Significance
Hematopoietic stem and progenitor cells (HSPCs) cannot only replace depleted progeny pools, but act as the primary immune response to various pathological conditions. Here we show that the circulating HSPCs from various patients with solid tumors exhibit a generalized myeloid bias with a skew toward granulocytic differentiation. Importantly, circulating hematopoietic precursors were highly enriched in tumor tissues and positively correlated with disease progression. We developed an in vitro short-term culture model to effectively induce the rapid generation of myeloid-derived suppressor cells (MDSCs), defined as cord blood-derived MDSCs. In addition, we suggest that the altered circulating HSPCs may serve as an important link between dysregulated hematopoiesis and accumulated immunosuppressive MDSCs in patients with cancer.
Abstract
Cancer is associated with a profound perturbation in myelopoiesis that results in the accumulation of myeloid-derived suppressor cells (MDSCs) to promote disease progression. Recent studies in mice suggest that tumor-derived factors could regulate the differentiation of hematopoietic stem and progenitor cells (HSPCs) in the bone marrow and subsequently contribute to dysregulation of hematopoiesis. However, the nature and role of HPSCs in patients with cancer remain unknown. Here we show, in detailed studies of the peripheral blood from 133 untreated patients with seven different types of tumors, that the composition of circulating HSPCs was significantly altered in patients with solid tumors. The frequencies of circulating granulocyte–monocyte progenitors (GMPs) were increased four to seven fold in all types of tumors examined, and the circulating hematopoietic precursors exhibited myeloid bias with a skew toward granulocytic differentiation in patients with solid tumors. These myeloid precursors are selectively enriched in tumor tissues, and the high levels of circulating GMPs were positively correlated with disease progression. By using cord blood-derived CD34+ cells, we developed an in vitro short-term culture model to effectively induce the rapid generation of MDSCs. We found that, among the factors produced by various tumors, GM-CSF, granulocyte colony-stimulating factor, and IL-6 could not only promote the myeloid-biased differentiation, but also induce the differentiation of myeloid precursors into functional MDSCs. These findings suggest that the altered circulating HSPCs may serve as an important link between dysregulated bone marrow hematopoiesis and accumulated MDSCs in patients with cancer.
The development of blood cell lineages in the steady state is tightly controlled by endogenous signals that drive the sequential differentiation of hematopoietic stem and progenitor cells (HSPCs) to the downstream and highly proliferative lineage-committed progenitors, e.g., the common myeloid progenitors and granulocyte–monocyte progenitors (GMPs) (1–5). These progenitors in the bone marrow and peripheral blood can differentiate into immature myeloid cells (IMCs) and produce a large number of terminally differentiated myeloid cells. Normally, IMCs migrate to different peripheral organs, where they differentiate into macrophages, dendritic cells, or granulocytes. However, recent studies have shown that HSPCs are far more sensitive to exogenous environmental cues than previously anticipated, in part because of the expression of receptors for various microbial products and inflammatory cytokines. For example, proinflammatory cytokines (IL-1, IL-6, interferons, and others) and TLR4 stimulation can influence the pace and direction of HSPC differentiation (4, 6, 7). These data strongly suggest that HSPCs play a direct and vital role in the primary immune response to pathological conditions; however, the nature and role of HPSCs in human tumors remain largely unknown.
Tumor progression is associated with a profound alteration in myelopoiesis that results in the recruitment and expansion of myeloid-derived suppressor cells (MDSCs). MDSCs are a heterogeneous population of IMCs and myeloid progenitors that negatively regulate immune responses, enhance the “stemness” of cancer cells, and facilitate tumor metastasis and angiogenesis (8–11). Although the precise underlying mechanisms are not yet clear, the accumulation of MDSCs is generally thought to be elicited by tumor-derived factors (12, 13). Growth factors (GM-CSF, macrophage colony-stimulating factor, and others) and proinflammatory cytokines produced by malignant cells and stromal cells induce a rapid generation of MDSCs from precursors present in human bone marrow (12, 14). These findings agree with clinical studies showing that the serum concentrations of these cytokines are usually elevated in patients with cancer, and, in some cases, the levels are associated with a poor prognosis (15, 16). However, it should be noted that IMCs with the same phenotype as the MDSCs are continually generated in the bone marrow of healthy individuals and differentiate into mature myeloid cells without causing immunosuppression, whereas this process can be redirected toward the differentiation of pathological MDSCs in cancer (8, 9). Thus, a more detailed characterization of HSPCs and MDSCs in patients with cancer would be helpful for understanding the roles and potential mechanisms of these cells in tumor immunopathogenesis.
Here, we have characterized the frequency of HSPC subsets and other hematopoietic progenitor populations in the peripheral blood of 133 patients with seven different types of tumors and in 102 age-matched healthy individuals. We found the composition of circulating HSPCs to be significantly altered in patients with solid tumors, with a four- to sevenfold increase in the level of circulating GMPs and a generalized myeloid bias of HSPCs toward granulocytic differentiation. The frequency of circulating GMPs was associated with clinical stages and negatively correlated with the time to progression in patients. In addition, we used cord blood (CB)-derived CD34+ cells to establish an in vitro short-term culture model and found that, among the factors produced by various tumors, GM-CSF, granulocyte colony-stimulating factor (G-CSF), and IL-6 could not only promote the myeloid-biased differentiation, but could also induce the differentiation of myeloid precursors into functional MDSCs.
Results
Characterization of HSPCs in Peripheral Blood of Patients with Solid Tumors.
