Significance
Physical exercise causes profound changes in energy metabolism in humans. In this study we show that resting skeletal muscle has a crucial role in the metabolic response to acute exercise. During endurance exercise, selective induction of the protein angiopoietin-like 4 (ANGPTL4) in nonexercising muscle reduces local fatty acid uptake, presumably to prevent fat overload, while directing fatty acids to the active skeletal muscle as fuel. Our data thus suggest that nonexercising muscle has a key role in governing lipid homeostasis during exercise.
Abstract
Physical activity increases energy metabolism in exercising muscle. Whether acute exercise elicits metabolic changes in nonexercising muscles remains unclear. We show that one of the few genes that is more highly induced in nonexercising muscle than in exercising human muscle during acute exercise encodes angiopoietin-like 4 (ANGPTL4), an inhibitor of lipoprotein lipase-mediated plasma triglyceride clearance. Using a combination of human, animal, and in vitro data, we show that induction of ANGPTL4 in nonexercising muscle is mediated by elevated plasma free fatty acids via peroxisome proliferator-activated receptor-δ, presumably leading to reduced local uptake of plasma triglyceride-derived fatty acids and their sparing for use by exercising muscle. In contrast, the induction of ANGPTL4 in exercising muscle likely is counteracted via AMP-activated protein kinase (AMPK)-mediated down-regulation, promoting the use of plasma triglycerides as fuel for active muscles. Our data suggest that nonexercising muscle and the local regulation of ANGPTL4 via AMPK and free fatty acids have key roles in governing lipid homeostasis during exercise.
Acute exercise greatly increases the cellular demand for ATP, oxygen, glucose, and fatty acids. To meet these demands, acute exercise is associated with marked changes in skeletal muscle activity of key transporters and enzymes involved in glucose and fatty acid transport and oxidation (1). Much of the regulation occurs via allosteric regulation and covalent modification of rate-limiting enzymes. In addition, alterations at the level of mRNA increasingly are believed to represent an important regulatory mechanism in the acute response to exercise (2). Indeed, acute exercise induces mRNA expression of many genes involved in a variety of processes, including energy metabolism, hypertrophy, and signaling (3–6). Not surprisingly, most studies have focused on the events occurring in exercising muscle. In contrast, much less is known about the exercise-induced changes in nonexercising muscle. Studies have shown that resting skeletal muscle is crucial in the removal of lactate from the circulation during high-intensity exercise (7) and also plays a role in adrenaline and noradrenaline production during exercise (8). In addition, similar to exercising muscle, resting muscle exhibits enhanced phosphorylation of mTOR following resistance exercise (9). Overall, however, the impact of exercise on metabolic processes and gene expression in nonexercising muscles remains ill-defined. It can be envisioned that exercise may elicit changes in gene expression in nonexercising muscle via circulating mediators including muscle-derived myokines and metabolites (10). The present study was undertaken to try to elucidate the role of inactive muscle in the metabolic response to acute exercise.
Results
To investigate the molecular events occurring during exercise in nonexercising muscle, we carried out an acute exercise trial in which 12 human subjects performed moderate- to high-intensity cycling exercise with one leg, and muscle biopsies were taken before and after exercise from the exercising and nonexercising (resting) leg. One-legged cycling allows the direct analysis of the effects of acute exercise in exercising muscle, with the nonexercising leg serving as control leg. Microarray analysis was performed on all four muscle biopsies of nine subjects (4). Microarrays from two subjects failed to meet quality control criteria and were excluded from analysis, and one subject refused to have biopsies taken.
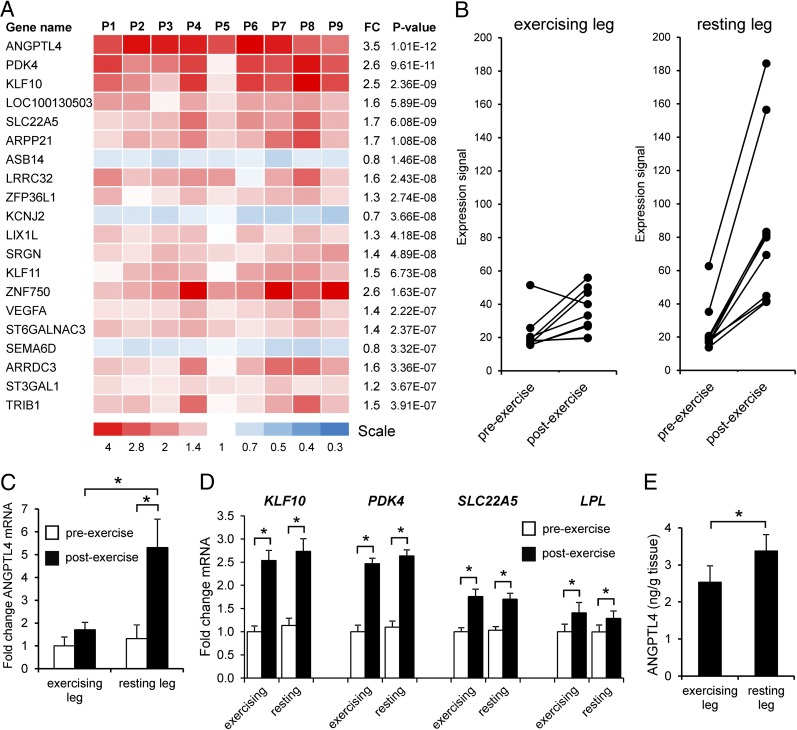
Surprisingly, the most significantly induced gene in the nonexercising leg was angiopoietin-like 4 (ANGPTL4) (Fig. 1A), a sensitive target of the peroxisome proliferator-activated receptor (PPAR) transcription factors that encodes a secreted inhibitor of the enzyme lipoprotein lipase (LPL) (11–13). LPL catalyzes hydrolysis of circulating triglycerides (TG) and therefore plays a key role in uptake of fatty acids in skeletal muscle (14). Paired individual gene-expression profiles in muscle biopsies from both legs clearly showed that ANGPTL4 was induced much more strongly in the nonexercising leg than in the exercising leg (Fig. 1B), and this finding was confirmed by quantitative PCR (qPCR) analysis (Fig. 1C). In fact, the microarray analysis indicated that ANGPTL4 was one of the few genes that was induced more highly in the nonexercising leg than in the exercising leg: Other PPAR targets such as PDK4, SLC22A5, and KLF10 were induced to the same extent in both legs (Fig. 1D). The same was true for LPL mRNA levels (Fig. 1D). In parallel with changes in ANGPTL4 mRNA, the content of ANGPTL4 protein in muscle after exercise was significantly higher in the nonexercising leg than in the exercising leg, as determined by ELISA (Fig. 1E).
