Significance
Apoptotic cell-mediated suppression is critical to prevent inflammatory pathology and fatal autoimmunity. Integral to this process is early recognition by innate phagocytes driving downstream suppressive mechanisms. Though significant progress has been made identifying adaptive immune components involved in apoptotic cell-driven tolerance, early innate mechanisms involved in this process are relatively unknown. Here we report that apoptotic cell capture by CD169+ macrophages promotes rapid expression of the chemokine CCL22, inducing migration and activation of FoxP3+ Tregs and dendritic cells. Moreover, we found CCL22 function is required for generation of stable allograft tolerance and prevention of apoptotic cell-driven autoimmunity. Thus, our findings highlight a previously unknown mechanism whereby stromal macrophages coordinate early cellular interactions required for stable apoptotic cell-driven immune tolerance.
Keywords: transplantation, autoimmunity, migration, spleen, regulation
Abstract
Tolerance to apoptotic cells is essential to prevent inflammatory pathology. Though innate responses are critical for immune suppression, our understanding of early innate immunity driven by apoptosis is lacking. Herein we report apoptotic cells induce expression of the chemokine CCL22 in splenic metallophillic macrophages, which is critical for tolerance. Systemic challenge with apoptotic cells induced rapid production of CCL22 in CD169+ (metallophillic) macrophages, resulting in accumulation and activation of FoxP3+ Tregs and CD11c+ dendritic cells, an effect that could be inhibited by antagonizing CCL22-driven chemotaxis. This mechanism was essential for suppression after apoptotic cell challenge, because neutralizing CCL22 or its receptor, reducing Treg numbers, or blocking effector mechanisms abrogated splenic TGF-β and IL-10 induction; this promoted a shift to proinflammatory cytokines associated with a failure to suppress T cells. Similarly, CCR4 inhibition blocked long-term, apoptotic cell-induced tolerance to allografts. Finally, CCR4 inhibition resulted in a systemic breakdown of tolerance to self after apoptotic cell injection with rapid increases in anti-dsDNA IgG and immune complex deposition. Thus, the data demonstrate CCL22-dependent chemotaxis is a key early innate response required for apoptotic cell-induced suppression, implicating a previously unknown mechanism of macrophage-dependent coordination of early events leading to stable tolerance.
Immunologic tolerance toward apoptotic cell-associated antigens is critical for maintenance of homeostasis and prevention of autoimmune disease (1). Though several soluble effectors required for immune suppression by apoptotic cells have been described, arguably the most notable examples being the cytokines TGF-β and IL-10 (2, 3), key cellular and molecular mechanisms that promote tolerance have been more difficult to characterize; however, studies have implicated regulatory T-cell populations as essential downstream components of long-term apoptotic cell-induced regulatory immunity (4–6).
Systemic tolerance to apoptotic cells is dependent on splenic function (7). Specifically, apoptotic cell-driven tolerance requires a CD8α+CD103+ dendritic cell (DC) population found in the splenic marginal zone (MZ), which induce Treg differentiation from naïve CD4 T-cell precursors and tolerize effector CD8 T cells (8–11). However, several recent reports have indicated an indispensible role for splenic MZ resident stromal macrophages in the establishment of tolerance to apoptotic cells. For example, we recently reported deletion of MARCO+ and CD169+ macrophages promoted loss of T-cell tolerance to apoptotic cell antigens and accelerated disease in an animal model of systemic lupus erythematosus (1), and Miyake et al. (12) reported a similar result using a mouse model of experimental autoimmune encephalomyelitis (EAE).
FoxP3+ Tregs are induced in a TGF-β–dependent manner after apoptotic cell exposure and are a key downstream cellular mechanism driving apoptotic cell tolerance, because inhibition of TGF-β or reduction of Treg numbers after apoptotic cell phagocytosis (efferocytosis) is sufficient to reduce or prevent apoptotic cell-mediated tolerance in vivo (4). However, there is no information on the role preformed Treg populations play in early apoptotic cell-driven suppression or the establishment of long-term tolerance. Our data has shown rapid production of IL-10 and TGF-β occur after apoptotic cell challenge, suggesting that a suppressive microenvironment is established within hours of exposure (13). Given the rapidity of the response, apoptotic cell antigen-induced Treg populations are not involved in this early response. However, natural Tregs (nTregs) show a significant degree of self-reactivity and are critical in maintenance of immune homeostasis (14, 15). Thus, we theorized nTregs might be recruited to the site of efferocytosis, playing a role in establishment of immunosuppressive conditions elicited by apoptotic cells. Tregs migrate in response to several CC motif chemokines (16, 17). In particular, CCL22 is a prominent chemotactic agent driving Treg accumulation in tumors, and antagonism of either CCL22 or its receptor (CCR4) has potent antitumor effects (18–21). Similarly, CCL22 expression in allografts promotes Treg accumulation and prevents graft rejection (20, 22, 23), and induction of CCL22 in the pancreas of nonobese diabetic mice promoted Treg accumulation, protecting against tissue destruction and autoimmune diabetes (24).
In this report we examined if efferocytosis in the spleen induced CCL22 and the mechanistic relationship between Tregs and phagocytes in apoptotic cell-dependent suppression and tolerance. Our data demonstrate that apoptotic cells induce rapid production of CCL22 in CD169+ macrophages that drives Treg and DC accumulation in the spleen, acquisition of a tolerogenic phenotype, and induction of long-term suppression to apoptotic cell antigens. Thus, the results demonstrate a new mechanism of CCL22-coordinated recruitment by macrophages as a requisite early mechanistic step in apoptotic cell-mediated immune regulation.
Results
Apoptotic Cells Induce CCL22 Expression Specifically in CD169+ Metallophillic Macrophages.