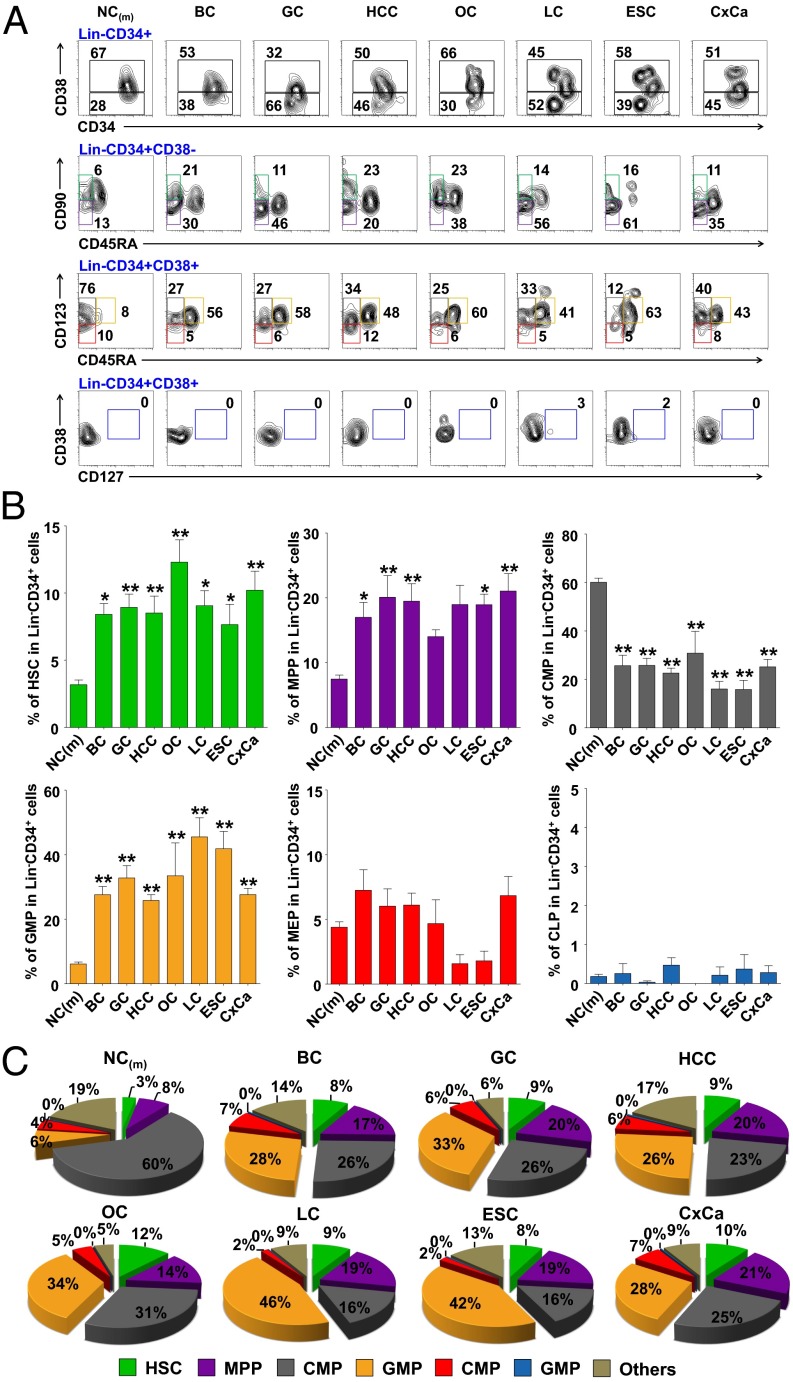
To determine whether the composition of the HSPCs is altered in patients with solid tumors, we first applied FACS to determine the frequency of HSPC subsets within the Lin−CD34+ population in peripheral blood of 90 patients with different types of carcinoma and 63 age-matched healthy donors. The classification of six HSPC subsets was based on the expression of combined surface markers (Fig. S1) (17, 18). The results showed that the frequency of GMPs in the peripheral blood of cancer patients was four- to sevenfold higher than that of healthy donors. The levels of hematopoietic stem cells (HSCs) and multipotent progenitors (MPPs) were also significantly increased in patients with cancer, albeit not as pronounced as those of GMPs (Fig. 1). In contrast, we observed a marked decrease of circulating common myeloid progenitors in patients with cancer. There was no obvious difference in the frequency of megakaryocyte–erythroid progenitors between the healthy adults and the patients with cancer, and common lymphoid progenitors had a very low frequency (0∼0.5% in Lin−CD34+ cells; Fig. 1).
Fig. 1.
Altered composition of circulating HSPC subsets with a skew toward GMPs in patients with cancer. (A) Representative FACS staining of HSPC subsets in the peripheral blood Lin−CD34+ cells from healthy donors and patients with cancer. HCC (n = 26); and BC, breast cancer (n = 9); CxCa, cervical cancer (n = 19); ESC, esophageal cancer (n = 4); GC, gastrointestinal cancer (n = 18); LC, lung cancer (n = 8); NC, age-matched healthy donors (n = 63); OC, ovarian cancer (n = 4). (B) Summary of the circulating HSPC subsets in Lin−CD34+ population. Statistical differences between groups were calculated by one-way ANOVA, followed by post hoc Bonferroni tests (*P < 0.05 and **P < 0.01 vs. healthy donors). (C) Pie charts summarizing the frequencies of HSPC subsets from healthy donors and patients.
Consistent with a recent finding in human bone marrow samples (18), we also observed similar trends of age-related alteration of the HSPCs in peripheral blood of healthy donors, including the increased frequency of HSCs and MPPs and decreased common lymphoid progenitors within the Lin−CD34+ population in aged individuals (Fig. S1). In contrast to the marked increased GMPs in patients with cancer, we observed a decreased GMP levels in elderly healthy individuals (Fig. S1).
The Frequency of Circulating Hematopoietic Precursors with Lymphoid Potential Was Significantly Reduced in Patients with Cancer.