Fig. 1.
Exercise induces ANGPTL4 gene expression in nonexercising human muscle. (A) Heatmap showing genes altered by exercise in the nonexercising leg muscle ranked by statistical significance. Values are displayed per subject (P1 to P9). Fold-change (FC) in gene expression is indicated. (B) mRNA expression profile of ANGPTL4 in the exercising and nonexercising legs according to microarray. (C) qPCR analysis of ANGPTL4 mRNA expression. (D) mRNA expression of PPARδ targets KLF10, PDK4, and SLC22A5, as well as LPL. (E) ANGPTL4 protein levels in postexercise muscle biopsies as determined by ELISA. Error bars represent SEM. *Significantly different according to paired Student t test (P < 0.01).
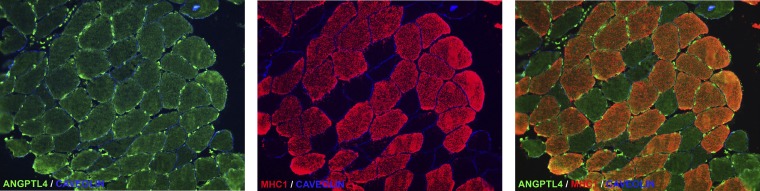
To localize ANGPTL4 protein in human muscle, we performed immunofluorescence staining, which revealed substantial staining of ANGPTL4 in human muscle fibers, with a slight preference for type 1 slow oxidative fibers (Fig. 2). Consistent with the function of ANGPTL4 as inhibitor of endothelial-bound LPL, most intense ANGPTL4 protein staining was observed in the capillaries (Fig. 2).
Fig. 2.
ANGPTL4 protein is detected in human muscle in myocytes and endothelial cells. Representative images of immunofluorescent staining of ANGPTL4 in a biopsy from human vastus lateralis muscle show ANGPTL4 in green, myosin heavy chain 1 (MHC1), a marker type I fiber, in red, and caveolin in blue.
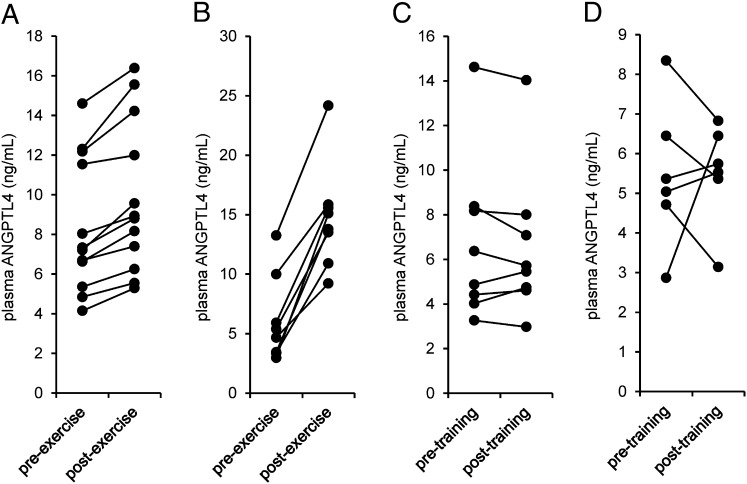
Induction of ANGPTL4 mRNA in skeletal muscle during one-legged exercise was associated with a significant increase in the concentration of plasma ANGPTL4 (Fig. 3A). Cycling with two legs for a more extended period (3 h) caused an even more significant increase in the concentration of plasma ANGPTL4 (Fig. 3B). In contrast, the concentration of plasma ANGPTL4 was not altered by 2 wk of intense endurance exercise training (Fig. 3C), or by a 12-wk endurance exercise program (Fig. 3D), indicating that ANGPTL4 is induced specifically by acute exercise.
Fig. 3.
Plasma ANGPTL4 levels are increased by acute exercise but not by exercise training. (A) Plasma ANGPTL4 levels before and after 1 h of one-legged cycling exercise at 50% of the one-legged Wmax (study A, n = 12). (B) Plasma ANGPTL4 levels before and after 3 h of cycling exercise at 40% Wmax (Study B, n = 8). (C) Fasting plasma ANGPTL4 levels before and after an intense 2-wk endurance training program on a cycling ergometer (study C, n = 8). (D) Fasting plasma ANGPTL4 levels before and after a moderate-intensity, 12-wk endurance training program on a cycling ergometer (study D, n = 6).
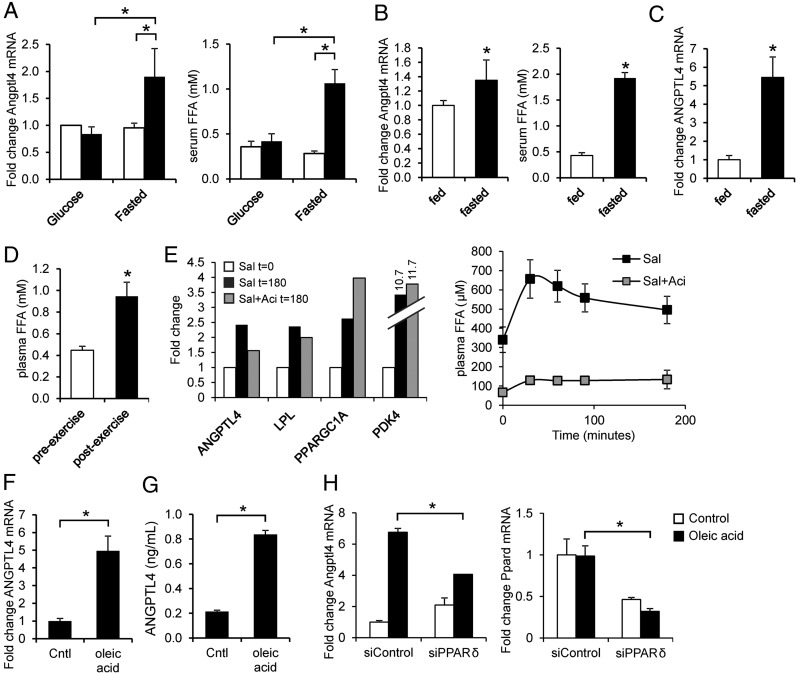
Because the nonexercising leg was inactive, changes in gene expression in the resting leg muscle cannot be caused by local contractile activity but must be related to systemic factors, including circulating metabolites. To verify this notion, serum from fasted human subjects collected before and after 2 h of cycling exercise was added to mouse C2C12 myotubes, and Angptl4 mRNA expression was determined by qPCR. Angptl4 mRNA was markedly increased in cells treated with postexercise serum as compared with preexercise serum (Fig. 4A). Intriguingly, no such effect was observed using pre- and postexercise plasma from subjects that received repeated glucose drinks during the cycling exercise (Fig. 4A). Angptl4 expression in C2C12 myotubes also was induced more strongly by plasma collected from subjects in the fasted state than by plasma from subjects in the fed state (Fig. 4B). In those subjects, ANGPTL4 expression in skeletal muscle also was markedly higher in the fasted state than in the fed state (Fig. 4C). Together, these data suggest that ANGPTL4 is induced by a circulating factor that is specifically induced by exercise in the fasted state but is not elevated when glucose is consumed during exercise and that also is enriched in the fasted state as compared with fed state. One such parameter is plasma free fatty acids (FFA) (Fig. 4 A and B, Right), levels of which also increased during one-legged cycling (Fig. 4D) and which previously had been shown to induce Angptl4 mRNA potently in various cell types, including cultured myocytes (15–18). In support of a role of plasma FFA in induction of ANGPTL4 gene expression in human muscle, raising plasma FFA levels by salbutamol treatment markedly increased muscle ANGPTL4 expression, and this increase was largely blunted when salbutamol was coadministered with the lipolysis inhibitor acipimox (Fig. 4E). This expression pattern was not found for other relevant genes such as PPARGC1A and PDK4.