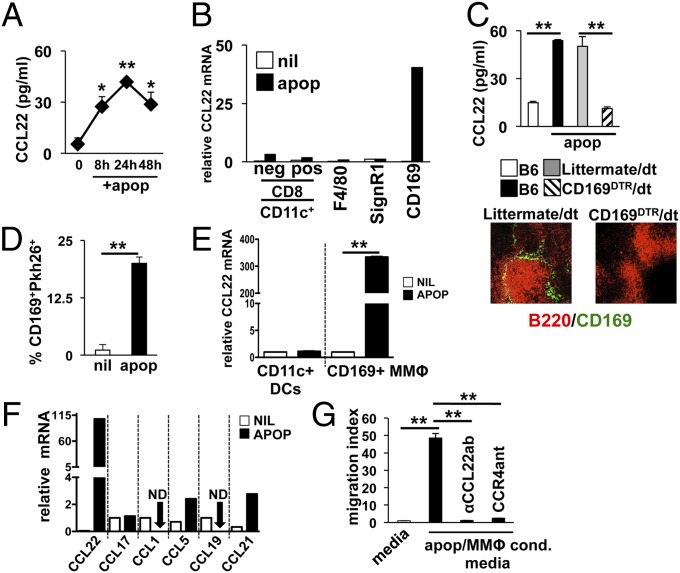
Systemic apoptotic cell challenge results in rapid production of IL-10 and TGF-β1 by MZ resident macrophages and DCs. Moreover, MZ macrophage IL-10 production plays a critical role in expansion of induced Tregs (iTregs) and efferent tolerance mechanisms (25). However, it is not known what role Tregs play in early afferent mechanisms of apoptotic cell-driven suppression. Because CCL22 is a key mediator of Treg chemotaxis and immune suppression, we hypothesized it may be essential in early innate suppression after apoptotic cell exposure. To test this, B6 mice were challenged with 107 syngeneic apoptotic cells i.v., and spleens were collected for measurement of CCL22. CCL22 protein rapidly increased in the spleen after apoptotic cell challenge with >fivefold increase observed 8 h after administration in whole-tissue preparations, peaking 24 h after apoptotic cell injection (41 ± 1.2 pg/mL) and remaining elevated for at least 48 h (Fig. 1A). Both macrophages and DCs can produce CCL22 in response to activation (26), and several MZ resident DCs and macrophage populations actively phagocytose apoptotic cells entering the spleen from circulation (27). When FACS-sorted splenic phagocytes (for sorting strategy, see Fig. S1) were examined, only CD169+ metallophillic macrophages (MMΦs) showed increased CCL22 message (288-fold increase at 4 h; Fig. 1B). To confirm apoptotic cell-driven CCL22 expression was dependent on CD169+ MΦs, we used a transgenic model in which the human diphtheria toxin receptor is expressed on CD169+ cells (12, 13). When the CD169+ macrophages were depleted before apoptotic cell injection, CCL22 was not induced (Fig. 1C), confirming CD169+ MMΦs responded selectively to apoptotic cells challenge by induction of CCL22.
Fig. 1.
Apoptotic cells induce CCL22 expression in splenic CD169+ metallophillic macrophages. (A) B6 mice were injected with 107 apoptotic thymocytes i.v. and at indicated time points, whole-spleen lysates were tested for CCL22. (B) At 4 h after apoptotic cell injection as in A, phagocytes were sorted based on indicated markers by FACS, and CCL22 message was measured by sqPCR. (C) B6.CD169DTR mice were depleted of MMΦs as described (13) and injected with apoptotic thymocytes as described in A. At 24 h later, CCL22 protein was measured in whole-spleen lysates by ELISA. Immunofluorescence shows representative spleen sections from B6.CD169DTR and littermate control groups 48 h after last injection with diphtheria toxin stained for B-cell markers (αB220, red) and αCD169 (green). (D) Splenic CD169+ cells were analyzed by FACS for uptake of Pkh26-labeled apoptotic cells 30 min after injection with 107 cells i.v. (E) CD169+ and CD11c+ cells were sorted from B6 mice and cultured in complete RPMI with apoptotic thymocytes at a 10:1 apoptotic cell/phagocyte ratio for 4 h. CCL22 message was then measured by sqPCR as described. (F) At 4 h after apoptotic cell injection as in A, CD169+ MΦs were sorted by FACS and chemokine message for the species indicated was measured by sqPCR. (G) FACS-purified splenic CD169+ macrophages were incubated with apoptotic cells at a 10:1 ratio. At 24 h, supernatant from the cultures were analyzed for chemotactic activity against MACS-purified CD4+CD25+ Tregs. In some wells, neutralizing αCCL22 antibody or CCR4 antagonist was added to confirm the dependence of migration on CCL22-dependent mechanisms as described in Materials and Methods. Bars represent mean value for five mice ± SD (A, C, and D), pooled samples from three mice (B and F), or triplicate wells (E and G). *Pval ≤ 0.05; **Pval < 0.01 as determined by Student t test. Experiments were repeated at least three times with similar results. ND, not detected.
MMΦs are positioned at the outer edge of the B-cell follicle, underneath the MadCAM+ cells lining the marginal sinus (27), and thus may have limited access to apoptotic cells entering the spleen. However, FACS analysis showed MMΦs are robust apoptotic cell phagocytes, and ∼20% of the CD169+ macrophages costained with an apoptotic cell tracer dye 30 min after i.v. injection (Fig. 1D). This result raised the possibility that apoptotic cells may induce CCL22 in MMΦs by direct induction rather than by trans mechanisms. To test this hypothesis, FACS-sorted splenic CD11c+ DCs and CD169+ MMΦs were cultured with apoptotic thymocytes at a 1:10 phagocyte/apoptotic cell ratio for 4 h, and CCL22 mRNA was measured by semiquantitative PCR (sqPCR; for cell viability, see Fig. S2). In agreement with the in vivo data, splenic CD11c+ DCs failed to induce CCL22 mRNA in coculture conditions, whereas apoptotic cells induced a 337-fold increase in CCL22 message relative to baseline in MMΦs (Fig. 1E). In contrast to CCL22, apoptotic cells did not induce significant expression of other tested CC motif chemokines in sorted CD169+ MΦs, suggesting the CCL22 response was specific and unique to apoptotic cell exposure (Fig. 1F).
