Abstract
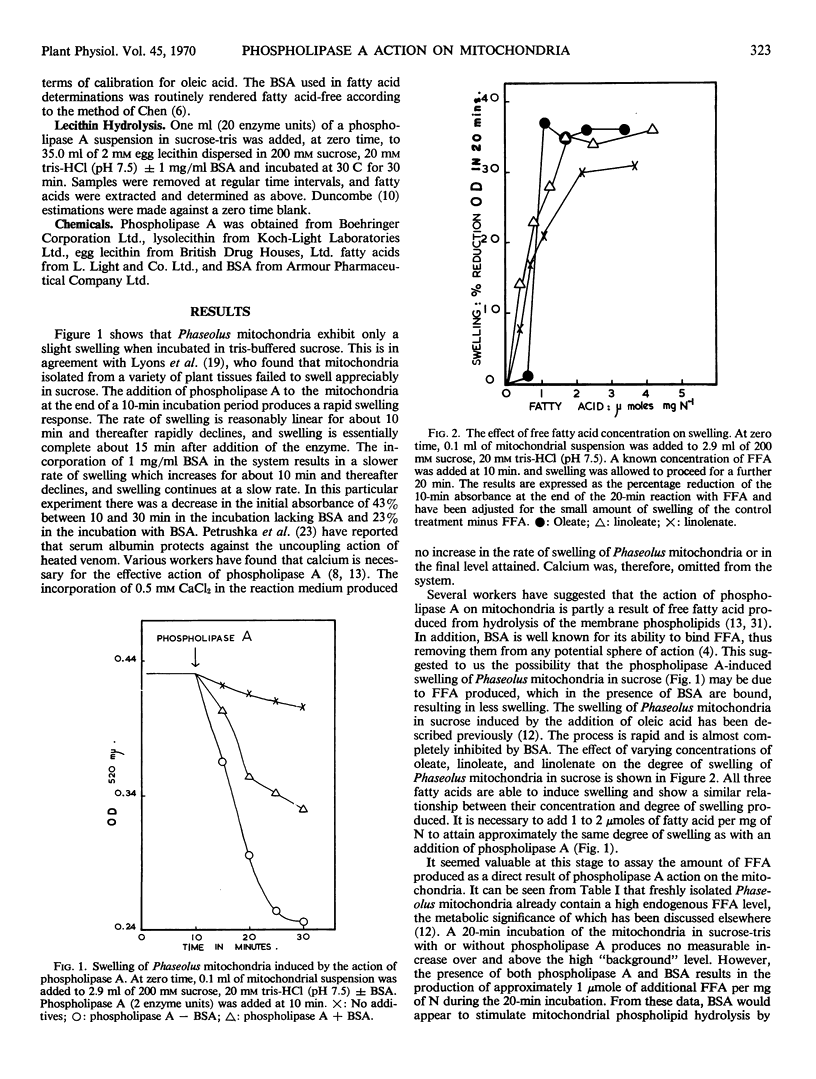
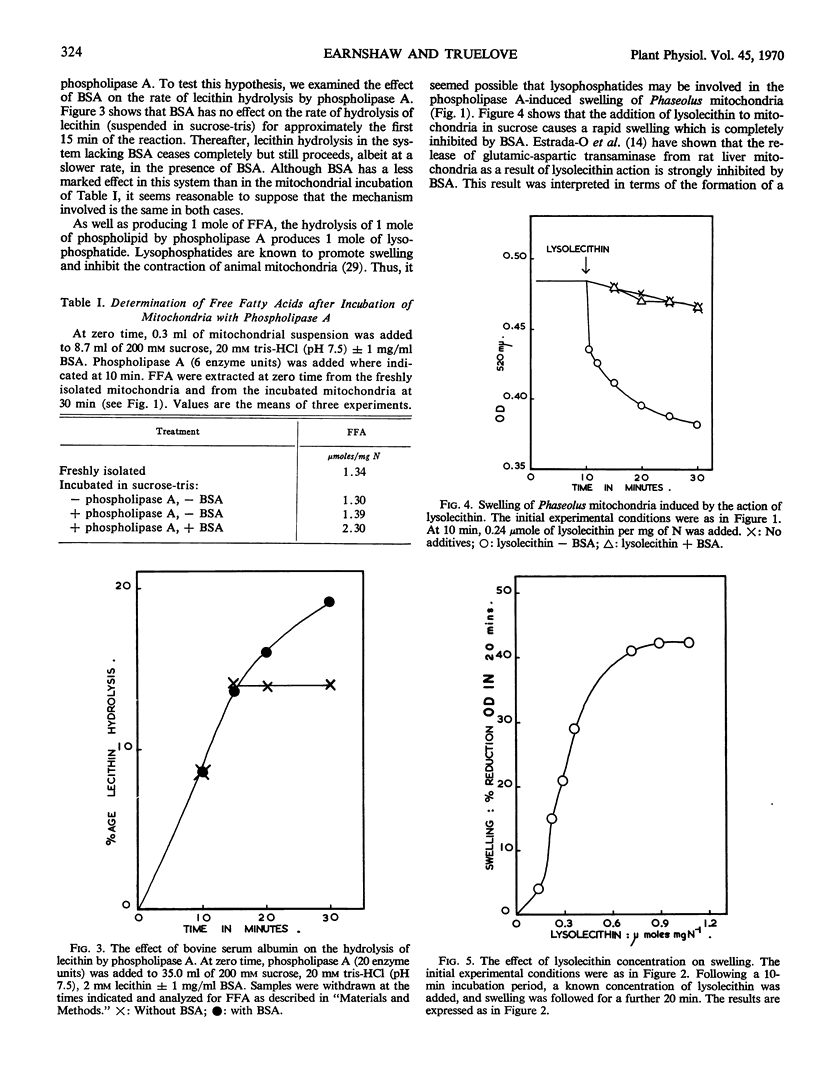
Phaseolus vulgaris mitochondria incubated in sucrose swell rapidly upon the addition of phospholipase A. Bovine serum albumin inhibits the swelling. The release of free fatty acids as a result of phospholipase A action on the mitochondria is detected only in the presence of bovine serum albumin, which promotes the hydrolysis of both mitochondrial phospholipids and purified lecithin. Either free fatty acid or lysolecithin is able to initiate an extensive mitochondrial swelling in sucrose. It is suggested that phospholipase A-induced swelling results from the release of lysophosphatides plus free fatty acids and their subsequent detergent action on the membranes rather than phospholipid loss per se.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBE K. S., CRANE F. L. Phospholipase-induced release of cytochrome c from the electron transport particle. Science. 1959 Jan 9;129(3341):98–99. doi: 10.1126/science.129.3341.98. [DOI] [PubMed] [Google Scholar]
- ARAVINDAKSHAN I., BRAGANCA B. M. Oxidative phosphorylation in brain and liver mitochondria of animals injected with cobra venom. Biochim Biophys Acta. 1959 Feb;31(2):463–466. doi: 10.1016/0006-3002(59)90022-8. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Condrea E., Rosenberg P. Demonstration of phospholipid splitting as the factor responsible for increased permeability and block of axonal conduction induced by snake venom. II. Study on squid axons. Biochim Biophys Acta. 1968 Mar 1;150(2):271–284. doi: 10.1016/0005-2736(68)90170-3. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M. ON THE MECHANISM OF ACTION OF PHOSPHOLIPASE A. Biochem J. 1963 Sep;88:414–423. doi: 10.1042/bj0880414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Duncombe W. G. The colorimetric micro-determination of long-chain fatty acids. Biochem J. 1963 Jul;88(1):7–10. doi: 10.1042/bj0880007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS S. W., BALL E. G. The action of phospholipases on succinate oxidase and cytochrome oxidase. J Biol Chem. 1954 Aug;209(2):619–633. [PubMed] [Google Scholar]