Fig. 4.
Sensitive induction of the ANGPTL4 gene by FFAs in human and mouse myocytes. (A) C2C12 myotubes were incubated for 6 h with 10% serum from subjects (n = 5) before exercise (white bar) and after exercise (black bar) performed in fasted state or with provision of glucose (study E). (Left) Angptl4 mRNA. (Right) Serum FFA levels. (B) C2C12 myotubes were incubated for 3 h with 10% serum from subjects (n = 12) at the end of a 60-h fast or after 60 h in the normal fed condition (study F). (Left) Angptl4 mRNA levels. (Right) Serum FFA levels. (C) ANGPTL4 mRNA in muscle biopsies collected at the end of the 60-h fast or after 60 h in the normal fed condition (study F). (D) Plasma FFA levels before and after one-legged exercise (n = 12). (E) (Left) Pooled mRNA expression of selected genes in muscle biopsies collected before and after salbutamol infusion with and without prior acipimox administration (study G, n = 9). (Right) Plasma FFA levels during salbutamol (Sal) infusion. Error bars represent SEM. (F and G) ANGPTL4 mRNA (F) and ANGPTL4 (G) concentration in medium in primary human myotubes treated with oleic acid. (H) Angptl4 and Ppard mRNA in C2C12 myotubes transfected with control (nontargeting) or with PPARδ siRNA and treated with oleic acid. *Significantly different according to Student t test (P < 0.05). Error bars represent SD unless otherwise indicated. Cells were treated for 12 h unless otherwise indicated.
To study ANGPTL4 gene regulation by fatty acids further, we used cultured myocytes. Oleic acid markedly induced ANGPTL4 mRNA in human primary myotubes (Fig. 4F), accompanied by a similar increase in the secretion of ANGPTL4 protein (Fig. 4G). Oleic acid also markedly induced Angptl4 expression in C2C12 myotubes, and this expression was significantly blunted upon siRNA-mediated knockdown of PPARδ (Fig. 4H). Consistent with the high sensitivity of ANGPTL4 gene regulation to stimulation by fatty acids, microarray analysis indicated that ANGPTL4 was the second most highly induced gene by oleic acid in human primary myotubes and mouse C2C12 myotubes (Fig. S1). Taken together, these data strongly suggest that the marked induction of ANGPTL4 mRNA in the nonexercising human muscle is caused by increased plasma FFA levels associated with exercise.
Despite being exposed to elevated plasma FFA, exercising human muscle shows only a very minor increase in ANGPTL4 expression. Accordingly, we hypothesized that induction of ANGPTL4 mRNA in exercising muscle by FFA is mitigated by a contraction-related factor. If so, expression of ANGPTL4 should increase strongly after cessation of exercise when performed in the fasted state, because plasma FFA levels remain high for hours after such exercise. Indeed, in the continued fasted state, ANGPTL4 mRNA in exercising human muscle is increased 20-fold at 4 h postexercise as compared with baseline postexercise, and the increase in ANGPTL4 mRNA is concurrent with sustained elevation of plasma FFA (Fig. S2).
