The role of WMH as a diagnostic and prognostic biomarker of cerebrovascular disease is now commonly accepted; however, the question remains whether WMH plays an active role in the pathophysiology of cerebral dysfunction linked to the severity and progression of leukoaraiosis. Novel methods of clinical research, including genome-wide association studies (GWAS) and advanced neuroimaging techniques may provide evidence of underlying disease biology in WMH. Of particular interest is the recent report on a shared genetic contribution between WMH severity assessed on brain MRIs of the healthy aging adults enrolled through the multiple population-based cohorts of the CHARGE consortium [54] and hospital-based cohorts of patients with acute ischemic stroke. [55] In a meta-analysis of WMH GWAS in 9,361 stroke–free individuals of European descent, the CHARGE consortium identified 6 novel single nucleotide polymorphisms (SNPs) from a locus on chromosome 17q25 associated with WMH burden.[54] The association of these SNPs, especially rs9894383 (p=0.0006) with WMHv in ischemic stroke patients has been replicated by the International Stroke Genetics Consortium study,[55] and it represents a major breakthrough in understanding of the shared genetic contribution to leukoaraiosis across the spectrum of small cerebral vessel disease.
Future breakthroughs are also expected to emerge from advanced neuroimaging of white matter in health and disease, among which diffusion tensor imaging (DTI) is most promising. DTI provides detailed data on the white matter structure, and in recent studies, the association between common vascular risk factors, such as hypertension and serum lipids, and DTI measures of white matter integrity has been explored in healthy adults.[56–57] If validated, these preliminary reports of the effect of resting blood pressure and serum LDL on white matter integrity warrant further assessment in subjects with advanced WMH.
Conclusion
The body of literature supports the role of WMH as a biomarker of long-standing cerebrovascular disease. Advanced neuroimaging and future studies of genetic architecture of leukoaraiosis will unravel the underlying biology of white matter disease and its role in pathophysiology of stroke, dementia and the total burden of cerebrovascular dysfunction.
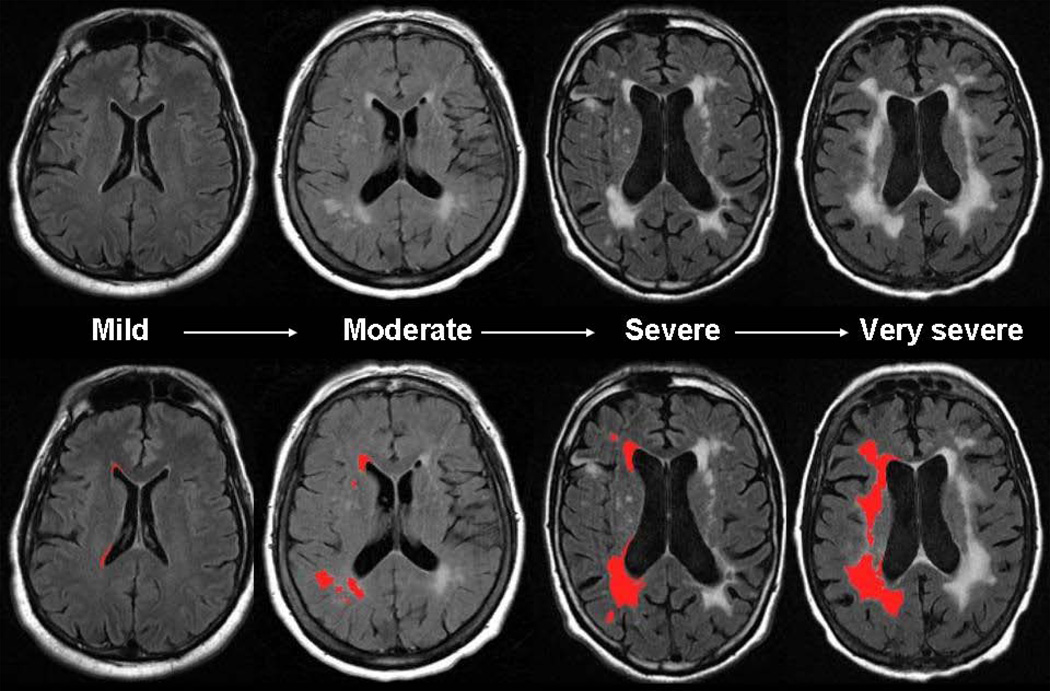
Figure 1.