Because CCL22 is a potent Treg chemokine, we reasoned that the increase in CCL22 expression might result in increased Treg chemotaxis to the spleen, promoting increased retention of Tregs in the organ. To test this, we examined the ability of CD169+ MΦ/apoptotic culture-conditioned media to induce migration of Tregs in vitro. Media conditioned with CD169+ MΦs only had no impact on Treg migration (Fig. 1G); however, when the MMΦs were cultured with apoptotic thymocytes, the conditioned media induced robust Treg migration, an effect that could be blocked by the addition of either a CCL22-neutralizing antibody or the soluble CCR4 antagonist C-021 dihydrochloride (28, 29) (Fig. 1G; for dosage, see SI Material and Methods). Thus, apoptotic cell-driven MMΦ activation potently stimulated Treg migration in a manner dependent on CCL22-mediated recruitment of Tregs via CCR4.
Apoptotic Cells Induce Rapid Treg and DC Accumulation in the Follicle Following Apoptotic Cell Challenge.
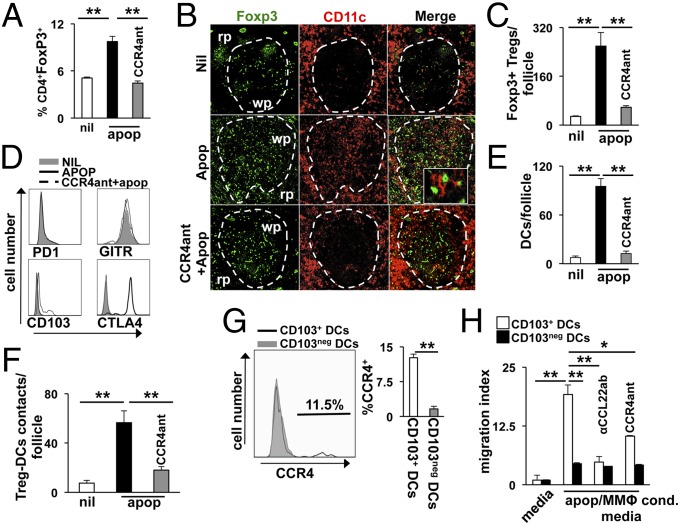
To test if this mechanism is relevant in vivo, we challenged mice i.v. with 107 apoptotic cells and examined FoxP3+ Treg recruitment 4 h after injection ± pretreatment with CCR4 antagonist. Mice injected with apoptotic cells showed a twofold increase in the percentage of splenic FoxP3+ Tregs compared with controls, an effect that was completely inhibited by pretreatment with CCR4 antagonist (Fig. 2A). Treg accumulation was primarily in the white pulp, with scattered cells found throughout the red pulp (Fig. 2B), suggesting CCL22 drives specific recruitment to lymphoid areas of the spleen. This idea was supported by image analysis that showed a ninefold increase in the number of Foxp3+ cells stained per follicle (Fig. 2C). Pretreatment with CCR4 antagonist prevented this increase in Tregs, resulting in splenic FoxP3 staining that was similar to control groups (Fig. 2B). We had similar results if mice were pretreated with αCCL22-neutralizing IgG or lacked CCR4 expression (Fig. S3A), demonstrating CCL22 and CCR4 are required for apoptotic cell-driven FoxP3+ Treg accumulation. We have found that 3 d after apoptotic cell challenge there is no appreciable difference in splenic Treg percentages or expression of effector molecules (13). However, the data presented herein indicated a significant, rapid Treg response to apoptotic cell challenge occurring within the first few hours after exposure. Thus, we examined expression of receptors known to mediate Treg-dependent immune suppression during this timeframe. FACS analysis revealed splenic Tregs rapidly up-regulated surface CTLA-4 in response to apoptotic cell exposure, whereas GITR, CD103, and PD-1 were relatively unchanged (Fig. 2D). Moreover, this effect was dependent on CCR4, because pretreatment with antagonist prevented surface CTLA-4 staining (Fig. 2D).
Fig. 2.
Apoptotic cell exposure drives rapid Treg and DC migration into the splenic follicle. B6 mice were pretreated with CCR4 antagonist as described in SI Materials and Methods 6 h before injection of 107 apoptotic thymocytes i.v. Four hours after apoptotic cell administration, the spleen was collected for analysis. (A) Splenocytes analyzed by flow cytometry to quantify CD4+FoxP3+ Treg accumulation after exposure to apoptotic cells. (B) Representative immunofluorescence staining of splenic sections to determine localization of CD11c+ DCs (red) and FoxP3+ Tregs (green) 4 h after apoptotic cell challenge. Rp, red pulp; wp, white pulp. (C) Image analysis of the number of FoxP3+ cells per follicle in splenic sections from mice treated as described in B. (D) Representative FACS analysis histograms showing surface expression of the markers indicated in CD25+FoxP3+ Tregs 4 h after apoptotic cell challenge. (E and F) Image analysis of splenic sections from mice treated as in B for semiquantitative analysis of follicular CD11c+ DC accumulation and Treg/DC interactions after apoptotic cell challenge. Distance between Tregs and DCs considered contacts was 0.02 μm or less. Distance was quantified by Applied Precision Software (Softworx) on images captured as described in Materials and Methods. (G) Histogram analysis of CCR4 expression in the DC populations indicated (i.e., CD11c+CD8α+CD103+/−). (H) Chemotaxis of DCs in response to apoptotic cell/MMΦ-conditioned media was done as described in Fig. 1F using FACS-purified DCs with the phenotype indicated. Bars represent mean value for triplicate samples (H) or five or more mice per group (A and G) ± SD. Images in B are representative for five or more mice and are 200× magnification. *Pval < 0.05 and **Pval < 0.01 as determined by Student t test. Experiments were repeated three times with similar results.