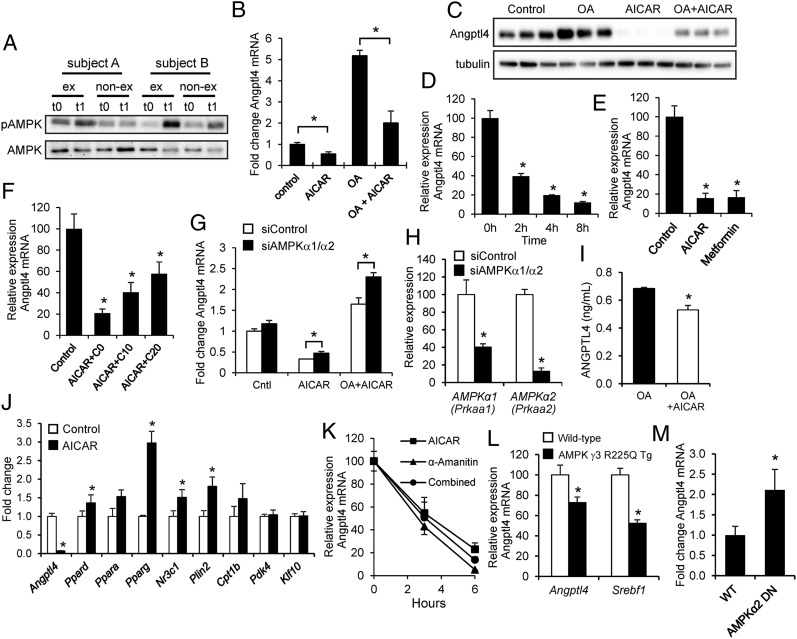
A candidate factor that may suppress ANGPTL4 in exercising muscle is AMP-activated kinase (AMPK), which is activated specifically in exercising muscle but not in nonexercising muscle during one-legged exercise (19, 20). In our study, even though muscle biopsies were collected ∼10–15 min postexercise, we found enhanced AMPK phosphorylation in some, but not all, subjects (Fig. 5A). To study the impact of AMPK activation on Angptl4 expression, we treated mouse C2C12 myotubes with the AMPK activator 5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide (AICAR), leading to phosphorylation of AMPK (Fig. S3A). Strikingly, AICAR treatment markedly reduced Angptl4 mRNA (Fig. 5B) and protein (Fig. 5C) in C2C12 myotubes and blunted the induction of Angptl4 expression by oleic acid. The suppressive effect of AICAR on Angptl4 mRNA was very fast (Fig. 5D), was mimicked by the alternative AMPK activator metformin (Fig. 5E), could be partially abolished by the AMPK inhibitor compound C (Fig. 5F and Fig. S3B), and was modestly relieved upon combined knockdown of the AMPK α1 and α2 subunits (Fig. 5 G and H). AICAR also modestly but significantly reduced the oleic acid-induced up-regulation of ANGPTL4 secretion in primary human myotubes (Fig. 5I). The reduction of Angptl4 mRNA in C2C12 myotubes was not mediated by down-regulation of PPARδ, PPARα, or PPARγ, because expression of all three PPARs was increased rather than decreased by AICAR treatment (Fig. 5J). Target genes of PPARδ (the main PPAR isotype in muscle), including Plin2, Pdk4, Klf10, and Cpt1b, also either were increased or remained unchanged by AICAR, as was the glucocorticoid receptor Nr3c1, another transcriptional inducer of Angptl4 (Fig. 5J) (21). These data suggest that down-regulation of Angptl4 mRNA by AMPK activation is not mediated by any of the known transcriptional regulators of Angptl4. Time-course studies in C2C12 myotubes indicated that AICAR reduces Angptl4 gene expression with nearly the same speed as the transcriptional inhibitor α-amanitin. No additive effect of α-amanitin and AICAR was observed, suggesting that AMPK activation almost completely blocks Angptl4 gene transcription (Fig. 5K). In vivo overexpression of an activating mutant of the muscle-specific isoform of the AMPKγ subunit supported the suppressive effect of AMPK on Angptl4 gene expression (Fig. 5L) (22). Conversely, in vivo overexpression of a dominant-negative mutant of the AMPKα2 subunit led to a significant induction of Angptl4 mRNA (Fig. 5M) (23). The data suggest that the stimulatory effect of plasma FFA on skeletal muscle ANGPTL4 mRNA is counteracted by AMPK activation in exercising muscle.
Fig. 5.
AMPK activation suppresses Angptl4 mRNA. (A) Immunoblot for AMPK and phospho-AMPK in skeletal muscle biopsies from two selected subjects before (t0) and after (t1) exercise. (B) Expression of Angptl4 mRNA in C2C12 myotubes treated with oleic acid (200 μM) and/or AICAR (1 mM) for 3 h. (C) Immunoblot for ANGPTL4 in C2C12 myotubes treated with oleic acid and/or AICAR. (D) Time-course of the effect of AICAR on Angptl4 mRNA in C2C12 myotubes. (E) Comparison of the effect of AICAR (1 mM) and metformin (0.5 mM) on Angptl4 mRNA in C2C12 myotubes. (F) Effect of AICAR (1 mM) and compound C cotreatment on Angptl4 mRNA in C2C12 myotubes. Concentrations are indicated in millimolars. (G) Angptl4 mRNA in C2C12 myotubes transfected with control (nontargeting) or AMPKα1/AMPKα2 siRNA and treated with AICAR. (H) Effective knockdown of AMPKα1 and AMPKα2 by AMPKα1/AMPKα2 siRNA. (I) ANGPTL4 levels in medium of human primary myotubes treated with oleic acid and AICAR. (J) Expression of PPARs and PPAR targets in C2C12 myotubes treated with AICAR. (K) Angptl4 mRNA in C2C12 myotubes preincubated with 50 μg/mL α-Amanitin for 1 h and treated with AICAR for 3 h or 6 h. (L) Angptl4 mRNA in the gastrocnemius of mice that overexpress an activating mutant of the muscle-specific isoform of the AMPKγ subunit. Error bars represent SEM. Data were extracted from GSE4065 (22). (M) Angptl4 mRNA in the gastrocnemius of mice that overexpress a dominant-negative mutant of the AMPKα2 subunit. Cells were treated for 12 h unless otherwise indicated. Error bars represent SEM. *Significantly different according to Student t test (P < 0.05). Error bars represent SD unless otherwise indicated.
