Significance
Ionotropic glutamate receptors are critical for excitatory transmission and plasticity in the brain and have been implicated in several neurological diseases. The GluA4 subunit of the AMPA-type glutamate receptors is expressed transiently in hippocampal CA1 principal neurons when synaptic connectivity is forming, but its physiological significance is unknown. Here we show that GluA4 expression is sufficient to alter the signaling mechanisms of synaptic plasticity and can fully explain the switch in the kinase dependency of long-term potentiation from PKA to Ca2+/calmodulin-dependent protein kinase II during synapse maturation. GluA4 expression at developing synapses confers a minimal mechanism for activity-dependent AMPA-receptor regulation to facilitate silent synapse activation during early development of glutamatergic synapses.
Keywords: glutamate receptor, hippocampus, synaptic transmission
Abstract
The AMPA-receptor subunit GluA4 is expressed transiently in CA1 pyramidal neurons at the time synaptic connectivity is forming, but its physiological significance is unknown. Here we show that GluA4 expression is sufficient to alter the signaling requirements of long-term potentiation (LTP) and can fully explain the switch in the LTP kinase dependency from PKA to Ca2+/calmodulin-dependent protein kinase II during synapse maturation. At immature synapses, activation of PKA leads to a robust potentiation of AMPA-receptor function via the mobilization of GluA4. Analysis of GluA4-deficient mice indicates that this mechanism is critical for neonatal PKA-dependent LTP. Furthermore, lentiviral expression of GluA4 in CA1 neurons conferred a PKA-dependent synaptic potentiation and LTP regardless of the developmental stage. Thus, GluA4 defines the signaling requirements for LTP and silent synapse activation during a critical period of synapse development.
Activity-dependent plasticity at immature glutamatergic synapses is thought to underlie fine tuning of the synaptic circuitry and optimize the network for its adult functions. The synaptic mechanisms of plasticity at immature contacts differ from those in the adult because of developmental alterations in the expression of several molecules that are critical in mediating and modulating synaptic transmission. For example, in area CA1 of the hippocampus, the signaling cascades necessary for long-term potentiation (LTP) are altered during the first weeks of postnatal life, corresponding to the time of formation and maturation of glutamatergic synapses. In the neonate, LTP is dependent mainly on the activation of PKA, but later in development LTP requires the activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) together with other kinases (1, 2). In parallel, expression of the AMPA-receptor subunit GluA4 in the hippocampal pyramidal neurons is strongly down-regulated and replaced by other subunits, including GluA1 (3, 4).
Both GluA4 and GluA1 and a splice variant of GluA2, GluA2L, contain a long intracellular C-terminal domain (CTD) that is thought to be involved in activity-dependent synaptic incorporation of AMPA receptors (5–8, but also see ref. 9). Spontaneous synaptic activity and consequent activity-dependent PKA phosphorylation is sufficient to drive recombinant GluA4, but not GluA1, into synapses (4, 10), suggesting that the switch in the subunit composition of AMPA receptors may explain some of the developmental changes in the mechanisms of LTP. However, the exact role of the developmentally restricted expression of GluA4 in synaptic transmission and plasticity remains unknown.
Here we show that GluA4 expression is sufficient to alter the signaling mechanisms underlying LTP and to confer PKA-dependent postsynaptic potentiation. Thus, the expression of GluA4 can explain fully the developmental switch in the LTP kinase dependency in CA1 pyramidal neurons.
Results
Activation of Postsynaptic PKA Leads to a Large Increase In Excitatory Synaptic Transmission at Immature Synapses That Is Dependent on C-Terminal Protein Interactions of GluA4.
To study the mechanisms regulating functional AMPA receptors at immature synapses, GST-fusion proteins corresponding to the CTD sequence of GluA4, GluA1, and GluA2L were applied to immature [postnatal day 4 (P4)–P6] CA1 pyramidal neurons via a patch electrode while their effects on excitatory postsynaptic currents (EPSCs) were monitored. Loading the cells with the recombinant proteins is expected to scavenge proteins interacting with the corresponding endogenous proteins and thereby perturb any physiological processes dependent on these interactions (e.g., ref. 11). None of the proteins with the long CTD significantly affected EPSC amplitude within 30 min of recording, but the GST-fusion protein containing the GluA2 short CTD, which was used as a positive control, caused a rundown of transmission (to 64 ± 7% of baseline, n = 5) (Fig. S1), as shown previously (12–14). This result indicates that dynamic protein interactions with long C-terminal tails of AMPA receptors are not involved in regulation of low-frequency transmission at immature synapses.
Proteins interacting with CTDs might selectively regulate the mobilization of AMPA receptors in response to activity (for review, see ref. 8). Therefore, we next studied the effect of forskolin, an activator of adenylate cyclase and consequently of PKA, on synaptic transmission in the neonatal hippocampus.