An unexpected observation was CCL22- and CCR4-dependent follicular accumulation of CD11c+ cells following apoptotic cell challenge (Fig. 2 B and E); this was not associated with an overall increase in the percent of splenic CD11c+ DCs, suggesting they were recruited from the MZ and red pulp rather than systemically. Moreover, image analysis showed that increased CD11c+ cell presence in the follicle significantly elevated the number of follicular Treg–DC interactions (Fig. 2F and Fig. S3B). CD8+CD103+ DCs preferentially acquire apoptotic cells in the MZ and are responsible for a significant portion of apoptotic cell-driven TGF-β production, Teff cell tolerization, and induction of Tregs from naive populations (5, 8, 11, 30). We found that a significant percentage of CD8+CD103+ DCs expressed the CCR4 receptor in apoptotic cell-challenged mice (Fig. 2G). In contrast, CD103neg DCs did not show appreciable surface staining for CCR4, suggesting CD103+ DCs may migrate preferentially in response to CCL22 production. In agreement with this, we found apoptotic cell/MMΦ coculture-conditioned media induced significant migration of CD8+CD103+ DCs in a CCL22- and CCR4-dependent mechanism in Transwell assays and in vivo (Fig. 2H and Fig. S4). In contrast, CD8+CD103neg DCs showed reduced apoptotic cell-dependent migratory capacity, which was independent of CCL22/CCR4 (Fig. 2H).
Treg Recruitment Is Required for Suppression of Innate and Adaptive Inflammatory Immune Responses Elicited by Apoptotic Cells.
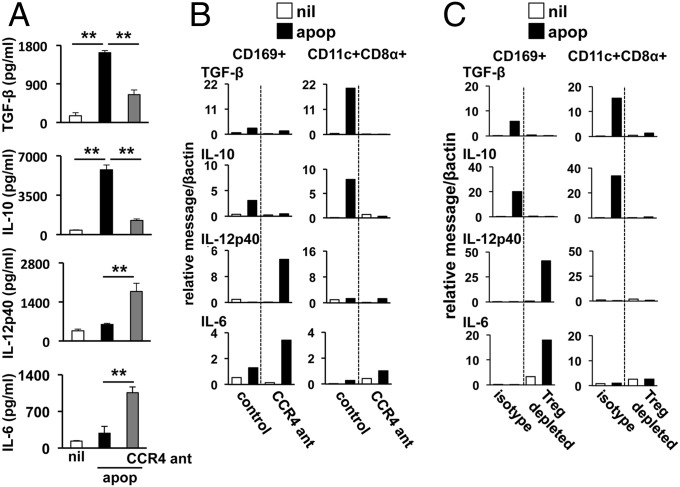
We have shown that i.v. apoptotic cell administration induces TGF-β and IL-10 expression by splenic phagocytes within a few hours of challenge (13). Because CCL22 expression occurred rapidly after apoptotic challenge and recruits Tregs to sites of inflammation, we hypothesized that regulatory cytokine induction may be dependent on CCL22-mediated Treg recruitment. Thus we pretreated mice with CCR4 antagonist 12 h before apoptotic cell challenge and examined the impact on cytokine induction. Consistent with our previous reports (1, 13), apoptotic cells induced prominent increases in splenic TGF-β and IL-10 (Fig. 3A). In stark contrast, when CCR4 was blocked, IL-10 and TGF-β induction were muted compared with controls, and IL-12 and IL-6 protein induction was significantly increased (Fig. 3A). Similar results were observed when CCL22 was inhibited or CCR4KO mice were challenged with apoptotic cells (Fig. S5), demonstrating a responsive shift had occurred favoring a proinflammatory environmental milieu in the absence CCL22-dependent Treg recruitment.
Fig. 3.
CCR4 inhibition promotes an apoptotic cell-driven proinflammatory cytokine response in the spleen. (A) B6 mice were injected i.p. with CCR4 antagonist as described in SI Materials and Methods. At 12 h later, the mice were challenged with 107 apoptotic syngeneic thymocytes i.v., and 18 h after apoptotic cell administration, spleens were collected and cytokine protein concentrations were determined on whole-spleen lysate by ELISA. (B) B6 mice were treated with CCR4 antagonist and injected with apoptotic cells as in A. At 4 h after apoptotic cell challenge, CD169+ MMΦs and CD11c+CD8α+ DCs were sorted by FACS, and cytokine message for the indicated species was determined by sqPCR. (C) CD25+ Treg numbers were depleted by administration of 250 μg of αCD25 monoclonal antibody (clone PC61) as described in Materials and Methods. At 24 h later, mice were challenged with 107 apoptotic cells i.v., and macrophages and DCs were analyzed for cytokine message induction as in B. For A, bars represent mean value for five mice ± SD, and for B and C, bars represent the value for pooled samples from three mice. **Pval < 0.01 as determined by Student t test. Experiments were repeated at least three times with similar results.
Recently, we reported CD8+DCs were the primary TGF-β–producing antigen presenting cells (APCs) after apoptotic challenge (13). Accordingly, TGF-β transcription rapidly increased (55-fold at 4 h) after apoptotic cell challenge in CD8+ DCs (Fig. 3B). Similarly, IL-10 message increased in sorted CD8+ DCs and CD169+ MΦs when apoptotic thymocytes were injected i.v. However, when CCR4 function was inhibited by pretreatment with antagonist, apoptotic cell-driven IL-10 and TGF-β mRNA induction was completely abrogated (Fig. 3B) with a concomitant increase in IL-12p40, predominately in CD169+ MΦs (Fig. 3B). Likewise, though both CD8+ DCs and MMΦs showed an induction in IL-6 message in CCR4-antagonized groups, it was the CD169+ macrophages that showed the most rapid and significant induction of IL-6 expression after apoptotic cell exposure (Fig. 3B). This finding is consistent with studies suggesting that tolerogenic DC populations in the spleen and other organs have a limited capacity to produce proinflammatory cytokines (30), and implies CCL22-mediated Treg recruitment functions to promote acquisition of a regulatory phenotype in DCs and prevent of inflammatory cytokine production in macrophages involved in apoptotic cell clearance.