To confirm the altered composition of circulating HSPCs in patients with cancer, we further analyzed the frequency and lineage potentials of CD133-expressing cells, a marker that has been linked to stem cell-fate decisions in human HSCs (19). The coexpression of CD3 or CD33 on CD133+ cells was used to evaluate their lymphoid or myeloid potentials, respectively. In general, the percentage of CD133+ cells in the peripheral blood of healthy donors (n = 39) was slightly higher than that in patients with cancer (n = 45), but the difference was not significant (Fig. S2A). Approximately 30% of the circulating CD133+ cells in healthy donors coexpressed lymphoid and myeloid markers (CD3 and CD33, respectively), and this population was markedly reduced to less than 7% in patients with various cancers (Fig. S2A). This reduction was primarily caused by the loss of CD3 expression on CD133+ cells (37.6 ± 2.6% vs. 11.0 ± 1.1% for control subjects and patients with cancer, respectively; Fig. S2 B and C), particularly on those cells that coexpressed the myeloid marker CD33, CD14, or CD15 (Fig. S2 B and C). A detailed analysis of the differentiation potentials of the circulating CD133+ cells revealed that only the granulocytic CD15+CD133+ subset and CD15+ precursors (CD3−CD33int) were selectively up-regulated, whereas the other subsets, including the CD3+, CD14+, CD3+CD14+, CD3+CD15+, CD14+CD15+, and CD3+CD14+CD15+ cells, were all decreased in the peripheral blood of patients with various cancers (Fig. S2 B and C).
In addition to the phenotypic analyses, we also tested the differentiation capacity of HSPCs by using a methylcellulose assay. The results showed that the ratio of total CFU of granulocyte–monocyte (CFU-GM), granulocyte (CFU-G), or monocyte (CFU-M) from patients with cancer were significantly higher than those from healthy donors (Fig. S3). These results, together with the selective elevation of circulating GMPs (Fig. 1) in various tumors, suggest that patients with cancer exhibited a generalized myeloid bias of HSPCs with a skew toward granulocytic differentiation.
The High Frequency of Circulating GMPs Was Correlated with Tumor Progression.
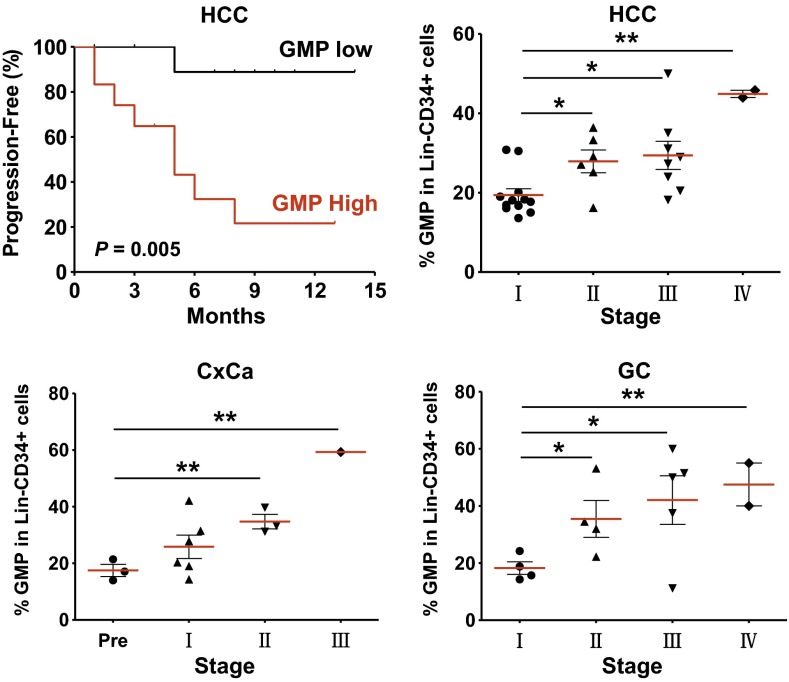
To identify the potential role of GMPs in tumor progression, we next analyzed relevant clinical information and correlated the data with the frequencies of circulating GMPs. In patients with hepatocellular carcinoma (HCC; n = 28), the levels of GMPs were significantly increased and correlated with clinical stages (Fig. 2). When the patients with HCC were divided into two groups according to the median value of circulating GMPs, Kaplan–Meier analysis revealed that the frequency of GMPs was negatively correlated with the time to progression (P = 0.005). The patients with lower GMP levels had significantly longer time to progression (median, 14 mo) than patients with higher GMP levels (median, 5.7 mo). Moreover, 11 of the 14 patients who did not experience disease progression within 1 y after treatment were in the low GMP group (Fig. 2). This positive correlation between the levels of circulating GMPs and clinical stages was also found in patients with cervical (n = 13) and colorectal carcinoma (n = 15; P = 0.001 and P = 0.013, respectively).
Fig. 2.
Increased circulating GMPs predicted poor survival in patients with cancer. Twenty-eight patients with HCC were divided into two groups according to the median value of GMP frequency. Individuals in the low GMP group (black) had significantly improved survival compared with those in the high GMP group (red). The time to progression was estimated by Kaplan–Meier method and compared by using the log-rank test. The frequency of GMPs in the Lin−CD34+ cells from patients with HCC (Upper Right), cervical cancer (CxCa, Lower Left), and gastrointestinal cancer (GC, Lower Right) are shown separated by their clinical stages (*P < 0.05 and **P < 0.01).
Myeloid Precursors Accumulated in Colon Cancer Tissues.