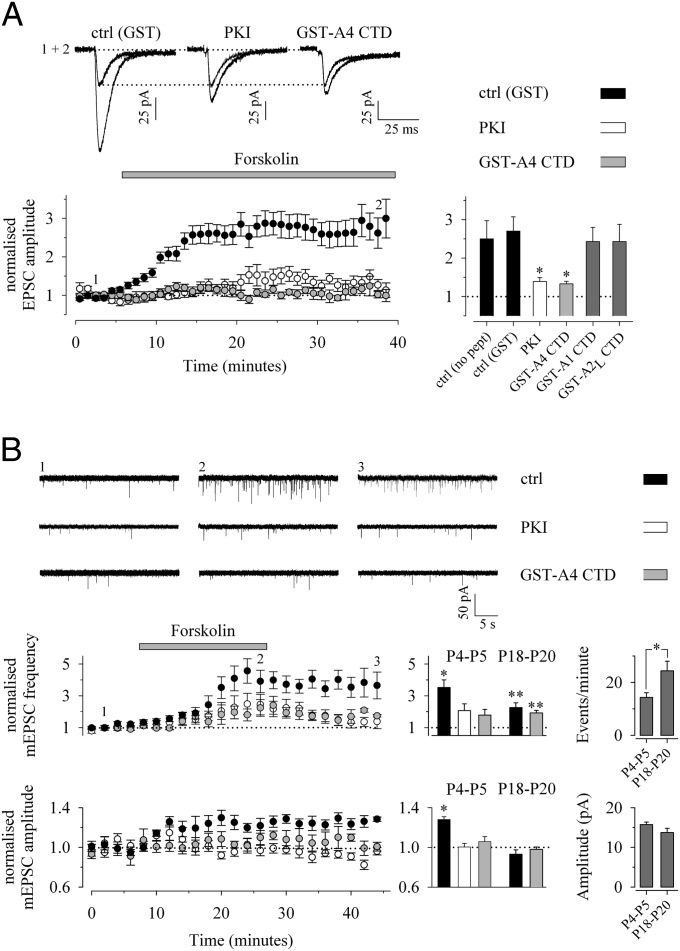
Extracellular application of forskolin (50 μM) induced a pronounced increase in EPSC amplitude (to 250 ± 48% of control, n = 9) when applied within 5–10 min after obtaining whole-cell access. After postsynaptic application of a peptide that selectively inhibits the activation of PKA (PKI) (0.1 mM), forskolin induced an increase in the EPSC amplitude to 139 ± 10% (n = 10), indicating that at this developmental stage the majority of the effect of forskolin on EPSCs was mediated by activation of postsynaptic PKA (Fig. 1A). The effect of forskolin on EPSC amplitude was strongly attenuated in the presence of GST-GluA4 CTD in the intracellular solution (133 ± 6%, n = 7) (Fig. 1A), whereas the presence of GST alone had no effect (270 ± 37%, n = 23). Also, the effect of forskolin on EPSC amplitude was not significantly affected by postsynaptic infusion of GST-GluA1 CTD (243 ± 37%, n = 6) or GST-GluA2L CTD (243 ± 45%, n = 6) (Fig. 1A). These data suggest that forskolin enhances the function of synaptic AMPA receptors in the neonatal hippocampus by activating postsynaptic PKA and that this effect is dependent on proteins selectively interacting with the GluA4 C-terminal sequence.
Fig. 1.
Activation of postsynaptic PKA induces a robust LTP and silent synapse activation at immature synapses via the mobilization of GluA4. (A) Activation of adenylate cyclase and consequently PKA by application of forskolin (50 µM) leads to a large increase in EPSC amplitude at immature (P4–P6) CA1 pyramidal neurons. Examples of traces (Upper) and a time-course plot (Lower Left) Illustrate the effect of forskolin on EPSC amplitude during postsynaptic application of GST (n = 23), the PKA inhibitor PKI (0.1 mM, n = 10), and GST-GluA4 CTD (n = 7). (Lower Right) Summary statistics on the effects of various proteins on forskolin-induced potentiation [GST-GluA1 CTD (n = 6) and GST-GluA2L CTD (n = 6)]. (B) The effect of forskolin on mEPSC frequency and amplitude under control conditions (n = 11) and in the presence of PKI (n = 7) or GST-GluA4 CTD (n = 9) in the postsynaptic cell. Examples of traces from the indicated time points (Upper) and time-course plots (Lower Left) from the recordings at P4–P5 are shown. (Lower Center) The histogram depicts pooled data on the effect of forskolin on mEPSC amplitude and frequency at P4–P5 and P18–P20 under the different recording conditions. (Lower Right) The average mEPSC frequency and amplitude at the beginning of the experiment at the two developmental time points (P4–P5: n = 27; P18–P20: n = 17). *P < 0.05; **P < 0.01.
To characterize further the PKA-dependent regulation of AMPA-receptor function at immature synapses, we studied the effect of forskolin on spontaneous, action potential-independent glutamatergic transmission [miniature EPSCs (mEPSCs)]. At P4–P5, forskolin application caused an increase in both the amplitude and frequency of mEPSCs (128 ± 3% and 353 ± 48% of control, respectively, n = 11) (Fig. 1B). Postsynaptic PKI as well as the GST-GluA4 CTD fully blocked the forskolin-induced increase in mEPSC amplitude (100 ± 4%, n = 7, and 106 ± 5%, n = 9, respectively (Fig. 1B). Interestingly, infusion of PKI or GST-GluA4 CTD to the postsynaptic neuron also significantly reduced the effect of forskolin on mEPSC frequency (206 ± 43%, and 179 ± 35%, respectively) (Fig. 1B). This result suggests that, in addition to influencing AMPA-receptor function at existing synapses, PKA activation leads to incorporation of AMPA receptors in previously silent sites, a mechanism particularly implicated in the plasticity of immature synapses (reviewed in ref. 15).