Our data shows TGF-β is rapidly induced by apoptotic cells, and others have reported that this is critical for iTreg development and downstream tolerance (4). However, it is unclear if TGF-β expression is a result of early, innate immune suppression or a key driver of the innate suppressive immune response; to test this, we pretreated mice with αTGF-β–neutralizing antibodies before apoptotic cell exposure. Blockade of TGF-β abrogated apoptotic cell-driven TGF-β or IL-10 in MMΦs and DCs that instead showed prominent increases in IL-12p40 and IL-6 expression (Fig. 6SA), implying innate TGF-β induction in phagocytes after efferocytosis is an essential mechanistic driver of suppressive cytokine production.
To confirm Tregs were required for apoptotic cell-driven suppression, we depleted them before apoptotic cell challenge by administration of an anti-CD25 antibody. In a pattern similar to that observed in CCR4-antagonized animals, Treg depletion altered cytokine message induction, preventing TGF-β and IL-10 production in DCs and MΦs and, rather, promoting IL-12p40 and IL-6 transcription (Fig. 3C). Tregs can promote immune suppression by several mechanisms, including production of the soluble effector TGF-β and by direct contact with DCs (e.g., CTLA4/B7 interactions). We surmised TGF-β production was not a likely candidate because we did not observe a significant increase in Treg TGF-β expression within 24 h of apoptotic cell challenge. However, it has been reported that regulatory costimulation signals delivered by CTLA4 are potent drivers of an immunosuppressive phenotype in DCs (31); moreover, our own observations demonstrated a dramatic and specific increase in surface CTLA-4 on Tregs (Fig. 2D), leading us to hypothesize Tregs may promote apoptotic cell suppression via signals delivered by CTLA4. In agreement with this theory, blockade of CTLA4 with a neutralizing antibody before apoptotic cell administration inhibited apoptotic cell-driven splenic IL-10 or TGF-β induction with contrasting significant increases in IL-12 and IL-6 (Fig. S6B). Thus, the data indicate CTLA4 is a critical driver of suppression in the microenvironment promoting a tolerogenic phenotype (i.e., TGFβ+) in DCs.
Previously, we reported that apoptotic cell-associated antigens elicited minimal effector T-cell proliferative responses (1, 13); this was absolutely dependent on MZ MΦs, because their deletion promoted vigorous T-cell proliferation and generation of adaptive inflammatory immunity (1). We reasoned that CCR4 antagonism, which prevented regulatory cytokine production, would also compromise suppression of apoptotic cell-associated antigen-specific T-cell responses; to test this, carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled transgenic OTII T cells (2.5 × 106 per mouse) were adoptively transferred into B6 mice ± CCR4 antagonist, followed 24 h later by challenge with 107 apoptotic OVA+ thymocytes, and we assessed proliferation (CFSE dye dilution). Treatment with CCR4 antagonist had no impact on OTII localization to the spleen because vehicle-treated and CCR4 antagonist-treated mice had similar numbers of CFSE-labeled OTII T cells in the absence of apoptotic cell exposure. In control mice there was no evidence of proliferation after apoptotic cell injection, demonstrated by the lack of CFSE fluorescence reduction (Fig. S6C). Interestingly, OTII T cells were not deleted after apoptotic cell challenge because the total number of OTII cells present in the spleen was comparable to untreated controls. In contrast, when CCR4 activity was inhibited, OTII T cells underwent significant proliferation, with the majority of T cells showing a reduction of CFSE fluorescence intensity (Fig. S6C).
Apoptotic Cell-Induced Allograft Tolerance Requires CD169+ Macrophages and CCR4 Function.
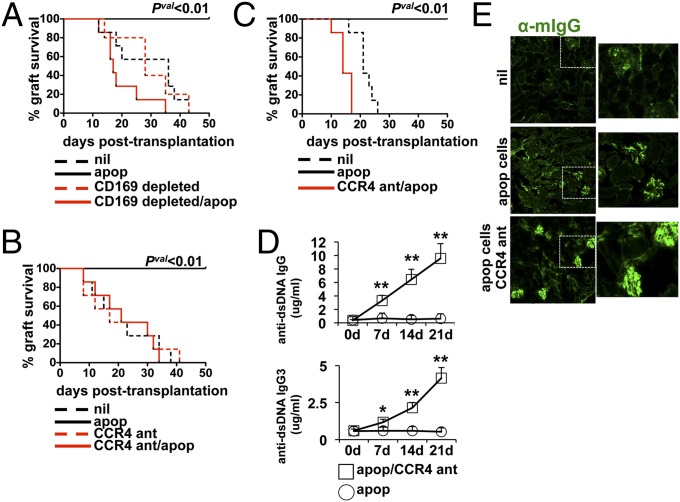
Systemic apoptotic cell exposure results in long-term tolerance to associated antigens. Because we have shown that apoptotic cells drive early innate regulatory responses via recruitment of Tregs to the spleen by MMΦ-driven, CCL22/CCR4-dependent mechanisms, we reasoned that mice deficient in CD169+ MΦs or CCR4 function would have defective downstream generation of stable tolerance. To test the role MMΦs and CCL22 in long-term regulatory immunity, we used a mouse model of skin allograft tolerance where systemic challenge of female mice with male apoptotic cells leads to long-term, whole-animal tolerance to H-Y antigens (32). CD169+ MΦ depletion is associated with an abrogation of apoptotic cell-driven immune suppression to associated antigens (1, 12); however, it has never been shown if this cell population is required for stable tolerance induction. Therefore, we depleted the CD169+ MΦs in CD169DTR mice, followed by challenge with apoptotic male thymocytes and engraftment of male skin. In control groups (i.e., untreated), the male skin was rejected by all recipient mice with a graft mean survival time (MST) of 36 d (Fig. 4A), which was in stark contrast to apoptotic cell-treated mice where there was 100% survival of the grafts (Fig. 4A). Control female MMΦ-depleted mice rejected allografts with a time course similar to control untreated mice (MST 34.5 d), suggesting the absence of MMΦs did not impact the ability to reject H-Y mismatched grafts. In agreement with our hypothesis, apoptotic cell-induced tolerance was completely dependent on the presence of an intact MMΦ population, because CD169+ cell depletion abrogated the protective effect of apoptotic cells and complete graft rejection with a MST of 28 d (Fig. 4A). Similarly, when female B6 mice were treated with a single injection of CCR4 antagonist 12 h before i.v. apoptotic cell injection, apoptotic cell-driven protection of male allografts from rejection was completely abrogated (MST 24 d; Fig. 4B). Tolerance was transferrable to secondary recipients, because mice receiving 107 splenocytes from apoptotic cell-tolerized mice showed complete acceptance of allografts (Fig. 4C). In contrast, groups receiving splenocytes from mice treated with apoptotic cells + CCR4 antagonist rejected skin allografts in an accelerated fashion compared with controls (MST 14 d for CCR4 antagonist/apoptotic vs. MST 21 d for nil, Pval = 0.0024; Fig. 4C). Thus, the data strongly indicate apoptotic cells drive stable, infectious tolerance by a mechanism that depends on early innate recognition events associated with recruitment of CCR4+ regulatory cells.