Severity of MRI-detected white matter hyperintensity. Total burden of white matter disease varies significantly among asymptomatic adults and patients with known cerebrovascular disease. In age-matched individuals, white matter hyperintensity (WMH) volume may vary from mild to very severe (upper panel). Using validated, semi-automated volumetric protocol, WMH volume can be quantified (lower panel, in red, WMH maps are derived from contiguous, supratentorial axial T2-FLAIR MRI slices using previously published method [10]) with a high degree of accuracy and precision.
Table 1.
Association of WMH with stroke, recurrent stroke, intracerebral hemorrhage, hemorrhagic transformation, and mortality
| Author | Population | Results |
|---|---|---|
|
Risk of first ever stroke | ||
| Wong TY 2002 [14] | ARIC Study | 5 year cumulative incidence of clinical stroke: WML vs without WML: 6.8% vs 1.4%; RR 3.4; 95%CI 1.5–7.7 |
| Vermeer SE 2003 [15] | Rotterdam Scan Study | 3rd vs 1st PVH tertile: HR 4.7; 95%CI 2.0–11.2 3rd vs 1st SWML tertile: HR 3.6; 95%CI 1.4–9.2 |
| Kuller LH 2004 [16] | CHS | WML grades ≥ 5 vs grades 0−1: 2.8% vs 0.6%; HR 3.0; 95%CI 1.9–4.7 |
| Bokura H 2006 [17] | Shimane Study | marked vs mild PVH: OR 2.08; 95% CI 1.04–4.17 marked vs no SWML: OR 2.73; 95% CI 1.32–5.63 |
| Buyck JF 2009 [18] | 3-City Dijon Study | 4th vs 1st total WMHv quartile: HR 5.7; 95%CI 2.0–16.4 4th vs 1st PVH volume quartile: HR 6.2; 95%CI 2.0–19.5 4th vs 1st deep WMHv quartile: HR 4.1; 95%CI 1.5–11.3 |
| Debette S 2010 [19] | Framingham Offspring Study | extensive WMHv: HR 2.28; 95% CI 1.02–5.13 |
| Debette S 2010 [20] | Meta-analysis | WMH: HR 3.1; 95% CI 2.3–4.1 |
| Folsom AR 2012 [21] | ARIC and CHS Study | Risk of ICH: WMH grade 4–9 vs WMH grade 0–1: HR 3.96; 95%CI 1.90–8.27 WMH grade 3 vs WMH grade 0–1: HR 3.52; 95%CI 1.80–6.89 |
| Risk of recurrent stroke | ||
| Yamauchi H 2002 [22] | Japanese | WML score at baseline: HR 1.60; 95%CI 1.02–2.54 |
| Appelros P 2005 [23] | Swedish | WML score at baseline: HR 1.7 95%CI 1.2–2.7 |
| Fu JH 2005 [24] | Chinese | Severity of WML: HR 4.18; 95%CI 2.0–8.6 |
| Gerdes VE 2006 [25] | Amsterdam Vascular Medicine Group | PVH vs without PVH: HR 3.2; 95%CI 1.3–8.4 deep vs without deep WML: HR 1.5; 95%CI 0.6–3.8 |
| Naka H 2006 [26] | Japanese | For ischemic stroke: advanced WMH: HR 10.7; 95%CI 2.6–43.7 |
| Debette S 2010 [20] | Meta-analysis | WMH: HR 7.4; 95% CI 2.4–22.9 |
| Risk of ICH and hemorrhagic transformation | ||
| Smith EE 2004 [10] | American | For recurrent ICH: CT-WMH (grade 1) vs without CT-WMH: HR 3.7; 95%CI 1.1–12.3 |
| Naka H 2006 [26] | Japanese | For ICH: advanced WMH: HR 0.016; 95%CI 0.001–0.258 |
| Shi ZS 2012 [43] | American | Moderate or severe deep WMH on preintervention MRI: Predict hemorrhagic transformation: OR 3.43; 95% CI 1.23–9.57, after mechanical thrombectomy Predict parenchymal hematoma: OR 6.26; 95% CI 1.74–22.45, after mechanical thrombectomy |
| Mortality | ||
| Bokura H 2006 [17] | Shimane Study | marked vs mild PVH: OR 4.01; 95% CI 1.91–8.45 marked vs no SWML: OR 1.06; 95% CI 0.45–2.53 |
| Ikram MA 2009 [32] | Rotterdam Scan Study | For all cause mortality: 4th vs 1st WMH quartile: HR 2.05; 95% CI 1.32–3.20 WML per SD: HR 1.38; 95% CI 1.16–1.65 For cardiovascular mortality: WML per SD: HR 2.52; 95% CI 1.65–3.84 |
| Debette S 2010 [19] | Framingham Offspring Study |
Risk of death: WMHv: HR 1.38; 95% CI 1.13–1.69 extensive WMHv: HR 2.27; 95% CI 1.41–3.65 Risk of vascular death: WMHv: HR 1.96; 95% CI 1.13–2.92 extensive WMHv: HR 4.18; 95% CI 1.72–10.15 Risk of cardiovascular death: WMHv: HR 1.86; 95% CI 1.20–2.89 extensive WMHv: HR 3.49; 95% CI 1.30–9.37 |