The effect of forskolin on mEPSCs and the expression of the GluA4 subunit in the CA1 pyramidal neurons are developmentally down-regulated (2, 4, 10). At P10, very little GluA4 subunit is expressed, and by P20 its levels are practically undetectable (4). In accordance, at P18–P20, no effect of forskolin on mEPSC amplitude was detected (93 ± 4%, n = 11), and its effect on mEPSC frequency (227 ± 30%) was significantly smaller than at P4–P5 (P < 0.05). At this developmental stage, inclusion of the GST-GluA4 CTD at the postsynaptic neuron had no effect on the forskolin-induced increase in transmission (n = 6) (Fig. 1B). Therefore, in CA1 pyramidal neurons, the postsynaptic effects of PKA activation are down-regulated in parallel with the expression of GluA4.
PKA Activity-Induced Increase in AMPA-Receptor Function Depends on GluA4 Expression.
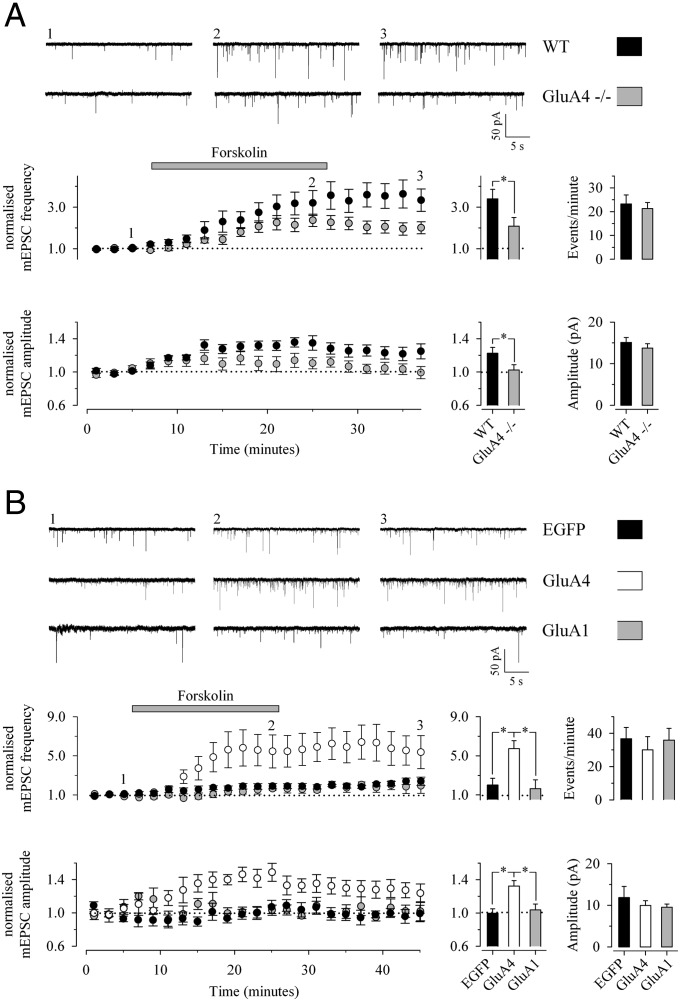
To confirm further that the expression of GluA4 accounts for the potentiation of transmission in response to PKA activation, we used genetically modified mice lacking GluA4 (16). As expected, application of forskolin in the newborn (P4–P6) WT mice caused a robust increase in both the amplitude and frequency of mEPSCs (123 ± 7% and 340 ± 45%, respectively, n =10) (Fig. 2A). In the GluA4−/− mice, however, the effect of forskolin on mEPSC frequency was significantly smaller (209 ± 41%, n = 12), and there was no effect on mEPSC amplitude (102 ± 6%, n = 12) (Fig. 2A). No differences between the genotypes in baseline frequency (WT, 23 ± 4 events/min, n = 20; GluA4−/−, 21 ± 3 events/min, n = 22) or amplitude (WT, 15 ± 1 pA; GluA4−/−, 14 ± 1 pA) were observed. The small remaining increase in mEPSC frequency in the GluA4−/− mice was similar to that observed in the presence of postsynaptic PKI in rats and thus likely was mediated by presynaptic mechanisms.
Fig. 2.
PKA activity-induced potentiation of glutamatergic transmission correlates with expression of GluA4. (A) (Upper) Examples of traces from the time points indicated. (Lower Left) Time-course plots showing the effect of forskolin on mEPSC frequency and amplitude in neonatal (P4–P6) GluA4−/− mice (n = 12) and WT controls (n = 10). (Lower Center) Summary statistics depict pooled data on the effects of forskolin on mEPSCs. (Lower Right) The average baseline mEPSC frequency and amplitude in the two genotypes (WT: n = 20; GluA4−/−, n = 22). (B) Effect of forskolin on mEPSCs in CA1 neurons that are lentivirally transduced to express EGFP (n = 7), GFP-GluA4 (n = 5), or GFP-GluA1 (n = 4) at P13–P18. Data are presented as in A. *P < 0.05.
Given that the early developmental effects of postsynaptic PKA activation on AMPA-receptor function depend on GluA4, reexpression of this subunit at more mature synapses, when endogenous expression already is down-regulated, should recapitulate the developmental phenotype. To test this hypothesis, we used lentiviral vectors to produce persistent expression of GFP-tagged GluA4 at the CA1 pyramidal neurons in vivo and studied the effect of forskolin on mEPSCs in the infected neurons at P13–P18 in vitro. Lentiviral vectors encoding for GFP-GluA1 and EGFP were used as a controls.