Fig. 4.
Apoptotic cell-mediated allograft tolerance requires an intact CD169+ MMΦ population and CCR4 function. (A) Female B6.CD169DTR mice were injected 3× with saline ± 100 ng diphtheria toxin (DT) to deplete the MMΦ population. At 48 h after the last DT injection, 107 male B6 thymocytes were adoptively transferred i.v. followed by male skin engraftment 7 d later (as described in Materials and Methods). (B) Female B6 mice were injected with CCR4 antagonist and challenged with male apoptotic cells receiving male skin allografts as in A. (C) Female mice were injected with apoptotic cells ± CCR4 antagonist pretreatment, and 7 d later 107 splenocytes were adoptively transferred i.v. to secondary recipient female B6 mice. At 1 d after the transfer, mice received male B6 skin. For A–C, graphs represent the cumulative survival of the skin allografts over a 50-d time period posttransplantation (n = 7–10 mice per group). Significance determined as described. (D) B6 mice were injected once per week (3× total) i.v. with apoptotic cells and i.p. with CCR4 antagonist as described in Materials and Methods. The serum was collected at the indicated time points, and concentrations of total IgG and IgG3 reactive against dsDNA as a marker of systemic autoimmunity were determined by ELISA. (E) Kidneys from mice in D were collected after the terminal bleed, and frozen sections were stained with α-mouse IgG to measure immune complex deposition. For D, each data point represents the mean value for five mice per group ± SD. *Pval <0.05 and **Pval < 0.01 as determined by Student t test. Images in C are representative images shown at 200× magnification. Experiments were repeated three times with similar results.
CCR4 Inhibition Promotes Apoptotic Cell-Driven Tolerance Breakdown and Autoimmunity.
We have previously shown that depletion of marginal zone and metallophillic macrophages impairs apoptotic cell-activated regulatory mechanisms, resulting in a breakdown of self-tolerance and the development of autoimmunity (1). Because our data has demonstrated antagonizing CCL22 or CCR4 promotes a proinflammatory splenic response to apoptotic cell challenge i.v., we tested if repeated exposure to apoptotic cells in CCR4 inhibition conditions would elicit an autoimmune response. Mice were challenged weekly with syngeneic apoptotic thymocytes i.v. and parallel i.p. treatments with CCR4 antagonist (for a total of three injections), and serum autoreactivity to dsDNA was monitored by ELISA. We found that one injection of apoptotic cells/CCR4 antagonist was sufficient to induce a 10-fold increase in serum anti-dsDNA IgG 7 d after administration (Fig. 4D). Moreover, repeated injection of apoptotic cells (3× in total) resulted in a 26-fold increase in anti-dsDNA IgG compared with baseline levels and a significant increase in cryoglobulin (anti-dsDNA IgG3; Fig. 4D). Because increased circulating anti-dsDNA Ig generally parallels increase immune complex (IC) deposition in the glomeruli, we examined kidney sections for IC deposition. Mice challenged with apoptotic cells alone showed minimal changes in IgG staining relative to controls (Fig. 4E). However, when CCR4 was antagonized there was a significant increase in IC staining upon chronic exposure to apoptotic cells, paralleling increased anti-dsDNA IgG in serum (Fig. 4E). Renal function was preserved in the mice, and there were no overt signs of pathology; however, this is likely due to the short timeframe of the experiment. Nevertheless, the data clearly show that in that absence of CCR4 function, apoptotic cells can elicit a strong autoimmune response, suggesting that Treg recruitment is required for the regulation of tolerance to apoptotic corpse-associated antigens and, in particular, the regulation of adaptive autoimmunity.
Discussion
Efferocytosis of apoptotic cells leads to stable, long-term tolerance mediated by induced regulatory lymphocytes. Studies have reported that CD8α+ DCs are critical drivers of this process, cross-presenting apoptotic cell antigens to tolerize CD8+ T cells and promote iTreg development in an antigen and TGF-β–dependent manner. However, this induction occurs several days after primary exposure and does not account for the cellular or molecular mechanisms involved in the early innate response to apoptotic cells. Our previous data has shown that MZ MΦ populations (CD169+ and SignR1+) play a key mechanistic role as immune sentinels in the spleen, capturing apoptotic material entering from circulation and promoting immune suppression immediately after contact. Importantly, MZ MΦs influence the activity of DCs modulating TGF-β production and phagocytosis of apoptotic cells (1, 13). Thus, it is likely that MΦ–DC communication is critical for initial generation of a suppressive microenvironment and acquisition of a regulatory phenotype.