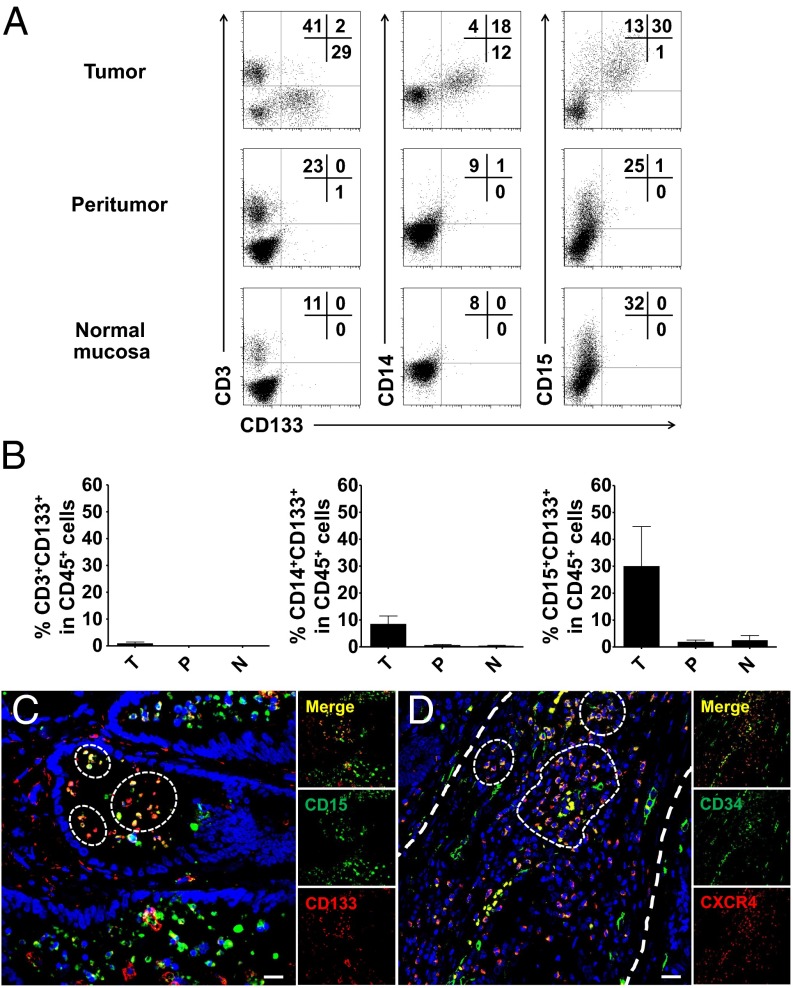
Tumors can recruit IMCs to tumor sites, where they then differentiate into MDSCs to suppress antitumor immunity. To determine whether this mechanism is involved in the presently observed positive association between GMP levels and disease progression in patients with solid tumors, we initially investigated the infiltration and distribution of myeloid precursors in paired colon tumor tissues, peritumor tissues, and normal mucosa tissues (n = 8). FACS analysis of tissue-infiltrating leukocytes revealed that CD133-expressing cells were rarely detected (0∼0.3%) in the peritumor and normal mucosa tissues, but were markedly increased in tumor tissues (30.0 ± 14.7%; Fig. 3 A and B). Consistent with the results for circulating CD133+ cells in patients with cancer, we observed that most of the tumor-infiltrating CD133+ cells were negative for CD3, but coexpressed the myeloid markers CD15 or CD14 (Fig. 3 A and B).
Fig. 3.
IMCs are enriched in colon tumor tissues. (A) Representative FACS analysis of CD133+CD3+, CD133+CD14+, and CD133+CD15+ cells in tissue CD45+ cells isolated from eight paired colon tumor, peritumor, and relative normal mucosal tissues. (B) Summary of the results from A. (C) Multiple staining of CD133 (red), CD15 (green), and DAPI (blue) in frozen sections were analyzed by confocal microscopy. The coexistence of CD133 and CD15 confirmed that a proportion of CD133+CD15+ cells in colon tumor tissues. One of 10 representative micrographs is shown. (D) Multiple staining of CXCR4 (red), CD34 (green), and DAPI (blue) in paraffin-embedded sections shows the coexistence of CXCR4- and CD34-positive cell in tumor tissues. One of 10 representative micrographs is shown. (Scale bar, 20 μm.)
We subsequently examined the in situ distribution of these myeloid precursors in frozen sections of colon cancer tissues. By using confocal microscopy, we confirmed that CD133 protein was expressed on intraepithelial neoplasia and tumor stroma, and remarkable proportions of them were CD133+CD15+ cells (Fig. 3C). Interestingly, a substantial amount of CD34+ cells coexpressed the HSC homing marker C-X-C chemokine receptor type 4 (CXCR4) (Fig. 3C), indicating that these myeloid precursors might be mobilized and homing to tumor tissues (20).
GM-CSF, G-CSF, and IL-6 Promoted the GMP Expansion and MDSC Differentiation from CB-Derived CD34+ Cells.
Cytokines are critical regulators for hematopoietic lineage choice. Studies in other systems have shown that G-CSF, GM-CSF, and IL-6 are the dominant cytokines that regulate myelopoiesis of bone-marrow HSPCs and induce subsequent expansion and accumulation of tumor-infiltrating IMCs (9, 12, 21). To investigate whether these mechanisms are also responsible for the increased circulating GMP levels in patients with cancer, we first determined the plasma levels of these cytokines in 17 healthy donors and 96 patients with various tumors. The results showed that the levels of G-CSF and GM-CSF were significantly higher in patients with cancer than that in healthy donors (means and ranges: G-CSF, 18.3 pg/mL and 3.9–70.8 pg/mL vs. 11.4 pg/mL and 4.4–17.2 pg/mL; GM-CSF, 2.2 pg/mL and 0–45.2 pg/mL vs. 0–0 pg/mL; P = 0.005 for both; Fig. S4). The plasma IL-6 level was also significantly higher in patients with cancer, as shown in previous studies (16).