Forskolin application induced a large increase in mEPSC frequency in cells expressing GFP-GluA4 (573 ± 81%, n = 5) that was similar to or even greater than the increase observed in neonates and significantly larger than the increase in control (EGFP) or GFP-GluA1–infected neurons (202 ± 69%, n = 7, and 164 ± 91%, n = 4, respectively) (Fig. 2B). A forskolin-induced increase in mEPSC amplitude was observed in the neurons expressing GluA4 (132 ± 6%) but not in control (EGFP) or GFP-GluA1–infected neurons (100 ± 5% and 104 ± 7%, respectively) (Fig. 2B). No significant differences were observed in the baseline frequency or amplitude of mEPSCs in the neurons infected with the various constructs (Fig. 2B). Thus, reexpression of GluA4 at the mature synapses is sufficient for the PKA-dependent potentiation of synaptic AMPA-receptor function.
Neonatal LTP Is PKA Independent in the Absence of GluA4.
Having established that GluA4 is critical for the large PKA-induced increase in transmission at immature synapses, we went on to study whether this mechanism contributes to neonatal LTP.
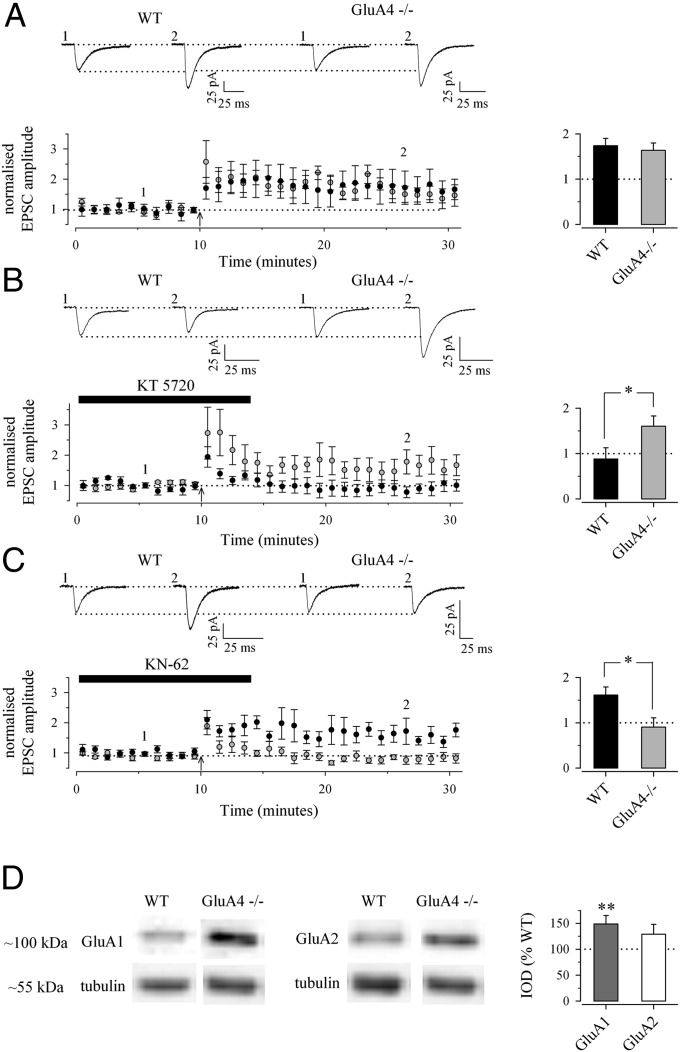
Perforated patch-clamp recordings were made from CA1 pyramidal neurons in WT and GluA4−/− mice, and EPSCs were evoked by Schaffer collateral stimulation in the presence of picrotoxin. LTP was induced by pairing postsynaptic depolarization (delivered at −10 mV) with short bursts (10× five pulses at 50 Hz, with 5-s intervals) of afferent stimulation. This protocol induced stable input-specific LTP (Fig. S2). There was no significant difference in the level of potentiation between WT and GluA4−/− slices 30 min after induction (173 ± 16%, n = 7, and 164 ± 16%, n = 7, respectively) (Fig. 3A).
Fig. 3.
LTP in neonatal GluA4−/− mice is PKA independent and CaMKII dependent. (A) Perforated patch-clamp recordings of neonatal LTP in WT (n = 7) and GluA4−/− (n = 7) mice (P5–P8). Examples of traces (Upper), time-course plots (Lower Left), and summary statistics (Lower Right) show no differences in the average level of pairing-induced LTP between the genotypes. (B) The effect of the PKA antagonist KT5720 (1 µM) on neonatal LTP in WT (n = 5) and GluA4−/− (n = 6) mice. Data are presented as in A. *P < 0.05. (C) The effect of the CaMKII antagonist KN62 (3 µM) on neonatal LTP in WT (n = 10) and GluA4−/− mice (n = 8) mice. Data are presented as in A. *P < 0.05. (D) (Left) Western blot showing significantly higher expression of the GluA1 subunit and a slightly increased level of the GluA2 subunit of AMPA receptors in the CA1 area of P5–P6 GluA4−/− mice as compared with WT mice. Tubulin blots from the same samples indicate equal protein levels. (Right) The histogram shows quantified data (values normalized to the tubulin level in each sample) from WT (n = 4) and GluA4−/− (n = 3) mice. **P = <0.01.