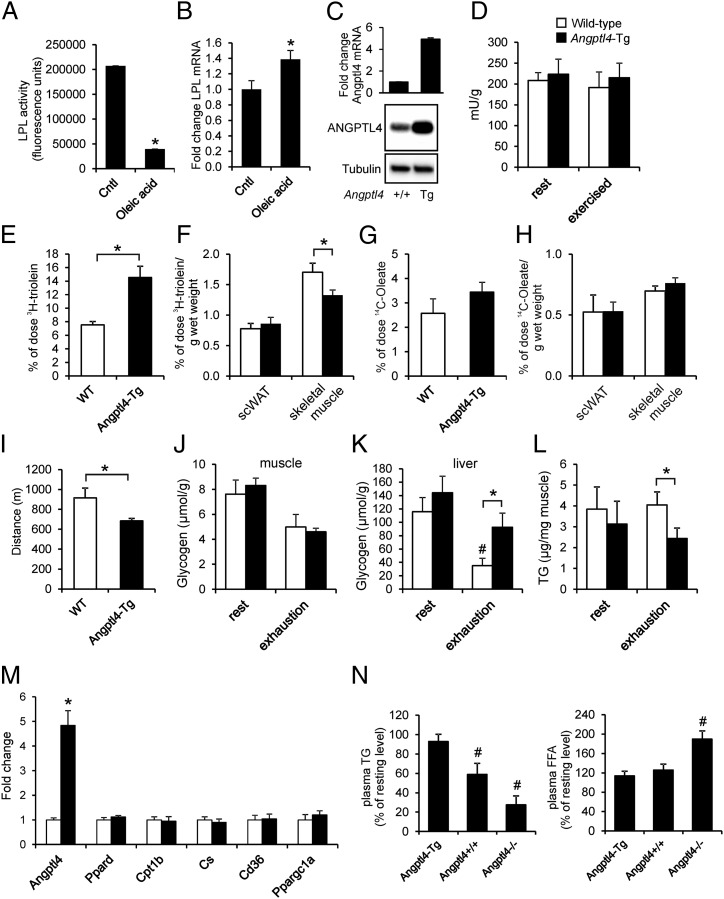
As previously observed for the PPARδ agonist GW501516 (17), induction of Angptl4 mRNA in C2C12 myotubes by oleic acid was associated with a pronounced decrease in heparin-releasable LPL activity (Fig. 6A) but induced Lpl mRNA (Fig. 6B). To study the impact of Angptl4 up-regulation on skeletal muscle lipid uptake in vivo, we used Angptl4-transgenic mice characterized by overexpression of Angptl4 mRNA and protein in a variety of tissues, including skeletal muscle (Fig. 6C) (24). Transgenic Angptl4 overexpression did not affect muscle weights or lean body mass percentage (Fig. S4). To assess the functional effect of Angptl4 overexpression during exercise, we subjected WT and Angptl4-transgenic (Angptl4-Tg) mice to an acute moderate exercise bout on a motorized treadmill. Total LPL protein levels in skeletal muscle (gastrocnemius) after exercise were not different in the WT and Angptl4-Tg mice (Fig. 6D). However, Angptl4-Tg mice showed markedly reduced plasma clearance of [3H]triolein-labeled very-low-density lipoprotein (VLDL)-like particles during exercise (Fig. 6E) and reduced fatty acid uptake from the labeled VLDL-like particles into skeletal muscle but not into subcutaneous adipose tissue (Fig. 6F). Plasma clearance of [14C]-oleic acid and uptake into skeletal muscle and subcutaneous adipose tissue was unaffected in Angptl4-Tg mice (Fig. 6 G and H). Plasma levels of FFA, glucose, glycerol, and β-hydroxybutyrate were not different between the WT and Angptl4-Tg mice in the exercised or resting state (Fig. S5A). To assess whether reduced muscle uptake of plasma TG-derived fatty acids in Angptl4-Tg mice had any influence on muscle performance, we determined the maximal endurance capacity using an incremental treadmill protocol characterized by a gradual increase in speed and slope of the treadmill until exhaustion. Strikingly, Angptl4-Tg mice ran significantly less far than WT mice (Fig. 6I). Strength, as determined using the horizontal wire test, was not different in the two sets of animals (Fig. S5B). Depletion of muscle glycogen stores during exhaustive exercise was comparable in WT and Angptl4-Tg mice (Fig. 6J), whereas liver glycogen levels remained higher in Angptl4-Tg mice after exhaustive exercise, a difference that reached statistical significance (Fig. 6K). Intramuscular TGs were lower in Angptl4-Tg mice, and this difference also reached significance after exhaustive exercise (Fig. 6L). No differences in baseline expression of markers of oxidative capacity were observed in the two genotypes, suggesting that Angptl4 does not influence oxidative capacity (Fig. 6M). Finally, we determined the relative decrease in plasma TG in the exercised state as compared with resting state in WT, Angptl4-Tg, and Angptl4−/− mice, as a measure of relative plasma TG utilization. The relative decrease in plasma TG in the exercised vs. the resting state was much more pronounced in Angptl4−/− mice than in WT and especially Angptl4-Tg mice (Fig. 6N), likely because of the preferential use of plasma TG in Angptl4−/− mice. Conversely, the relative increase in plasma FFA in the exercised vs. resting state was more pronounced in Angptl4−/− mice, likely because of the sparing of plasma FFA in favor of the use of TG-derived fatty acids. Overall, these data indicate that up-regulation of Angptl4 impairs skeletal muscle uptake of fatty acids from circulating TG-rich lipoproteins and reduces liver glycogen utilization, leading to decreased performance of exhaustive exercise.
Fig. 6.
Angptl4 up-regulation impairs LPL activity and uptake of plasma TG-derived fatty acids in muscle. (A and B) Heparin-releasable LPL activity (A) and Lpl mRNA (B) in mouse C2C12 myotubes treated with oleic acid (400 µM) for 24 h. (C) ANGPTL4 protein abundance and Angptl4 mRNA expression in mouse skeletal muscle. (D) Total LPL activity in skeletal muscle (gastrocnemius) of WT and Angptl4-Tg mice at rest and after 90 min of moderate running exercise (12 m/min). (E) Serum 3H activity after 15 min of running in WT and Angptl4-Tg mice injected with [14C]-oleate together with glycerol tri[3H]oleate-labeled VLDL-like particles. (F) 3H-activity in subcutaneous adipose tissue and gastrocnemius after 15 min of running. (G) Serum 14C activity after 15 min of running in WT and Angptl4-Tg mice injected with [14C]-oleate together with glycerol tri[3H]oleate-labeled VLDL-like particles. (H) 14C activity in subcutaneous adipose tissue and gastrocnemius after 15 min of running. (I) Distance covered, excluding warm-up, by WT and Angptl4-Tg mice subjected to an incremental exercise test to exhaustion. (J–L) Muscle glycogen (J), liver glycogen (K), and muscle TG (L) levels in WT and Angptl4-Tg mice at rest or after exhaustive running exercise. (M) mRNA expression of selected genes in skeletal muscle (gastrocnemius) of WT and Angptl4-Tg mice at rest. (N) The relative levels of plasma TG (Left) and FFA (Right) in the exercised state (90 min of moderate running exercise) compared with the resting state (90 min rest) in WT, Angptl4-Tg, and Angptl4−/− mice. *Significantly different from WT mice according to Student t test (P < 0.05). #Significantly different from resting mice according to Student t test (P < 0.05). Error bars represent SEM; n = 6–10 mice per group.
Discussion
The activity of LPL and the associated uptake in tissue of fatty acids derived from TG in plasma is under the control of different physiological stimuli in different tissues (14). In white adipose tissue LPL activity is decreased by fasting, which has been demonstrated unequivocally to be mediated by up-regulation of Angptl4 (25). In brown adipose tissue LPL activity is increased by exposure to cold; such exposure is associated with a decrease in Angptl4 mRNA (26), hinting at a potential role of ANGPTL4. Our data suggest that ANGPTL4 also plays an important role in LPL-dependent plasma clearance of TG in skeletal muscle, particularly during acute exercise, by coordinating lipid uptake in exercising and nonexercising muscles.