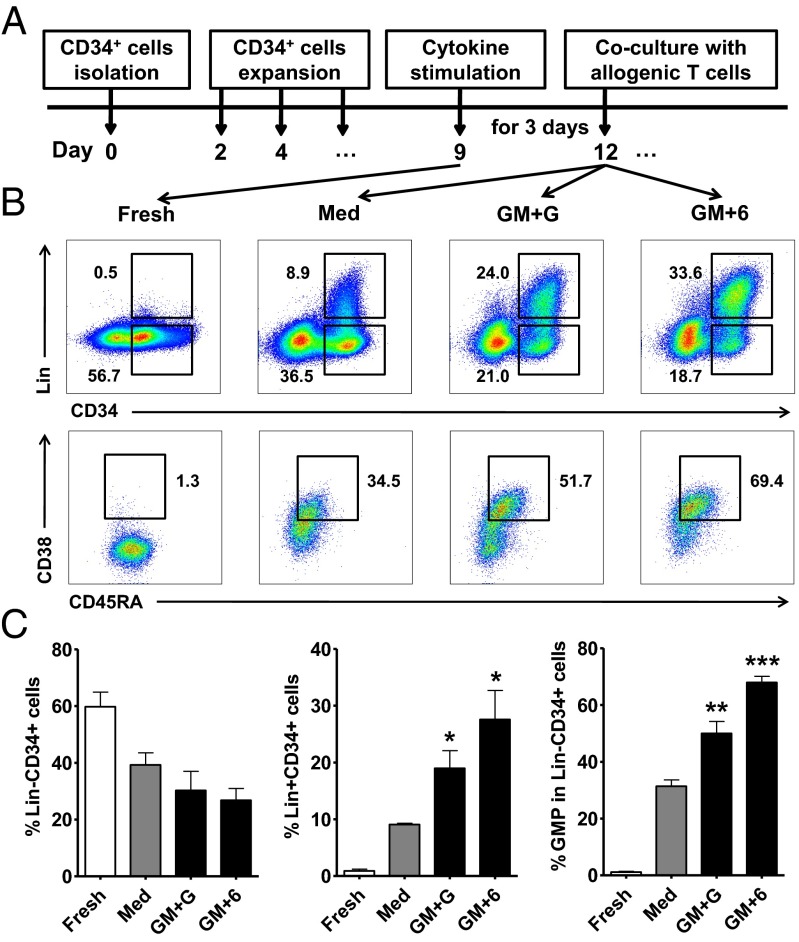
These results showed that patients with cancer exhibited a generalized myeloid bias of circulating HSPCs and that these myeloid precursors are enriched in tumor tissues. We next determined whether G-CSF, GM-CSF, and IL-6 could affect the differentiation of hematopoietic precursors and formation of functional MDSCs. We used CB-derived CD34+ cells to establish short-term culture conditions under which this process could be reliably reproduced in vitro. Purified CD34+ cells were cultured in HSC expansion media. After 8–10 d of expansion, most of the cells were still kept at Lin− status, and approximately half these cells retained their expression of CD133 and CD34 (Fig. 4 B and C). The expanded cells were then incubated with GM-CSF, G-CSF, and IL-6, alone or in combination, for 3–4 d. Compared with the untreated cells, we found that all three cytokines, alone or in combination, could increase the frequency of Lin+ cells, and GM-CSF was the most potent. Interestingly, most of these Lin+ cells still expressed CD34, indicating their immature phenotype (Fig. 4 B and C). Phenotypic analysis revealed distinct roles for the cytokines in inducing the differentiation of hematopoietic precursors. GM-CSF and IL-6 markedly increased the frequency of GMPs (Lin−CD34+CD38+CD123+CD45RA+; Fig. 4 B and C) and the cells expressing high levels of myeloid markers (CD11b, CD14 and CD15). G-CSF had the most potent impact on induction of CD14- and CD15-expressing cells, although it alone did not affect the GMP frequency. A combination of GM-CSF with G-CSF or IL-6 gave rise to a significant increase in CD11b+CD14+ cells (Fig. S5 A and B and Fig. S6 A and B). Taken together, these data indicated that G-CSF, GM-CSF, and IL-6 effectively promote the differentiation of hematopoietic progenitors into IMCs.
Fig. 4.
GM-CSF, G-CSF, and IL-6 promote the differentiation of GMP and Lin+CD34+ cells. (A) Freshly isolated CD34+ cells from CB mononuclear cells were cultured in HSC expansion media for 8–10 d. The expanded cells were then stimulated with cytokines for 3 d, followed by coculture with allogeneic T cells. (B) Representative flow cytometry data of Lin−CD34+ cells, Lin+CD34+ cells, and GMPs after stimulation. (C) Summary of the frequency of Lin−CD34+ and Lin+CD34+ cells in total nucleated cells and of the GMPs in Lin−CD34+ cells after stimulation. The values given represent the mean (±SE) of four separate experiments; *P < 0.05, **P < 0.01, and ***P < 0.001 vs. untreated expanded cells (Med).
To characterize the phenotypic features of these myeloid cells, we monitored the induction of macrophage colony-stimulating factor receptor (M-CSFR, CD115) and IL-4Rα (CD124), two key components for the activation of the MDSC suppressive program (8, 9, 22). Compared with the untreated cells, G-CSF treatment significantly up-regulated the expression of M-CSFR and IL-4Rα on CD14+ and CD15+ cells. GM-CSF and IL-6 appeared to augment M-CSFR and IL-4Rα expression on these myeloid cells, but this increase was not statistically significant, partially because of their lower ability to induce CD14+ and CD15+ cells than G-CSF (Fig. S6B). In parallel, we also assessed the expression of other MDSC markers and molecules related to the suppressive functions of these cultured myeloid cells. CD14+MHC Class II (HLA-DR)low/− cells were recently identified as a new type of MDSC in the blood of patients with various cancers, and programmed death ligand 1 (PD-L1) is a key molecule that mediates immune evasion in tumor environments (23). We found that G-CSF and IL-6, alone or in combination, significantly increased the frequency of CD14+HLA-DRlow/− and CD14+PD-L1+ cells (Fig. S6B). Moreover, G-CSF alone or in combination with IL-6 also stimulated the expression of arginase I and CCAAT/enhancer-binding protein beta (C/EBPβ), two critical mediators for the immunosuppressive modalities of MDSCs (Fig. S6C).