As reported previously, the PKA antagonist KT5720 (1 µM) fully blocked LTP in the WT mice at this developmental stage (2, 17). However, in the GluA4−/− mice, application of KT5720 had no effect on LTP, and a potentiation similar to that observed under control conditions was induced (Fig. 3B). In contrast, antagonism of Ca2+/calmodulin-dependent protein kinase II (CaMKII) by KN-62 (3 µM) blocked LTP in the GluA4−/− mice but had no effect on LTP in the WT slices (Fig. 3C). This result indicates that GluA4 is responsible for the PKA dependency of LTP at the immature synapses. In the absence of GluA4, LTP is CaMKII dependent already at the early developmental stages. Consistently, Western blot analysis indicated a significant increase in the expression of GluA1 (149 ± 16%) and a small, nonsignificant increase in the expression of the GluA2 subunit (129 ± 19%) in hippocampal extracts from the GluA4−/− mice (n = 3) as compared with extracts from WT mice (n = 4) (Fig. 3D).
Expression of GluA4 at Mature Synapses Is Associated with PKA-Dependent LTP.
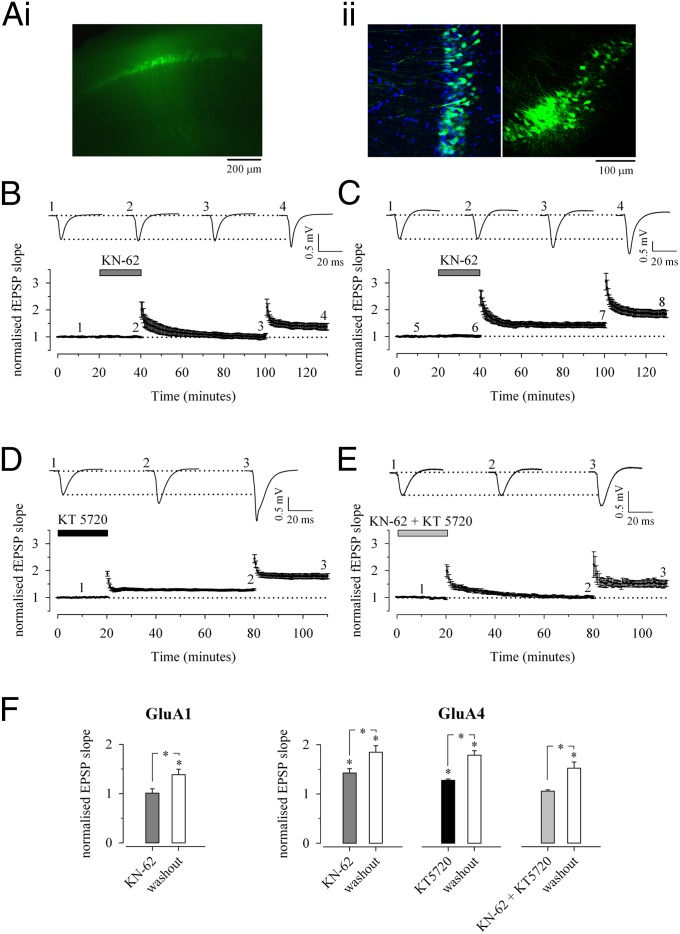
To study whether GluA4 expression would change the signaling requirements of LTP at mature synapses, we again used the lentiviral vectors to produce the lasting expression of GFP-GluA4 in the CA1 pyramidal neurons in vivo. Acute slices were cut from adult rats (on average 41 ± 3 d old), and slices with a clear, widespread fluorescence in the CA1 region were selected for electrophysiological recordings (Fig. 4 A, i). Based on confocal imaging, GFP-GluA4 was expressed on average in 52 ± 9% of all DAPI-stained cells in the CA1 pyramidal layer, with the infection rate reaching 79% at the site of injection (Fig. 4 A, ii).
Fig. 4.
Expression of GluA4 in the adult confers PKA-dependent LTP. (A) Expression of lentivirally transduced GFP-GluA4 in CA1 pyramidal neurons. (i) Fluorescent visualization of the GFP signal in an acute slice. (ii) (Left) A higher-resolution confocal image (single plane) illustrating GFP-GluA4 signal (green) in approximately half of the DAPI-stained nuclei (blue) in the CA1 pyramidal region. (Right) The site of injection has a locally very high (∼80%) infection rate. (B) fEPSP recordings of LTP from >P27 mice lentivirally transduced to express GFP-GluA4 or GFP-GluA1 in the CA1 region. Examples of traces (Upper) and averaged data (Lower) show that LTP was fully and reversibly blocked by the CaMKII antagonist KN-62 (3 µM) in slices expressing GFP-GluA1 (n = 8). (C) Similar data show that KN-62 (3 µM) has no effect on LTP in slices expressing GFP-GluA4 (n = 8). (D) Partial block of LTP in slices expressing GFP-GluA4 (n = 14) by application of the PKA antagonist KT5720 (1 µM). (E) Block of LTP in slices expressing GluA4 (n = 9) by the coapplication of KN-62 (3 µM) and PKA antagonist KT-5720 (1 µM). (F) Pooled data on the reversible effects of the kinase inhibitors on LTP in slices expressing GFP-GluA4 or GFP-GluA1. *P < 0.05.