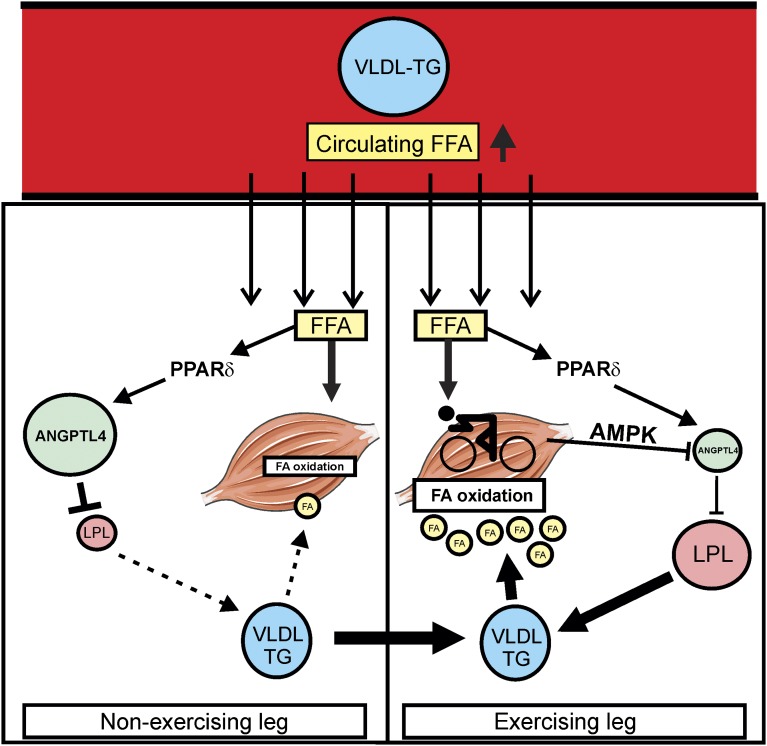
Acute exercise increases adipose tissue lipolysis and raises levels of FFA in plasma. Although the abundant plasma FFA are oxidized efficiently in exercising muscle, their increase leads to elevated intramuscular fat storage in nonexercising muscle, possibly leading to lipotoxicity (27). Previously, we found that ANGPTL4 functions as a fatty acid-inducible antilipotoxic factor in cardiomyocytes and macrophages (15, 28). The present work suggests that the exercise-induced increase in plasma FFA stimulates ANGPTL4 synthesis in nonexercising human muscle, leading to local inhibition of LPL activity and diminished uptake of fatty acids derived from plasma TG in compensation for elevated uptake of plasma FFA, presumably to mitigate lipid overload and associated lipotoxicity in nonexercising muscle during prolonged exercise. In contrast, in exercising muscle the stimulatory effect of FFA on ANGPTL4 is counteracted by AMPK-mediated suppression of ANGPTL4 mRNA, thereby maintaining LPL activity and supporting the use of plasma TG as fuel for the exercising muscle (Fig. 7).
Fig. 7.
Schematic representation of the proposed role of ANGPTL4 in providing lipid to exercising muscle. During exercise, circulating FFAs and VLDL particles are directed to exercising and nonexercising muscle. In the nonexercising leg, increased FFA levels provoke an increase in ANGPTL4 expression via PPARδ, leading to inhibition of LPL activity and consequent reduction in uptake of fatty acids derived from VLDL, which likely is aimed at preventing lipid overload. In contrast, in the exercising leg the stimulatory effect of FFA on ANGPTL4 mRNA is counteracted by AMPK-mediated suppression of ANGPTL4 mRNA. As a result, LPL activity remains high, allowing full exploitation of fatty acids derived from VLDL as the substrate for fatty acid oxidation to meet the energetic needs of exercising muscle.
Previously, in vivo AMPK activation by AICAR was found to lower plasma TG levels (29, 30). Moreover, AMPK activation by AICAR and metformin increased intralipid clearance and increased heparin-releasable LPL activity in rat hearts without causing any change in LPL mRNA (31, 32). Furthermore, AICAR and metformin enhanced LPL activity in rat L6 muscle cells (33). These findings suggest a role of LPL in the lowering of plasma TG by AMPK. Based on the data presented here, it is plausible that the stimulatory effect of AICAR and metformin on LPL activity and the concomitant lowering of plasma TG is mediated by suppression of ANGPTL4 in muscle and possibly other tissues. Future studies are needed to address this question in more detail.
Our data indicate that overexpression of Angptl4 reduces maximal performance in endurance exercise, most likely by limiting the provision of fatty acids derived from plasma TG to the muscle and by limiting the utilization of liver glycogen, which is an important fuel for exercising mice. Until recently, because of a number of methodological issues, the role of plasma TG as source of fatty acids for oxidation in exercising muscle and thereby the overall importance of plasma TG as fuel during endurance exercise likely have been underestimated (reviewed in ref. 1). Indeed, plasma TG uptake and clearance are increased many fold during leg exercise as compared with the resting situation (34). The relevance of plasma TG during exercise is suggested further by the adaptive increase in muscle LPL mRNA and activity in response to an acute exercise bout and after exercise training (35, 36). Unlike ANGPTL4, the expression of LPL is induced to the same extent in exercising and nonexercising muscle. The enhanced activity of LPL in skeletal muscle is believed to account for the low plasma TG concentrations in trained individuals (37, 38).
ANGPTL4 adds to a growing list of secreted proteins whose production in muscle is increased by acute exercise (39, 40). For many of these proteins it is unclear whether the change in production mainly impacts the muscle locally or whether the protein also exerts an endocrine effect and thus functions as a myokine. Even though acute exercise leads to elevated ANGPTL4 levels in the circulation, it is unclear whether this increase stems mainly from the increased mRNA and production of ANGPTL4 in skeletal muscle or whether other tissues contribute as well. Overall, the coexpression of ANGPTL4 with LPL in tissues such as muscle and adipose tissue and the tissue-specific regulation of ANGPTL4 expression suggest that ANGPTL4 may act mainly via local inhibition of LPL (17, 41).
In conclusion, our data indicate that, in addition to the responses in exercising muscle, molecular changes in nonexercising muscle likely play a key role in regulating the fuel supply during exercise. It can be speculated that the beneficial effects of exercise on various health parameters are conveyed by adaptive changes in nonexercising muscles.
Experimental Procedures
Human Intervention Studies.