We also examined the kinetic and dose effects of GM-CSF and G-CSF on myeloid differentiation and their expression of M-CSFR and IL-4Rα. The results showed that these cytokines induce myeloid and MDSC differentiation in dose-dependent manners. Comparison of the kinetics of myeloid differentiation and M-CSFR and IL-4Rα expression revealed that myeloid differentiation preceded M-CSFR and IL-4Rα expression in these cytokine-treated cells (Fig. S7). These findings imply that these cytokines not only promote myeloid differentiation, but also induce myeloid precursors to acquire MDSC features.
CB-Derived MDSCs Exhibit Strong Suppressive Activities.
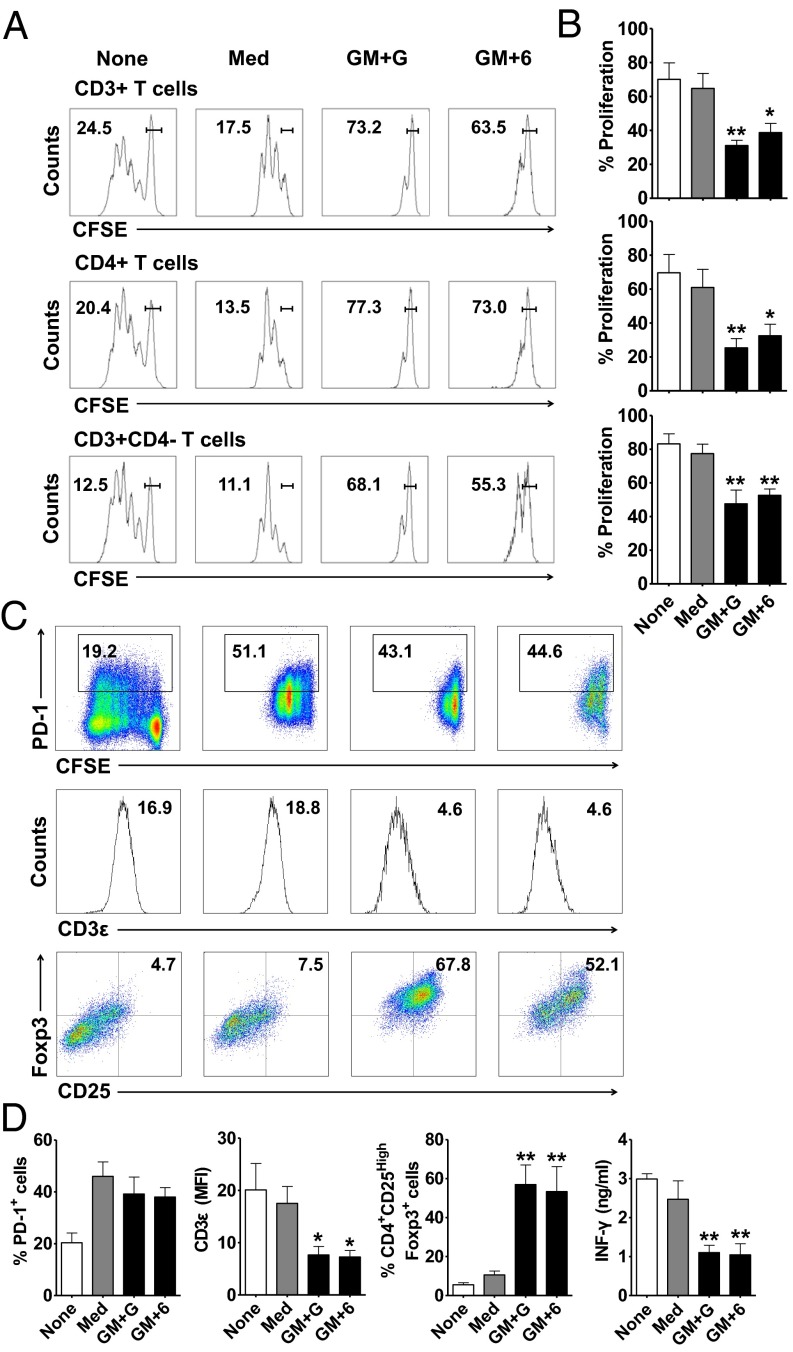
To confirm the immunosuppressive activity of the CB-derived MDSCs (CB-MDSCs), we examined their inhibitory effects on the proliferation and function of T cells. Control or cytokine-treated CB-MDSCs were cultured with purified allogenic circulating T cells at a ratio of 1:1 in the presence of anti-CD3/CD28 stimulation for 6 d. The carboxyfluorescein diacetate succinimidyl ester assay showed that GM-CSF plus G-CSF- or GM-CSF plus IL-6–treated CB-MDSCs effectively inhibited the proliferation of CD3+, CD3+CD4+, or CD3+CD4− T cells, whereas the control CB-MDSCs had only a marginal effect (Fig. 5 A and B). Similarly, these cytokine-treated CB-MDSCs suppressed the production of IFN-γ by T cells (Fig. 5 C and D). We also noted that CB-MDSCs significantly reduced the division cycle number of T cells upon CD3/CD28 stimulation, which was more evident for cytokine-treated CB-MDSCs (Fig. 5 A and B and Fig. S6D).
Fig. 5.