The high infection rate allowed us to study the signaling mechanisms of LTP in the CA1 area using field recordings. LTP was induced by tetanic stimulation (100 Hz, 1 s) in the presence of the antagonists for PKA and/or CaMKII, the key kinases implicated in LTP at immature and adult synapses, respectively. The capability for plasticity was confirmed by delivering a second tetanus after washout of the drug in each slice. In slices where GFP-GluA1 was expressed, LTP was fully blocked by the CaMKII antagonist KN-62 (101 ± 9%, n = 8) (Fig. 4B), as established previously for WT animals at this developmental stage (1, 2). In slices expressing GFP-GluA4, however, a significant and long-lasting potentiation was induced in the presence of KN-62 (142 ± 9%, n = 8) (Fig. 4C). In the slices expressing GFP-GluA4, LTP was partially blocked by the PKA antagonist KT5720 (128 ± 3%, n = 14) and was fully blocked by the coapplication of KN-62 and KT5720 (105 ± 3%, n = 9) (Fig. 4 D and E), indicating that the expression of GluA4 was sufficient to alter LTP-signaling mechanisms and render LTP PKA dependent at mature synapses.
Discussion
The mechanisms regulating AMPA receptors in synaptic plasticity are widely studied; these studies have focused mainly on the molecular determinants controlling synaptic targeting of GluA1 and GluA2, the subunits responsible for glutamatergic transmission between principal neurons in the adult hippocampus. Less is known about the corresponding mechanisms at immature CA3–CA1 synapses, where the GluA4 subunit is expressed transiently. Here, we show that the expression of GluA4 alters the signaling requirements of LTP. At pyramidal neurons expressing GluA4, PKA activation is both necessary and sufficient to drive functional AMPA receptors to synapses and to produce LTP. Physiologically, this mechanism is selective for immature synapses, because of developmental down-regulation of GluA4 expression.
CaMKII represents a key signaling molecule underlying LTP induction at mature CA3–CA1 synapses, as supported by strong experimental evidence (reviewed in ref. 18). In young animals, however, the contribution of CaMKII is less pronounced or nonexistent, and LTP critically depends on the activity of PKA (1, 2, 17).
Previous work has suggested a role for GluA4 in PKA-dependent neonatal LTP, based on findings that spontaneous neuronal activity or PKA phosphorylation is sufficient to deliver AMPA receptors containing recombinant GluA4, but not GluA1, to synapses (4, 10). In agreement, we found that the activation of PKA induced postsynaptic potentiation that is dependent on the C-terminal protein interactions of GluA4 at immature synapses. Interestingly, CTD interactions of GluA1 and GluA2L appeared to have little or no effect on PKA-dependent regulation of endogenous AMPA receptors at this developmental stage. These findings suggest that critical interactions are unique to GluA4 and do not involve those common to GluA4 and either GluA1 or GluA2L (e.g., interaction with protein 4.1N).
Postsynaptic application of the GluA4 CTD completely and selectively blocked the forskolin-induced increase in mEPSC amplitude and also strongly inhibited the associated increase in mEPSC frequency. The increase in mEPSC frequency manifests as an increase in the number of functional synapses that is postsynaptically best explained by insertion or activation of AMPA receptors at previously silent sites (15). Unsilencing high-potency synapses, for example by inserting homomeric GluA4 receptors with high conductance (19), also could explain the increase in average mEPSC amplitude. However, modulation of AMPA receptors at existing functional synapses (e.g., by direct phosphorylation or GluA4 insertion) is equally plausible. The regulation of both mEPSC frequency and amplitude in response to PKA activation was developmentally down-regulated in parallel with the loss of GluA4 expression and was highly correlated with the level of GluA4 expression in genetically modified systems. Therefore, our data strongly suggest that PKA-dependent activation of silent synapses at the immature hippocampus is mediated solely by synaptic insertion or activation of a GluA4 subunit containing AMPA receptors.
Given that neonatal LTP is fully blocked by PKA antagonism (2, 17) and that regulation of GluA4 underlies PKA- dependent synaptic potentiation, the genetic absence of GluA4 might be expected to perturb synaptic transmission and LTP at immature synapses. However, GluA4−/− mice apparently had unaltered spontaneous glutamatergic drive (mEPSCs) and LTP similar to that in the WT mice, which was PKA independent. This finding indicates that the PKA/GluA4-dependent mechanism is not indispensable for activity-dependent plasticity and the development of immature synapses but may be compensated for by other mechanisms. In fact, we observed that genetic loss of GluA4 was associated with increased expression of the GluA1 subunit, which underlies CaMKII-dependent LTP at mature synapses (7, 8). Indeed, LTP induction in the immature GluA4−/− slices was blocked fully by CaMKII antagonism, suggesting that the CaMKII/GluA1-dependent mechanism compensates for the loss of PKA-dependent LTP at the immature GluA4−/− synapses.
A widely accepted model for activity-dependent regulation of synaptic AMPA receptors involves a two-step mechanism, with PKA -dependent insertion of AMPA receptors to extrasynaptic membranes followed by synaptic incorporation via lateral diffusion (5, 7, 8, 20–23). Early in development, spines, postsynaptic and extrasynaptic scaffolds, which may limit lateral diffusion of AMPA receptors at the postsynaptic membranes, are not yet present (24, 25). Their absence could explain, in part, why PKA-dependent regulation of AMPA receptors is sufficient for LTP at immature but not mature synapses. However, our data show that this mechanism also can operate in the mature neurons; thus the inherent properties of GluA4 and GluA1 are responsible for the developmental switch in LTP-signaling mechanisms.