| Kuller LH 2007 [33] | CHS | Ventricular grade ≥ 6: HR 1.58; 95% CI 1.21–2.07 White matter grade ≥ 5: HR 1.87; 95% CI 1.43−2.32 |
| Tveiten A 2013 [34] | Norwegian | 30-day mortality: WMH score: OR 1.6; 95%CI 1.06–2.5 Long-term mortality: WMH score: OR 1.6; 95%CI 1.2–2.1 |
| Fu JH 2005 [24] | Chinese | Survival: severity of WML: HR 2.02; 95% CI 1.032–3.960 |
| Debette S 2010 [20] | Meta-analysis | WMH: HR 2.3; 95% CI 1.9–2.8 |
Abbreviations: ARIC – Atherosclerosis Risk In Communities study, CHS – Cardiovascular Health Study, CI – confidence interval, CT – computed tomography, HR – hazards ratio, ICH – intracerebral hemorrhage, OR – odds ratio, PVH – periventricular hyperintensity, SD – standard deviation, SWML – subcortical WML, WML – white matter lesions, WMH – whit matter hyperintensity, WMHv – WMH volume
Table 2.
Association of WMH with cognitive impairment, dementia, gait abnormalities, and urinary incontinence
| Author | Population | Results |
|---|---|---|
|
Risk of vascular cognitive impairment | ||
| Smith EE 2008 [27] | American | High WMHv: HR 3.30; 95%CI 1.33–8.22 |
| Debette S 2010 [19] | Framingham Offspring Study | For MCI WMHv: OR 1.06; 95% CI 0.83–1.36 extensive WMHv: OR 1.26; 95% CI 0.67–2.39 |
| For amnestic MCI WMHv: OR 1.24; 95% CI 0.98–1.57 extensive WMHv: OR 1.67; 95% CI 0.96–2.93 |
||
| For amnestic MCI, age ≥ 60 WMHv: OR 1.49; 95% CI 1.14–1.97 extensive WMHv: OR 2.47; 95% CI 1.31–4.66 |
||
| Schmidt R 2005 [28] | Austrian stroke prevention study | WMHv: β −0.025; 95% CI −0.047 to −0.004 (memory) WMHv: β −0.022; 95% CI −0.043 to 0.0004 (conceptualization) WMHv: β −0.035; 95% CI −0.059 to −0.011 (visuopractical skills) WMHv: β −0.017; 95% CI −0.036 to −0.002 (attention/speed) |
| Risk of dementia | ||
| Kuller LH 2003 [29] | Cardiovascular Health Cognitive Study | WMH ≥ 3: HR 1.7; 95% CI 1.36–2.10 (total dementia) WMH ≥ 3: HR 1.5; 95% CI 1.17–1.99 (AD) WMH ≥ 3: HR 2.1; 95% CI 1.36–3.11 (VaD/mixed dementia) |
| Prins ND 2004 [30] | Rotterdam Scan Study | PVH: HR 1.67; 95% CI 1.25–2.24 |
| Meguro K 2007 [31] | The Osaki-Tajiri Project | PVH: OR 0.78 (non significant) (AD) Deep WMH: OR 1.07, 1.02 (right, left, non significant) (AD) PVH: OR 4.14 (p < 0.005) (VaD) Deep WMH: OR 4.04, 3.27 (right , left, p < 0.05) (VaD) |
| Debette S 2010 [19] | Framingham Offspring Study | WMHv: HR 2.22; 95% CI 1.32–3.72 extensive WMHv: HR 3.97; 95% CI 1.10–14.30 |
| Debette S 2010 [20] | Meta-analysis | WMH: HR 2.9; 95% CI 1.3–6.3 |
| Gait abnormalities | ||
| Kreisel SH 2013 [45] | LADIS Study | Slope of the Short Physical Performance Battery (SPPB): Moderate ARWMC degree: −0.22; 95%CI −0.35 to −0.09 Severe ARWMC degree: −0.46; 95%CI −0.63 to −0.28 |
| Inzitari D 2007 [47] | LADIS Study | For patients with 0 or 1 activity limited at entry Severe vs mild ARWMC: HR 2.38; 95%CI 1.29–4.38 For patients with no activity limited at entry Severe vs mild ARWMC: HR 3.02; 95%CI 1.34–6.78 |
| Poggesi A 2013 [48] | LADIS Study | Severe vs mild white matter change: OR 2.34; 95%CI 1.52–3.60 |
| Silbert LC 2008 [49] | Oregon Brain Aging Study | Total WMHv and rate of changes in timed walking in seconds: R2= 0.08, p=0.0052 |
| Total WMHv and number of steps: R2= 0.12, p= 0.0125 | ||
| Increased PVH and rate of changes in timed walking in seconds: R2= 0.12, p= 0.0039 | ||
| Increased PVH and number of steps: R2= 0.13, p= 0.0075 | ||
| Urinary incontinence | ||
| Poggesi A 2008 [53] | LADIS Study | WMH: OR 1.74; 95%CI 1.04–2.90 |