- Earnshaw M. J., Truelove B., Butler R. D. Swelling of Phaseolus mitochondria in relation to free fatty acid levels. Plant Physiol. 1970 Mar;45(3):318–321. doi: 10.1104/pp.45.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw M. J., Truelove B. Swelling and contraction of phaseolus hypocotyl mitochondria. Plant Physiol. 1968 Jan;43(1):121–129. doi: 10.1104/pp.43.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. E., FLEISCHER S. THE ROLE OF LIPIDS IN MITOCHONDRIAL ELECTRON TRANSFER AND OXIDATIVE PHOSPHORYLATION. Biochim Biophys Acta. 1963 Oct 22;70:554–582. doi: 10.1016/0006-3002(63)90793-5. [DOI] [PubMed] [Google Scholar]
- Honjo I., Ozawa K., Kitamura O., Sakai A., Ohsawa T. Rapid change of phospholipid in pancreas mitochondria during aging. J Biochem. 1968 Sep;64(3):311–320. doi: 10.1093/oxfordjournals.jbchem.a128897. [DOI] [PubMed] [Google Scholar]
- Kenefick D. G., Hanson J. B. Contracted state as an energy source for ca binding and ca + inorganic phosphate accumulation by corn mitochondria. Plant Physiol. 1966 Dec;41(10):1601–1609. doi: 10.1104/pp.41.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzio A. J. Inhibitory properties of serum proteins on the enzymatic sequence leading to lysis of red blood cells by snake venom. Toxicon. 1967 Nov;5(2):97–103. doi: 10.1016/0041-0101(67)90160-2. [DOI] [PubMed] [Google Scholar]
- Lyons J. M., Wheaton T. A., Pratt H. K. Relationship between the Physical Nature of Mitochondrial Membranes and Chilling Sensitivity in Plants. Plant Physiol. 1964 Mar;39(2):262–268. doi: 10.1104/pp.39.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYGAARD A. P., SUMMER J. B. The effect of lecithinase A on the succinoxidase system. J Biol Chem. 1953 Feb;200(2):723–729. [PubMed] [Google Scholar]
- PETRUSHKA E., QUASTEL J. H., SCHOLEFIELD P. G. Role of phospholipids in mitochondrial respiration. Can J Biochem Physiol. 1959 Aug;37(8):975–987. [PubMed] [Google Scholar]