The data presented in this report adds an additional layer to this communication network because it suggests that rapid follicular accumulation of Tregs and DCs in response to CCL22 production by MMΦs is a key early mechanism involved in apoptotic cell-mediated immune suppression and tolerance. Tregs are potent suppressors of immunity, and at least a portion of nTregs is positively selected by self-antigen (14, 15). The importance of nTregs for immune homeostasis and the prevention of autoimmune disease is clear as even temporary depletion rapidly results in severe autoimmune/autoinflammatory disease and mortality, demonstrating the significant basal role Tregs play monitoring self-antigen presentation and potentially autoreactive naïve T cells (33). Moreover, recent data have shown that Tregs serve a critical function as “first responders” (34). In mice immunized with OVA/IFA/CpG, Sharma et al. (34) found Tregs provided a positive signal via CD40/CD40L within the first few hours after vaccine administration that is required for induction of immunity. In this context, functional participation of Tregs was TCR-dependent; however, because there were presumably no OVA-specific Tregs present, this result suggests the Tregs were reacting to endogenously presented self/MHCII in the context of CpG-driven activation. We envision a similar mechanism occurring in the spleen, whereby Tregs recruited as a result of MMΦ production of CCL22 are activated either by apoptotic cell antigens presented on capturing APCs or constitutively presented self in the context of tolerogenic stimulation.
The observation that suppression required CTLA4 function implies that contact-dependent communication is required for apoptotic cell-driven tolerogenic signals. The fact that the large majority of accumulated Tregs appear in the white pulp suggests Treg/APC interactions likely occur in the lymphoid areas of the spleen. CD8α+CD103+ DCs capture apoptotic material in the MZ and migrate to the white pulp where they present the captured antigens, driving downstream, stable tolerance (8, 30). Our report adds to this model because it suggests responsiveness to CCL22 results in coordinated recruitment of Tregs and tolerogenic CD103+ DCs to the white pulp, enhancing Treg/DC interactions (as shown in Fig. 2F).
When the total number of total follicular CD11c+ DCs was quantified after apoptotic cell challenge, there appeared to be a discrepancy compared with CD103+ DCs. Specifically, we observed CCL22/CCR4 inhibition had a larger impact on total CD11c+ DC migration vs. CD103+ DCs (as seen in Fig. 2 B and E and Fig. S3), suggesting CCL22 influences migration of CD103neg DC subsets. Though we do not know the precise reason for the difference, there are two related possibilities: (i) follicular recruitment of Tregs may activate a CCR4/CCL22-independent mechanism of DC chemotaxis, and/or (ii) recruitment of CCR4+CD103+ DCs may drive wider follicular migration of DCs. Regardless, inhibition of CCL22 or CCR4 would have the effect observed, reducing CD11c+ DC numbers in the follicle after apoptotic cell exposure.
Apoptotic cell-induced Treg recruitment to the spleen was clearly a critical mechanism for tolerance induction because either depletion of MMΦs or inhibition of CCR4 was sufficient to abrogate apoptotic cell-driven tolerance of H-Y mismatch allografts. Apoptotic cell-immune suppression is a mechanistically important phenomenon because it limits harmful autoreactivity. Our data suggests CCR4-mediated Treg recruitment plays an important role in this process, because cotreatment with antagonist resulted in rapid induction of serum autoimmunity after apoptotic cell injection associated with increased renal IC deposition. Thus, taken as a whole, the data strongly argue that early innate responses to apoptosis are dependent on functional cross-talk with Treg populations. It is not clear why apoptotic cells induce CCL22 specifically in splenic MMΦs; however, it is reasonable to assume that the specialized niche these MΦs populate has a significant influence on the apoptotic cell response. In conclusion, we show that selective CCL22 expression by a small population of CD169+ MΦs located in splenic marginal zone is essential for initiation of tolerogenic responses to apoptotic cells by rapid recruitment of Tregs. Moreover, the data indicates Tregs have a fundamental role in suppression induced by apoptosis in secondary lymphoid tissues, perhaps by providing initial instruction via CTLA4/B7 interactions and promoting a tolerogenic DC phenotype.
Materials and Methods
Animals.
C57BL/6J (B6), B6.CD169DTR, B6.Act-mOVA-II (Act-mOVA), OTII, B6.CCR4−/−, and B6.CD45.1+ congenic mice were obtained from a colony maintained under specific pathogen-free conditions at Georgia Regents University, in accordance with Institutional Animal Care and Use Committee guidelines. All mice used in the experiments were female 8- to 12-wk-old animals unless otherwise noted.
Apoptosis Induction and in Vivo Apoptotic Cell Administration.
Apoptotic cells were generated and administered as previously described (13). For cytokine measurements, the spleen was weighed and ground with a pestle and mortar containing 5 g sterile sand (Sigma) and 100 μL of PBS + protease inhibitor mixture (Sigma) per 10 mg of spleen as described (1). Assessment of CCL22, IL-6, IL-10, IL-12p40, and TGF-β1 was via ELISA (EBioscience).
Flow Cytometry.
Flow cytometric analysis and cell sorting were done using a standard approach as described in SI Materials and Methods.
CD169+ MMΦ Depletion, Treg Depletion, and Antibody Blockade.
B6.CD169+DTR mice were depleted of MMΦs as previously described (13). For Treg depletion, mice were injected i.p. with anti-CD25 mAb (clone PC61; 250 μg/mouse) 6 h before apoptotic cell injection. For TGF-β blockade, mice were injected i.p. with anti–TGF-β mAb (clone 1D11; 150 μg/mouse); for CCL22 blockade, mice were injected i.p. with 250 μg of anti-CCL22 mAb (clone 158132) 6 h before apoptotic cell injection; and for CTLA4 blockade, mice were injected i.p. with 100 μg/mouse α-CTLA4 mAb (clone 9H10) 24 h before apoptotic cell transfer.