GluA1 contains C-terminal motifs that are not present in GluA4, including a PDZ ligand-interacting site and a CaMKII phosphorylation site. These motifs are proposed to act as a retention mechanism to keep GluA1, but not GluA4, away from the synapses in a manner that is relieved by CaMKII phosphorylation (26, 27). Our data fully agree with this scenario and suggest that the retention sequence in GluA1, although not obligatory for LTP (9, 28), is critical for its CaMKII dependency.
Interestingly, when available, the PKA/GluA4-dependent mechanisms appeared to be dominant over the CaMKII/GluA1-dependent LTP. We estimated that lentiviral infection produced expression of GluA4 in ∼50% of the CA1 pyramidal neurons in adult slices. Field LTP in these slices was partially blocked by PKA antagonism, thus suggesting that PKA-dependent LTP was expressed in the neurons where GluA4 was available. On the other hand, in the absence of GluA4, the CaMKII/GluA1-dependent LTP was uncovered at the immature synapses.
To conclude, we show that GluA4 expression at developing synapses confers a minimal mechanism for activity-dependent regulation of AMPA receptors to facilitate the activation of silent synapses early in the development of glutamatergic synapses. Unsilencing allows the nascent synapses to respond to network activity in a precise and frequency-dependent manner, allowing fine tuning by the circuit activity. Indeed, the early (GluA4-containing) synapses are labile and highly susceptible to activity-dependent regulation both pre- and postsynaptically (17, 29–31). After the critical period of circuit development, parallel signaling requirements of LTP caused by the loss of GluA4 make plasticity more controlled and therefore suitable for the functions within the mature network.
Materials and Methods
Animals.
Experiments were performed on 4- to 55-d-old Wistar rats and 4- to 8-d-old WT or GluA4−/− mice kindly provided by Hannah Monyer (University of Heidelberg, Heidelberg) (13). All experiments with animals were done in accordance with the University of Helsinki Animal Welfare Guidelines.
Electrophysiology.
Acute parasagittal hippocampal slices (400 µm) were prepared using standard methods (17, 29). Whole-cell and perforated patch-clamp recordings were made from CA1 pyramidal neurons, and field recordings were made from the CA1 stratum radiatum as described in SI Materials and Methods. The relevant purified GST fusion proteins were prepared as described (32) and were included in the intracellular solution (0.5 µM) during whole-cell recording when indicated. Encoded residues were A1827–907 (UniProtKB no. P19490); A2Long834–901 (UniProtKB no. P19491-3); A4835–902 (UniProtKB no. P19493); and A2Short834-883 (UniProtKB no. P19491-1).
Lentiviral Vectors.
The cDNA encoding rat GluA1 and GluA4 (both as flip isoforms and both with GFP fused to the extracellular N terminus after the signal peptide) (33) were subcloned into pLen vector containing two separate synapsin1 promoters, the first driving the expression of GFP-tagged AMPA receptor and the second driving separate coexpression of EGFP, using standard methods. The lentiviral vectors were prepared and injected in the CA1 area of 0- to 5-d-old rat pups under isoflurane anesthesia as described in SI Materials and Methods and in ref. 34. Acute slices were cut at P13–P55 and were used for electrophysiological recordings or analysis of the GluA4 expression level. For estimating the infection rate, 250-µM-thick slices were fixed with 4% (wt/vol) paraformaldehyde, stained with 300 nM DAPI in 0.1% Triton X-100 in PBS, and mounted. The percentage of GFP+ CA1 pyramidal neurons of DAPI-stained cell nuclei in the CA1 pyramidal layer was counted from a 150 × 150 µm area of confocal images.
Western Blot.
The level of AMPA-receptor subunit expression was analyzed as described previously (35). Hippocampi were isolated from 5-d-old mice pups, and samples of equal protein concentration were submitted to Western blotting with the following antibodies: monoclonal rat anti–α-tubulin (1:5,000; Synaptic Systems), rabbit polyclonal anti-GluA2 (1:1,000; Synaptic Systems), rabbit anti-GluA1CTD (1:2,000; 36). Protein expression levels were quantified based on the optical density of the bands and were normalized against the tubulin level in each sample.
Data Analysis.
Data were collected and analyzed online using LTP software (www.ltp-program.com; 37) or Axoscope 9.2 (Axon Instruments). Uncompensated series resistance was monitored, and cells were discarded if this parameter varied by more than 20%. The amplitude of the evoked synaptic responses was measured as the peak relative to the average baseline level before the stimulation. mEPSCs were analyzed with the template search algorithm in the MiniAnalysis 6.0.3 program (Synaptosoft Inc.). All detected events were verified visually, and events with amplitude less than three times the baseline rms noise level were rejected. For time-course plots, detected events were calculated in 60-s or 120-s bins.
All pooled data are given as mean ± SEM for the number of cells indicated. For the time-course plots, the data are normalized to the baseline level before drug application or induction of LTP. For the histograms, the level of potentiation was calculated as an average of successive responses in the last 10 min of the relevant dataset. All the percentage values in the text are relative to the control (baseline) level.
For statistical analysis, Student’s two-tailed t test and two-way ANOVA were used, and P < 0.05 was considered as statistically significant.