- PETRUSHKA E., QUASTEL J. H., SCHOLEFIELD P. G. Role of phospholipids in oxidative phosphorylation and mitochondrial structure. Can J Biochem Physiol. 1959 Aug;37(8):989–998. [PubMed] [Google Scholar]
- RINGLER R. L., MINAKAMI S., SINGER T. P. Isolation and properties of the DPNH dehydrogenase of the respiratory chain from heart mitochondria. Biochem Biophys Res Commun. 1960 Oct;3:417–422. doi: 10.1016/0006-291x(60)90056-5. [DOI] [PubMed] [Google Scholar]
- ROSSI C. R., ROSSI C. S., SARTORELLI L., SILIPRANDI D., SILIPRANDI N. Protective action of phosphorylcholine on mitochondrial oxidative phosphorylation. Arch Biochem Biophys. 1962 Nov;99:214–221. doi: 10.1016/0003-9861(62)90003-6. [DOI] [PubMed] [Google Scholar]
- Sergio Estrada-O, Carabez A., Cabeza A. Effect of phospholipids on induced enzyme release from mitochondria. Biochemistry. 1966 Nov;5(11):3432–3440. doi: 10.1021/bi00875a007. [DOI] [PubMed] [Google Scholar]
- TAUB A. M., ELLIOTT W. B. SOME EFFECTS OF SNAKE VENOMS ON MITOCHONDRIA. Toxicon. 1964 Oct;2:87–92. doi: 10.1016/0041-0101(64)90008-x. [DOI] [PubMed] [Google Scholar]
- VIGNAIS P. M., VIGNAIS P. V., LEHNINGER A. L. IDENTIFICATION OF PHOSPHATIDYLINOSITOL AS A FACTOR REQUIRED IN MITOCHONDRIAL CONTRACTION. J Biol Chem. 1964 Jun;239:2011–2021. [PubMed] [Google Scholar]
- Vidal J. C., Badano B. N., Stoppani A. O., Boveris A. Inhibition of electron transport chain by purified phospholipase A from Bothrops neuwiedi venom. Mem Inst Butantan. 1966;33(3):913–920. [PubMed] [Google Scholar]
- WOJTCZAK L., LEHNINGER A. L. Formation and disappearance of an endogenous uncoupling factor during swelling and contraction of mitochondria. Biochim Biophys Acta. 1961 Aug 19;51:442–456. doi: 10.1016/0006-3002(61)90600-x. [DOI] [PubMed] [Google Scholar]
- ZIEGLER F. D., VAZQUEZ COLON L., ELLIOTT W. B., TAUB A., GANS C. ALTERATION OF MITOCHONDRIAL FUNCTION BY BUNGARUS FASCIATUS VENOM. Biochemistry. 1965 Mar;4:555–560. doi: 10.1021/bi00879a028. [DOI] [PubMed] [Google Scholar]