CB-MDSCs exhibit strong suppressive activity against T cells, down-regulate CD3ε expression, and induce Foxp3+ regulatory T cells. (A) Carboxyfluorescein diacetate succinimidyl ester-labeled pan-T cells were cocultured at a 1:1 ratio with or without CB-MDSCs for 6 d, and then analyzed by FACS. The percentages of undivided cells are shown. (B) Summary of the proliferation of T cells. (C) Representative staining of PD-1, CD3ε, and Foxp3 expression on T cells. (D) Summary of the frequency of PD-1+ and CD25+Foxp3+ and the mean fluorescence intensity of CD3ε expression on T cells. IFN-γ concentrations were determined from culture supernatants. The data shown represent the mean (±SE) of four separate experiments; *P < 0.05 and **P < 0.01 vs. pan-T cells cocultured with untreated expanded cells (Med).
In addition to their direct inhibition of T-cell proliferation, studies in other systems have shown that MDSCs could exert their suppressive activity by altering the phenotype and/or function of T cells, including the up-regulation of PD-1, down-regulation of CD3ε, and induction of regulatory T cells (8, 14, 24). Therefore, we next determined whether CB-MDSCs also equipped with these suppressive mechanisms. The results showed that control and cytokine-treated CB-MDSCs could effectively increase the levels of PD-1, a key immune-checkpoint receptor expressed by activated T cells. Interestingly, compared with the control cells, the cytokine-treated CB-MDSCs significantly attenuated the expression of CD3ε, a key component in TCR complex that is essential for T-cell survival and proliferation. Moreover, after coculture with cytokine-treated CB-MDSCs, approximately half of the CD4+T cells stained positive for FoxP3, a transcription factor generally used as a marker for regulatory T cells (Fig. 5 C and D).
Discussion
Cancer is associated with defects in hematopoiesis, leading to accumulations of immunosuppressive cells, which may predict adverse patient outcome. Recent studies have shown that HSPCs are far more sensitive to exogenous environmental cues than previously anticipated, and could act as part of the primary immune response to various pathological conditions (8, 25–28). The present study showed that the composition of circulating HSPCs is significantly altered in patients with various solid tumors, with myeloid-biased differentiation and declined lymphoid potential of the progenitor cells. These myeloid precursors are enriched in tumor tissues, and the high levels of circulating GMPs were correlated with tumor progression. By using an in vitro short-term culture model with CB-derived CD34+ cells, we found that GM-CSF, G-CSF, and IL-6 could not only promote the myeloid-biased differentiation, but could also induce the myeloid precursors to acquire the suppressive features of MDSCs. These findings suggest that the altered circulating HSPCs may serve as an important link between the dysregulated bone marrow hematopoiesis and the accumulated MDSCs in patients with cancer. It should be noted that our work applies only to solid tumors, as CD123, which is used to classify human HSPC subsets, is widely expressed in hematologic malignancies (29, 30).
Recent studies have shown that hematopoiesis functions not only to replace depleted progeny pools to maintain homeostasis, but also as part of the primary immune response to infectious diseases, inflammation, and tumors, because of the expression of receptors for various microbial products and cytokines on HSPCs (3, 6, 31, 32). Accordingly, tumor growth is associated with profound perturbations in hematopoiesis, including myelopoiesis and leukocytosis. Increased peripheral neutrophil numbers and neutrophil-to-lymphocyte ratio have been found in patients with different types of tumors, and could serve as indicators for poor prognosis in some cases (33). The present study showed that such cancer-associated myelopoiesis also occurred at the level of circulating HSPCs. The frequencies of circulating GMPs were increased four- to sevenfold in patients with different types of cancer, and their levels were correlated with disease progression. The circulating hematopoietic precursors in patients with cancer selectively lost their lymphoid potential, and were skewed toward granulocytic differentiation. Interestingly, these cells were highly enriched in tumor tissues, with similar phenotypic features, i.e., expressing myeloid but not lymphoid markers on these precursors. These observations indicated that in addition to their full maturation to produce cells of a given type, these myeloid precursors in patients with cancer may contribute to disease progression by homing to the tumor tissues. This notion was supported by our findings that tumor-infiltrating hematopoietic precursors expressed CXCR4, a major homing receptor for the chemokine SDF-1 to recruit and retain them in tumors (20).
The MDSCs are key modulators of immune dysfunction in tumor-bearing animals and patients with cancer. These cells comprise a heterogeneous population of IMCs capable of inhibiting immune responses and promoting angiogenesis. It should be noted that an increase in the generation and/or recruitment of IMCs following infection or vaccination does not necessarily reflect an expansion of immunosuppressive MDSCs (8, 34). Although the precise regulatory mechanisms are not yet clear, it is generally assumed that the expansion and subsequent activation of MDSCs are regulated by different factors with partially overlapping activities (8, 35). By establishing an in vitro short-term culture system with CB-derived CD34+ cells, the present study provided evidence that the tumor-derived cytokines, including GM-CSF, G-CSF, and IL-6, are sufficient to promote the differentiation of hematopoietic progenitors into IMCs and subsequent acquisition of the suppressive features of MDSCs. This conclusion is supported by our following observations. First, a short treatment with cytokine combinations markedly increased the frequencies of GMPs and the cells expressing high levels of myeloid markers, including CD14+HLA-DRlow/− cells that have been identified as a new type of human MDSCs. Accordingly, the levels of GM-CSF, G-CSF, and IL-6 were significantly increased in patients with cancer (Fig. S4) (15, 16). Second, stimulation with these cytokines up-regulated the expression of several key components for the activation of the MDSC suppressive program, including M-CSFR, IL-4Rα, arginase I, and C/EBPβ, on these cells. Third, when cocultured with allogenic T cells, the cytokine-stimulated MDSCs displayed strong immunosuppressive activity by directly inhibiting the proliferation and IFN-γ production of T cells. In addition to their direct inhibition of T cells, the cytokine-stimulated MDSCs also effectively induced the generation of regulatory T cells and attenuated the expression of CD3ε. These results also indicated that human MDSCs can be generated through a simple, short-term culture system by applying a combination of cytokines that are present in tumor environment.