Supplementary Material
Acknowledgments
We thank Juha Simola and Marina Tibeikina for expert help in producing the lentiviral vectors and contributing to the in vivo injections, respectively. This work was supported by the Academy of Finland, the Sigrid Juselius Foundation, and the Finnish Graduate School of Neuroscience.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315769111/-/DCSupplemental.
References
- 1.Wikström MA, Matthews P, Roberts D, Collingridge GL, Bortolotto ZA. Parallel kinase cascades are involved in the induction of LTP at hippocampal CA1 synapses. Neuropharmacology. 2003;45(6):828–836. doi: 10.1016/s0028-3908(03)00336-8. [DOI] [PubMed] [Google Scholar]
- 2.Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6(1):15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]
- 3.Monyer H, Seeburg PH, Wisden W. Glutamate-operated channels: Developmentally early and mature forms arise by alternative splicing. Neuron. 1991;6(5):799–810. doi: 10.1016/0896-6273(91)90176-z. [DOI] [PubMed] [Google Scholar]
- 4.Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: Delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3(11):1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- 5.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 6.Kolleker A, et al. Glutamatergic plasticity by synaptic delivery of GluR-B(long)-containing AMPA receptors. Neuron. 2003;40(6):1199–1212. doi: 10.1016/s0896-6273(03)00722-0. [DOI] [PubMed] [Google Scholar]
- 7.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40(2):361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 8.Collingridge GL, Isaac JTR, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5(12):952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 9.Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature. 2013;493(7433):495–500. doi: 10.1038/nature11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteban JA, et al. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6(2):136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- 11.Hirbec H, et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron. 2003;37(4):625–638. doi: 10.1016/s0896-6273(02)01191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimune A, et al. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21(1):87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- 13.Song I, et al. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. 1998;21(2):393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- 14.Lüscher C, et al. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24(3):649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 15.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9(11):813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs EC, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53(4):591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Luchkina NV, Sallert M, Clarke VRJ, Taira T, Lauri SE. Mechanisms underlying induction of LTP-associated changes in short-term dynamics of transmission at immature synapses. Neuropharmacology. 2013;67:494–502. doi: 10.1016/j.neuropharm.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13(3):169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci. 1997;17(1):58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8(2):101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 22.Heine M, et al. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320(5873):201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opazo P, Choquet D. A three-step model for the synaptic recruitment of AMPA receptors. Mol Cell Neurosci. 2011;46(1):1–8. doi: 10.1016/j.mcn.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Frischknecht R, et al. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12(7):897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- 25.Czöndör K, et al. Unified quantitative model of AMPA receptor trafficking at synapses. Proc Natl Acad Sci USA. 2012;109(9):3522–3527. doi: 10.1073/pnas.1109818109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi Y, et al. Driving AMPA receptors into synapses by LTP and CaMKII: Requirement for GluR1 and PDZ domain interaction. Science. 2000;287(5461):2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 27.Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105(3):331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 28.Kim CH, et al. Persistent hippocampal CA1 LTP in mice lacking the C-terminal PDZ ligand of GluR1. Nat Neurosci. 2005;8(8):985–987. doi: 10.1038/nn1432. [DOI] [PubMed] [Google Scholar]
- 29.Lauri SE, et al. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainate receptor-mediated inhibition of glutamate release. Neuron. 2006;50(3):415–429. doi: 10.1016/j.neuron.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Huupponen J, Molchanova SM, Taira T, Lauri SE. Susceptibility for homeostatic plasticity is down-regulated in parallel with maturation of the rat hippocampal synaptic circuitry. J Physiol. 2007;581(Pt 2):505–514. doi: 10.1113/jphysiol.2007.130062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanse E, Taira T, Lauri S, Groc L. Glutamate synapse in developing brain: An integrative perspective beyond the silent state. Trends Neurosci. 2009;32(10):532–537. doi: 10.1016/j.tins.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Coleman SK, Cai C, Kalkkinen N, Korpi ER, Keinänen K. Analysis of the potential role of GluA4 carboxyl-terminus in PDZ interactions. PLoS ONE. 2010;5(1):e8715. doi: 10.1371/journal.pone.0008715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman SK, et al. Isoform-specific early trafficking of AMPA receptor flip and flop variants. J Neurosci. 2006;26(43):11220–11229. doi: 10.1523/JNEUROSCI.2301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vesikansa A, et al. Expression of GluK1c underlies the developmental switch in presynaptic kainate receptor function. Sci Rep. 2012;2:310. doi: 10.1038/srep00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huupponen J, Molchanova SM, Lauri SE, Taira T. Ongoing intrinsic synchronous activity is required for the functional maturation of CA3-CA1 glutamatergic synapses. Cereb Cortex. 2013;23(11):2754–2764. doi: 10.1093/cercor/bhs262. [DOI] [PubMed] [Google Scholar]
- 36.Cai C, Li H, Rivera C, Keinänen K. Interaction between SAP97 and PSD-95, two Maguk proteins involved in synaptic trafficking of AMPA receptors. J Biol Chem. 2006;281(7):4267–4273. doi: 10.1074/jbc.M505886200. [DOI] [PubMed] [Google Scholar]
- 37.Anderson WW, Collingridge GL. Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J Neurosci Methods. 2007;162(1-2):346–356. doi: 10.1016/j.jneumeth.2006.12.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.