Twelve healthy men (age 51.5 ± 5.1 y, body weight 88 ± 17 kg, body mass index 26 ± 4) participated in study A (4). Subjects were asked to follow healthy eating guidelines the day before the experiment and to refrain from alcohol consumption. Subjects fasted from 10:00 PM the evening before the study began until the end of the intervention. During the exercise session subjects were allowed to drink water ad libitum. All subjects performed a single endurance exercise bout, consisting of 60 min of one-legged cycling at 50% of their one-legged maximal work load (Wmax) (determined by a graded one-legged cycling test). One-legged cycling was performed on a cycle ergometer (Excalibur Sport) adapted with a custom-made leg support. Skeletal muscle biopsies were taken before and after exercise from both legs of 11 subjects, with the average time of collection at ∼15 min pre- or postexercise. One subject became apprehensive about the needle biopsy immediately before the first biopsy collection. Two subjects later were excluded from the microarray analysis because the microarrays failed to meet quality control criteria. In addition, a venous blood sample was taken before and shortly after exercise. Other human intervention studies included in this paper have been published previously and are outlined briefly below.
In study B, eight young, untrained, healthy male subjects (age: 23.3 ± 3.2 y) performed 3 h of cycling on an electromagnetically braked ergometer at an intensity of 40% of the predetermined Wmax (42). To facilitate completion of the exercise test, subjects received two 125-mL servings of a maltodextrin drink during the second half of the test. Subjects were allowed to drink water ad libitum during the entire test. Plasma was collected before and after 3 h of cycling exercise and was used for measurement of ANGPTL4.
In study C, eight young, untrained, healthy male subjects (age: 23.3 ± 3.2 y) followed a 2-wk exercise training program on a cycling ergometer (42). Training consisted of alternating days of interval and endurance training and always started with 7.5 min of warming up at 40% Wmax and ended with 7.5 min of cooling down at 40% Wmax. Fasting plasma was collected before and after the 2- wk training program and was used for the measurement of ANGPTL4. Plasma was collected 3 d after the last training session.
In study D, six healthy, nonobese male subjects (age: 42.7 ± 2.0 y) followed a 12-wk exercise training program on a cycling ergometer (43). Subjects trained three times per week for 12 wk for 47.5 ± 2.5 min at 40% of predetermined maximal oxygen consumption (VO2 max). Fasting plasma was collected before and after the 12-wk training program and was used for measurement of ANGPTL4. Plasma was collected 3 d after the last training session.
Seven healthy, untrained male volunteers (age: 22.7 ± 0.6 y) participated in study E. After the subjects fasted overnight, a Teflon cannula was inserted in an antecubital vein for sampling of blood. Subjects rested on a bed, and a baseline blood sample was taken. Immediately thereafter, subjects ingested 1.4 g/kg bodyweight glucose or water. Subjects exercised at 50% VO2 max for 2 h and then rested for 4 h. At regular intervals subjects ingested 0.35 g/kg bodyweight glucose or water. Muscle biopsies were collected before exercise and after the 4-h rest period. All subjects underwent the experimental protocol twice, once with glucose ingestion and once while fasting (44).
Twelve healthy, lean male volunteers (age: 23.6 ± 1.0 y) participated in study F. Subjects stayed in the respiration chamber for 60 h in the normal fed condition or while being completely fasted, according to a randomized crossover design with a 2-wk washout period. Blood samples were collected at the end of the study after an overnight fast (fed condition) or after a cumulative 60-h fast. Around the same time, a muscle biopsy was taken (45).
Nine healthy, lean male volunteers (age: 24.4 ± 1.3 y) participated in study G. After subjects fasted overnight, two Teflon cannulas were inserted into an antecubital vein of each arm. One cannula was used for the infusion of salbutamol, and one was used for sampling blood. A first blood sample and muscle biopsy were taken, followed by a continuous infusion of salbutamol for 3 h. In addition, two doses (250 mg) of acipimox or placebo were given orally at time −120 min and time 0. Blood samples were taken at regular intervals throughout the study (46). A second muscle biopsy was taken after the 3-h infusion.
Informed consent was obtained from all subjects. The studies were approved by the Medical Ethics Committee of the institute involved (Wageningen University or Maastricht University).
Blood Samples.
Blood was collected in tubes containing EDTA and was centrifuged immediately for 10 min (1,000 × g, 4 °C). Blood samples were analyzed for FFA levels (Centre for Medical Diagnostics, Velp, The Netherlands) and ANGPTL4 levels (see below).
Skeletal Muscle Biopsies.
Percutaneous needle biopsies were taken before and shortly after exercise from the vastus lateralis muscle from both legs, using the Bergström technique with suction. All biopsies were taken from separate incisions; the second biopsy in the leg was located 2 cm proximal to the first biopsy. Biopsies were taken on average 10–15 min before and after the exercise bout. All biopsies were collected, processed, and frozen within 30 min postexercise. After each biopsy, the collected tissue sample was cleared carefully of adipose tissue and blood and was frozen directly in liquid nitrogen or was embedded into Tissue-Tek O.C.T. compound (Sakura Tissue Tek) and frozen in liquid nitrogen-cooled isopentane.
Muscle biopsy lysates were prepared using a lysis buffer consisting of 50 mM Tris⋅HCl (pH 7.5), 150 mM NaCL, 1 mM EGTA, 1 mM EDTA, 0.27 M sucrose, and 2% Triton X-100. Protease and phosphatase inhibitors (Complete and PhosSTOP; Roch Diagnostics GmbH) were added to the buffer. The buffer was added to tissue in a 15:1 (buffer: tissue) ratio, and the tissue was lysed using TissueLyser II (Qiagen).
Immunofluorescence.
Frozen muscle sections (5-μm thick) were treated with 0.1% Triton X-100 in PBS and were incubated for 45 min at room temperature with the primary antibody mix [a polyclonal rabbit hANGPTL4, a mouse monoclonal IgM antibody directed to MHC1 (type 1 muscle fibers; Developmental Studies Hybridoma Bank), and a mouse monoclonal IgG1 antibody to caveolin-3 (BD Biosciences)] diluted in 0.05% Tween20 in PBS. After three washing steps with PBS, sections were incubated for 45 min at room temperature with the appropriate fluorescent-labeled secondary antibodies. The specificity of the antibody for ANGPTL4 was demonstrated previously via immunoblot of human plasma using appropriate peptide controls and was corroborated by immunochemical and immunofluorescence staining of ANGPTL4 in human heart, intestine, and skin wounds, using appropriate negative controls (15, 47, 48).