Abbreviations: AD – Alzheimer disease, ARWMC – age-related white matter changes, CI – confidence interval, HR – hazards ratio, LADIS – Leukoaraiosis and Disability study, MCI – mild cognitive impairment, OR – odds ratio, PVH – periventricular hyperintensity, SD – standard deviation, WML – white matter lesions, WMH – white matter hyperintensity, WMHv – WMH volume, VaD – vascular dementia
Acknowledgments
Dr. Rost is supported by the National Institute of Neurological Disorders and Stroke (K23 NS064052 and R01 NS082285).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Dr. Chutinet reports no disclosure.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
* Of importance
** Of outstanding importance
- 1. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. This expert consensus statement provides the most updated evidence in support of the new definition of stroke.
- 2.Hachinski VC, Potter P, Merskey H. Leuko-araiosis: an ancient term for a new problem. Can J Neurol Sci. 1986;13(4 Suppl):533–534. doi: 10.1017/s0317167100037264. [DOI] [PubMed] [Google Scholar]
- 3.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 4.Fazekas F, Barkhof F, Wahlund LO, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis. 2002;13(Suppl 2):31–36. doi: 10.1159/000049147. [DOI] [PubMed] [Google Scholar]
- 5.Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 6.Scheltens P, Barkhof F, Leys D, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114:7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- 7.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 9.van Straaten EC, Fazekas F, Rostrup E, et al. Impact of white matter hyperintensities scoring method on correlations with clinical data: the LADIS study. Stroke. 2006;37:836–840. doi: 10.1161/01.STR.0000202585.26325.74. [DOI] [PubMed] [Google Scholar]
- 10.Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606–1612. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 11.Prins ND, van Straaten EC, van Dijk EJ, et al. Measuring progression of cerebral white matter lesions on MRI: visual rating and volumetrics. Neurology. 2004;62:1533–1539. doi: 10.1212/01.wnl.0000123264.40498.b6. [DOI] [PubMed] [Google Scholar]
- 12.Gouw AA, van der Flier WM, van Straaten EC, et al. Reliability and sensitivity of visual scales versus volumetry for evaluating white matter hyperintensity progression. Cerebrovasc Dis. 2008;25:247–253. doi: 10.1159/000113863. [DOI] [PubMed] [Google Scholar]
- 13.Gouw AA, Van der Flier WM, van Straaten EC, et al. Simple versus complex assessment of white matter hyperintensities in relation to physical performance and cognition: the LADIS study. J Neurol. 2006;253:1189–1196. doi: 10.1007/s00415-006-0193-5. [DOI] [PubMed] [Google Scholar]
- 14.Wong TY, Klein R, Sharrett AR, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002;288:67–74. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- 15.Vermeer SE, Hollander M, van Dijk EJ, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 16.Kuller LH, Longstreth WT, Jr, Arnold AM, et al. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 17.Bokura H, Kobayashi S, Yamaguchi S, et al. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: a prospective cohort study. Journal of stroke and cerebrovascular diseases. 2006;15:57–63. doi: 10.1016/j.jstrokecerebrovasdis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Buyck JF, Dufouil C, Mazoyer B, et al. Cerebral white matter lesions are associated with the risk of stroke but not with other vascular events: the 3-City Dijon Study. Stroke. 2009;40:2327–2331. doi: 10.1161/STROKEAHA.109.548222. [DOI] [PubMed] [Google Scholar]
- 19.Debette S, Beiser A, DeCarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. This meta-analysis provides a comprehensive and systematic approach to evaluation of the role of leukoaraiosis in cerebrolvascular disease.