Tumors can condition distant sites, such as the bone marrow and spleen, by releasing soluble factors that drive the accumulation of myeloid cells. Among factors produced by various tumors, GM-CSF, G-CSF, and IL-6 could induce the rapid generation of MDSCs from precursors present in bone marrow (12, 14) and CB (present study). However, these cytokines have different impacts on the generation and activation of MDSCs. In our in vitro culture model, we found that GM-CSF markedly increased the frequency of GMPs. G-CSF alone did not affect GMP frequency, but it had the most potent impact on the induction of CD14+ and CD15+ cells, as well as the expression of IL-4Rα and M-CSFR on these cells. Although IL-6 alone had only a marginal effect, it did yield an additive effect in combination with GM-CSF and G-CSF. These data support a two-stage model of MDSC involvement in cancer, which might allow for the flexible regulation of these cells in distinct tissue contexts.
Our results give important insights into the formation of MDSCs in human solid tumors. Cytokines and other soluble factors derived from cancer and/or stroma cells can alter the development of HSPCs and resulted in the increased level of circulating GMPs and myeloid-biased hematopoietic precursors. These IMCs are recruited and highly enriched in tumor tissues, where they are activated to acquire the suppressive property of MDSCs to promote tumor progression. This altered composition of circulating HSPCs can be found in all the different types of human solid tumors examined, and thus might serve as an important link between the dysregulated bone marrow hematopoiesis and accumulated MDSCs in patients with cancer. Given the increasing appreciation of MDSCs as a therapeutic target, studying mechanisms that selectively modulate or normalize the composition and differentiation of circulating HSPCs might provide a novel strategy for anticancer therapy.
Materials and Methods
Peripheral blood and tumor tissue samples or CB were obtained from the Cancer Center of Sun Yat-Sen University or the First Affiliated Hospital of Sun Yat-Sen University. All samples were coded anonymously in accordance with local ethical guidelines (as stipulated by the Declaration of Helsinki), and written informed consent was obtained. The protocol was approved by the review board of Sun Yat-Sen University.
Peripheral blood samples from 153 patients with pathologically confirmed hepatocellular (n = 62), breast (n = 9), colorectal (n = 38), ovarian (n = 4), lung (n = 13), esophageal (n = 4), and cervical (n = 23) carcinomas were taken before treatment. Paired fresh tumor and peritumor and normal mucosa (at least 5 cm away from tumor edge) tissues from eight patients with colon cancer were used for the isolation of tissue-infiltrating leukocytes and for immunofluorescence. The clinical stages were classified according to the International Union Against Cancer. The clinical characteristics of all patients are summarized in Table S1. Control blood samples were obtained from 112 healthy donors who took routine physical examinations at our cancer center, all of whom were negative for HCV, HBV, HIV, and syphilis. Detailed methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by National Basic Research Program of China Project Grants 2010CB529904 and 2011CB811305 and National Natural Science Foundation of China Grant 81230073.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320753111/-/DCSupplemental.
References
- 1.Kondo M, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 2.Laiosa CV, Stadtfeld M, Graf T. Determinants of lymphoid-myeloid lineage diversification. Annu Rev Immunol. 2006;24:705–738. doi: 10.1146/annurev.immunol.24.021605.090742. [DOI] [PubMed] [Google Scholar]
- 3.Essers MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458(7240):904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 4.King KY, Goodell MA. Inflammatory modulation of HSCs: Viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11(10):685–692. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: A human perspective. Cell Stem Cell. 2012;10(2):120–136. doi: 10.1016/j.stem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Nagai Y, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24(6):801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rieger MA, Hoppe PS, Smejkal BM, Eitelhuber AC, Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325(5937):217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68(13):5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui TX, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39(3):611–621. doi: 10.1016/j.immuni.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marigo I, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32(6):790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Corzo CA, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207(11):2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solito S, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118(8):2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsumata N, et al. Serum levels of cytokines in patients with untreated primary lung cancer. Clin Cancer Res. 1996;2(3):553–559. [PubMed] [Google Scholar]
- 16.Pang XH, et al. Preoperative levels of serum interleukin-6 in patients with hepatocellular carcinoma. Hepatogastroenterology. 2011;58(110-111):1687–1693. doi: 10.5754/hge10799. [DOI] [PubMed] [Google Scholar]
- 17.Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112(9):3543–3553. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang WW, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci USA. 2011;108(50):20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arndt K, et al. CD133 is a modifier of hematopoietic progenitor frequencies but is dispensable for the maintenance of mouse hematopoietic stem cells. Proc Natl Acad Sci USA. 2013;110(14):5582–5587. doi: 10.1073/pnas.1215438110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34(8):967–975. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Youn JI, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14(3):211–220. doi: 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma G, et al. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity. 2011;34(3):385–395. doi: 10.1016/j.immuni.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 24.Kuang DM, et al. Tumor-educated tolerogenic dendritic cells induce CD3epsilon down-regulation and apoptosis of T cells through oxygen-dependent pathways. J Immunol. 2008;181(5):3089–3098. doi: 10.4049/jimmunol.181.5.3089. [DOI] [PubMed] [Google Scholar]
- 25.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16(4):348–353. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 26.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin L, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5(1):31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Testa U, et al. Interleukin-3 receptor in acute leukemia. Leukemia. 2004;18(2):219–226. doi: 10.1038/sj.leu.2403224. [DOI] [PubMed] [Google Scholar]
- 31.Griseri T, McKenzie BS, Schiering C, Powrie F. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity. 2012;37(6):1116–1129. doi: 10.1016/j.immuni.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortez-Retamozo V, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci USA. 2012;109(7):2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung MR, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104(5):504–510. doi: 10.1002/jso.21986. [DOI] [PubMed] [Google Scholar]
- 34.Movahedi K, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111(8):4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 35.Gallina G, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116(10):2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.