Semiquantitative PCR.
RNA from sorted cells was purified using RNeasy RNA purification kits (Qiagen) and 250 ng of RNA was reverse-transcribed using a random hexamer cDNA reverse transcription kit (Clontech). cDNA was analyzed for the message species indicated as described in SI Materials and Methods.
Chemotaxis Assay.
Sorted CD169+ MΦs were incubated with apoptotic thymocytes at a 10:1 apoptotic cell/MMΦ ratio for 24 h. Culture supernatants were collected and prepared per manufacturer’s instructions for the QCM chemotaxis 5-μm cell migration assay (Millipore). For further details, see SI Materials and Methods.
Skin Transplantation.
Skin transplantation was carried out as described elsewhere (35). For further details, see SI Materials and Methods.
Immunofluorescence and Immunohistochemistry.
Frozen tissue cryosectioning and immunostaining was done using standard approaches and commercially available antibodies as described in SI Materials and Methods.
Assays for Autoantibodies.
Assays for serum anti-dsDNA autoantibodies have been described previously (36). For further details, see SI Materials and Methods.
Image and Statistical Analysis.
Image and statistical analysis was done as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by a Lupus Research Institute grant and National Institute of Health Grants AI092213 and AI105500 (to T.L.M.). The authors would like to thank Jeanene Pihkala for assistance with FACS-sorting.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320924111/-/DCSupplemental.
References
- 1.McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood. 2011;117(20):5403–5412. doi: 10.1182/blood-2010-11-320028. [DOI] [PubMed] [Google Scholar]
- 2.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101(4):890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voll RE, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390(6658):350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 4.Kleinclauss F, et al. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ. 2006;13(1):41–52. doi: 10.1038/sj.cdd.4401699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamazaki S, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181(10):6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith TS, et al. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J Immunol. 2007;178(5):2679–2687. doi: 10.4049/jimmunol.178.5.2679. [DOI] [PubMed] [Google Scholar]
- 7.Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med. 1981;153(5):1058–1067. doi: 10.1084/jem.153.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu K, et al. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196(8):1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315(5808):107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 10.Desch AN, et al. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med. 2011;208(9):1789–1797. doi: 10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu CH, et al. Novel subset of CD8alpha+ dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. J Immunol. 2009;182(7):4127–4136. doi: 10.4049/jimmunol.0803364. [DOI] [PubMed] [Google Scholar]
- 12.Miyake Y, et al. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117(8):2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravishankar B, et al. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc Natl Acad Sci USA. 2012;109(10):3909–3914. doi: 10.1073/pnas.1117736109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2(4):301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 15.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3(8):756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 16.Iellem A, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194(6):847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai T, et al. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem. 1998;273(3):1764–1768. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127(4):759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 19.Bayry J, et al. In silico identified CCR4 antagonists target regulatory T cells and exert adjuvant activity in vaccination. Proc Natl Acad Sci USA. 2008;105(29):10221–10226. doi: 10.1073/pnas.0803453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gobert M, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69(5):2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 21.Pere H, et al. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood. 2011;118(18):4853–4862. doi: 10.1182/blood-2011-01-329656. [DOI] [PubMed] [Google Scholar]
- 22.Lee I, et al. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201(7):1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu K, Chen Z, Khatri I, Gorczynski RM. CCR4 dependent migration of Foxp3+ Treg cells to skin grafts and draining lymph nodes is implicated in enhanced graft survival in CD200tg recipients. Immunol Lett. 2011;141(1):116–122. doi: 10.1016/j.imlet.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Montane J, et al. Prevention of murine autoimmune diabetes by CCL22-mediated Treg recruitment to the pancreatic islets. J Clin Invest. 2011;121(8):3024–3028. doi: 10.1172/JCI43048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Getts DR, et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol. 2011;187(5):2405–2417. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamashita U, Kuroda E. Regulation of macrophage-derived chemokine (MDC, CCL22) production. Crit Rev Immunol. 2002;22(2):105–114. [PubMed] [Google Scholar]
- 27.Ravishankar B, McGaha TL. O death where is thy sting? Immunologic tolerance to apoptotic self. Cell Mol Life Sci. 2013;70(19):3571–3589. doi: 10.1007/s00018-013-1261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow Z, Mueller SN, Deane JA, Hickey MJ. Dermal regulatory T cells display distinct migratory behavior that is modulated during adaptive and innate inflammation. J Immunol. 2013;191(6):3049–3056. doi: 10.4049/jimmunol.1203205. [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama K, et al. Potent and orally bioavailable CCR4 antagonists: Synthesis and structure-activity relationship study of 2-aminoquinazolines. Bioorg Med Chem. 2009;17(1):64–73. doi: 10.1016/j.bmc.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Jaensson E, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205(9):2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grohmann U, et al. Functional plasticity of dendritic cell subsets as mediated by CD40 versus B7 activation. J Immunol. 2003;171(5):2581–2587. doi: 10.4049/jimmunol.171.5.2581. [DOI] [PubMed] [Google Scholar]
- 32.Simpson E, Scott D, Chandler P. The male-specific histocompatibility antigen, H-Y: A history of transplantation, immune response genes, sex determination and expression cloning. Annu Rev Immunol. 1997;15:39–61. doi: 10.1146/annurev.immunol.15.1.39. [DOI] [PubMed] [Google Scholar]
- 33.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 34.Sharma MD, et al. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity. 2010;33(6):942–954. doi: 10.1016/j.immuni.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn LA, et al. Presentation of the HPV16E7 protein by skin grafts is insufficient to allow graft rejection in an E7-primed animal. Virology. 1997;235(1):94–103. doi: 10.1006/viro.1997.8650. [DOI] [PubMed] [Google Scholar]
- 36.McGaha TL, Sorrentino B, Ravetch JV. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 2005;307(5709):590–593. doi: 10.1126/science.1105160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.