- 21.Folsom AR, Yatsuya H, Mosley TH, Jr, et al. Risk of intraparenchymal hemorrhage with magnetic resonance imaging-defined leukoaraiosis and brain infarcts. Ann Neurol. 2012;71:552–559. doi: 10.1002/ana.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamauchi H, Fukuda H, Oyanagi C. Significance of white matter high intensity lesions as a predictor of stroke from arteriolosclerosis. J Neurol Neurosurg Psychiatry. 2002;72:576–582. doi: 10.1136/jnnp.72.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appelros P, Samuelsson M, Lindell D. Lacunar infarcts: functional and cognitive outcomes at five years in relation to MRI findings. Cerebrovasc Dis. 2005;20:34–40. doi: 10.1159/000086202. [DOI] [PubMed] [Google Scholar]
- 24.Fu JH, Lu CZ, Hong Z, et al. Extent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76:793–796. doi: 10.1136/jnnp.2003.032771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerdes VE, Kwa VI, ten Cate H, et al. Cerebral white matter lesions predict both ischemic strokes and myocardial infarctions in patients with established atherosclerotic disease. Atherosclerosis. 2006;186:166–172. doi: 10.1016/j.atherosclerosis.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Naka H, Nomura E, Takahashi T, et al. Combinations of the presence or absence of cerebral microbleeds and advanced white matter hyperintensity as predictors of subsequent stroke types. AJNR Am J Neuroradiol. 2006;27:830–835. [PMC free article] [PubMed] [Google Scholar]
- 27.Smith EE, Egorova S, Blacker D, et al. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol. 2008;65:94–100. doi: 10.1001/archneurol.2007.23. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt R, Ropele S, Enzinger C, et al. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian stroke prevention study. Ann Neurol. 2005;58:610–616. doi: 10.1002/ana.20630. [DOI] [PubMed] [Google Scholar]
- 29.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 30.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 31.Meguro K, Ishii H, Kasuya M, et al. Incidence of dementia and associated risk factors in Japan: The Osaki-Tajiri Project. J Neurol Sci. 2007;260:175–182. doi: 10.1016/j.jns.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 32.Ikram MA, Vernooij MW, Vrooman HA, et al. Brain tissue volumes and small vessel disease in relation to the risk of mortality. Neurobiol Aging. 2009;30:450–456. doi: 10.1016/j.neurobiolaging.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Kuller LH, Arnold AM, Longstreth WT, Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28:1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Tveiten A, Ljostad U, Mygland A, Naess H. Leukoaraiosis is Associated with Short- and Long-term Mortality in Patients with Intracerebral Hemorrhage. J Stroke Cerebrovasc Dis. 2013 Feb 21; doi: 10.1016/j.jstrokecerebrovasdis.2013.01.017. pii: S1052-3057(13)00023-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Liou LM, Chen CF, Guo YC, et al. Cerebral white matter hyperintensities predict functional stroke outcome. Cerebrovasc Dis. 2010;29:22–27. doi: 10.1159/000255970. [DOI] [PubMed] [Google Scholar]
- 36.Kissela B, Lindsell CJ, Kleindorfer D, et al. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race. Stroke. 2009;40:530–536. doi: 10.1161/STROKEAHA.108.521906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henninger N, Lin E, Baker SP, et al. Leukoaraiosis predicts poor 90-day outcome after acute large cerebral artery occlusion. Cerebrovasc Dis. 2012;33:525–531. doi: 10.1159/000337335. [DOI] [PubMed] [Google Scholar]
- 38.Caprio FZ, Maas MB, Rosenberg NF, et al. Leukoaraiosis on magnetic resonance imaging correlates with worse outcomes after spontaneous intracerebral hemorrhage. Stroke. 2013;44:642–646. doi: 10.1161/STROKEAHA.112.676890. [DOI] [PubMed] [Google Scholar]
- 39.Kang HJ, Stewart R, Park MS, et al. White matter hyperintensities and functional outcomes at 2 weeks and 1 year after stroke. Cerebrovasc Dis. 2013;35:138–145. doi: 10.1159/000346604. [DOI] [PubMed] [Google Scholar]
- 40.Arsava EM, Rahman R, Rosand J, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology. 2009;72:1403–1410. doi: 10.1212/WNL.0b013e3181a18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henninger N, Lin E, Haussen DC, et al. Leukoaraiosis and sex predict the hyperacute ischemic core volume. Stroke. 2013;44:61–67. doi: 10.1161/STROKEAHA.112.679084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ay H, Arsava EM, Rosand J, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–1413. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]
- 43.Shi ZS, Loh Y, Liebeskind DS, et al. Leukoaraiosis predicts parenchymal hematoma after mechanical thrombectomy in acute ischemic stroke. Stroke. 2012;43:1806–1811. doi: 10.1161/STROKEAHA.111.649152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.2001–2011: a decade of the LADIS (Leukoaraiosis And DISability) Study: what have we learned about white matter changes and small-vessel disease? Cerebrovasc Dis. 2011;32:577–588. doi: 10.1159/000334498. [DOI] [PubMed] [Google Scholar]
- 45.Kreisel SH, Blahak C, Bazner H, et al. Deterioration of Gait and Balance over Time: The Effects of Age-Related White Matter Change - The LADIS Study. Cerebrovasc Dis. 2013;35:544–553. doi: 10.1159/000350725. [DOI] [PubMed] [Google Scholar]
- 46.Pantoni L, Poggesi A, Basile AM, et al. Leukoaraiosis predicts hidden global functioning impairment in nondisabled older people: the LADIS (Leukoaraiosis and Disability in the Elderly) Study. J Am Geriatr Soc. 2006;54:1095–1101. doi: 10.1111/j.1532-5415.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- 47.Inzitari D, Simoni M, Pracucci G, et al. Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: the LADIS study. Arch Intern Med. 2007;167:81–88. doi: 10.1001/archinte.167.1.81. [DOI] [PubMed] [Google Scholar]
- 48.Poggesi A, Gouw A, van der Flier W, et al. Cerebral white matter changes are associated with abnormalities on neurological examination in non-disabled elderly: the LADIS study. J Neurol. 2013;260:1014–1021. doi: 10.1007/s00415-012-6748-3. [DOI] [PubMed] [Google Scholar]
- 49.Silbert LC, Nelson C, Howieson DB, et al. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71:108–113. doi: 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goto K, Ishii N, Fukasawa H. Diffuse white-matter disease in the geriatric population. A clinical, neuropathological, and CT study. Radiology. 1981;141:687–695. doi: 10.1148/radiology.141.3.7302224. [DOI] [PubMed] [Google Scholar]
- 51.Tarvonen-Schroder S, Roytta M, Raiha I, et al. Clinical features of leuko-araiosis. J Neurol Neurosurg Psychiatry. 1996;60:431–436. doi: 10.1136/jnnp.60.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sitoh YY, Sahadevan S. Clinical significance of cerebral white matter lesions in older Asians with suspected dementia. Age Ageing. 2004;33:67–71. doi: 10.1093/ageing/afh005. [DOI] [PubMed] [Google Scholar]
- 53.Poggesi A, Pracucci G, Chabriat H, et al. Urinary complaints in nondisabled elderly people with age-related white matter changes: the Leukoaraiosis And DISability (LADIS) Study. J Am Geriatr Soc. 2008;56:1638–1643. doi: 10.1111/j.1532-5415.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- 54. Fornage M, Debette S, Bis JC, et al. Genome-wide association studies of cerebral white matter lesion burden: the CHARGE consortium. Ann Neurol. 2011;69:928–939. doi: 10.1002/ana.22403. This is the first manuscript to provide eveidence in support of association between common genetic variants and severity of leukoaraiosis.
- 55.Adib-Samii P, Rost N, Traylor M, et al. 17q25 Locus is associated with white matter hyperintensity volume in ischemic stroke, but not with lacunar stroke status. Stroke. 2013;44:1609–1615. doi: 10.1161/STROKEAHA.113.679936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salat DH, Williams VJ, Leritz EC, et al. Inter-individual variation in blood pressure is associated with regional white matter integrity in generally healthy older adults. Neuroimage. 2012;59:181–192. doi: 10.1016/j.neuroimage.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams VJ, Leritz EC, Shepel J, et al. Interindividual variation in serum cholesterol is associated with regional white matter tissue integrity in older adults. Hum Brain Mapp. 2013;34:1826–1841. doi: 10.1002/hbm.22030. [DOI] [PMC free article] [PubMed] [Google Scholar]