Animal Experiments.
Exercise studies were carried out using the TSE PhenoMaster treadmill module (TSE Systems) that allowed four mice to run simultaneously. Four- to five-month-old littermate male WT and Angptl4-Tg mice bred on the C57BL/6 background for 15 generations were used for the exercise experiments. The number of mice per group varied from six to nine mice. All mice were acclimatized to the treadmill (10 m/min for 10 min) on three consecutive days. On the day of exercise, mice were fasted (4 h) and either were subjected to a moderate exercise protocol (12 m/min at 0% incline for 90 min) or ran until exhaustion (30 min at 12 m/min followed by an increase of 2.5 m/min and 2° of incline every 5 min until exhaustion). A mouse was considered exhausted if it failed to run after it was prompted by a slider with plastic bristles three times within 1 min. The moment of exhaustion was determined by an investigator unaware of the genotype of the mice. A control group of nonexercising mice from each genotype remained sedentary inside the treadmill. Upon cessation of exercise mice were anesthetized immediately using isoflurane, and blood was collected by orbital puncture. Mice then were killed by cervical dislocation, and tissues were excised quickly and were frozen immediately in liquid nitrogen.
The horizontal wire test was performed by placing a steel wire between a gray PVC stand at a height of 30 cm. Mice hung on the wire with their forepaws. Time measurements started at the moment that the researcher judged the mouse had a good grip on the wire. Time measurement ended when the animal grasped the wire with at least one hind paw. If the animal fell, it was placed back in its cage for a 3-min rest, and measurements were repeated.
Animal experiments were approved by the Animal Ethics Committee of Wageningen University.
Plasma TG and FFA Clearance.
After a 6-h fast, 6-mo-old male WT and Angptl4-Tg mice were put on a treadmill at a speed of 14m/min. After 45 min of running, mice were injected with a single bolus of [14C]-oleate and glycerol tri[3H]oleate-labeled VLDL-like particles and continued to run for another 15 min. After 15 min, blood was collected; then the animal was killed immediately and tissues were collected. Tissues and serum were processed to determine 3H and 14C activity. Preparation of VLDL-like particles, complexing of [14C]-oleate with BSA, the infusion protocol, and calculations were carried out as described previously (49–51).
Cell Culture and Treatment.
C2C12 myoblasts were maintained in DMEM (Lonza) supplemented with 10% (vol/vol) FCS and 1% penicillin/streptomycin/ amphotericin (PSA) under 5% CO2 at 37 °C. At ∼70–90% confluency, myoblasts were treated or full-growth medium was replaced with alphaMEM (Lonza) supplemented with 2% (vol/vol) FCS and 1% PSA to promote differentiation into myotubes. The differentiation medium was changed every 2–3 d. C2C12 myotubes were used for treatment after 1 wk of differentiation. All experiments with C2C12 myotubes were performed in differentiation medium.
C2C12 myotubes were treated with human serum by replacing FCS with human serum and were incubated for 3–6 h. For siRNA-mediated knockdown, C2C12 cells were transfected with siRNA sequences using Lipofectamine RNAiMax the day before differentiation was started. The ON-TARGETplus SMARTpool for PPARδ, AMPKα1, AMPKα2, and nontargeting was used (Dharmacon/Thermo-Fisher Scientific) at 100 pmol/mL The medium was replaced by differentiation medium (DMEM supplemented with 2% (vol/vol) horse serum, 100 µg/mL penicillin, and 100 µg/mL streptomycin) after 24 h. After 4 d of differentiation, cells were treated with oleic acid and AICAR as indicated below. Differentiated human primary myotubes were prepared as described previously (52).
Oleic acid (200 µM) was added to cells complexed with BSA (2.5:1). AICAR and metformin were used at 1 mM. The AMPK inhibitor compound C was used at concentrations indicated in the Fig. 5 legend. Cells were treated for 12 h unless otherwise indicated.
RNA Isolation and qPCR.
Total RNA was isolated using TRIzol reagent (Invitrogen) and was purified for microarray analysis using the Qiagen RNeasy Micro kit (Qiagen).
RNA was reverse transcribed using a First-Strand cDNA Synthesis Kit (Fermentas). Real-time PCR was carried out using SensiMiX (Bioline) on a CFX 384 Bio-Rad thermal cycler (Bio-Rad). Cyclophilin, GADPH, and/or 36B4 were used as housekeeping genes. Primers used are listed in Table 1.
Table 1.
Primer sequences
| Forward primer | Reverse primer | |
| m36B4 | ATGGGTACAAGCGCGTCCTG | GCCTTGACCTTTTCAGTAAG |
| Cyclophilin | CAGACGCCACTGTCGCTTT | TGTCTTTGGAACTTTGTCTGCAA |
| PGC1-a | AGACGGATTGCCCTCATTTGA | TGTAGCTGAGCTGAGTGTTGG |
| Cpt1b | ATCATGTATCGCCGCAAACT | CCATCTGGTAGGAGCACATGG |
| Lpl | CAGCTGGGCCTAACTTTGAG | GACCCCCTGGTAAATGTGTG |
| Angptl4 | GTTTGCAGACTCAGCTCAAGG | CCAAGAGGTCTATCTGGCTCTG |
| Ppard | TTGAGCCCAAGTTCGAGTTTG | CGGTCTCCACACAGAATGATG |
| Cd36 | TCCAGCCAATGCCTTTGC | TGGAGATTACTTTTCAGTGCAGAA |
| Plin2 | GGATGTGGTGACGACTACCAT | ACAGACTTGGTCCTTTCCACG |
| Pdk4 | TCTACAAACTCTGACAGGGCTTT | CCGCTTAGTGAACACTCCTTC |
| Ppara | TATTCGGCTGAAGCTGGTGTAC | CTGGCATTTGTTCCGGTTCT |
| Pparg | CACAATGCCATCAGGTTTGG | GCTGGTCGATATCACTGGAGATC |
| Prkaa1 | TTCGGGAAAGTGAAGGTGGG | TCTTCTGCCGGTTGAGTATCT |
| Prkaa2 | CAGGCCATAAAGTGGCAGTTA | AAAAGTCTGTCGGAGTGCTGA |
| Cs | GGACAATTTTCCAACCAATCTGC | TCGGTTCATTCCCTCTGCATA |
| Klf10 | ATGCTCAACTTCGGCGCTT | CGCTTCCACCGCTTCAAAG |
| ANGPTL4 | CACAGCCTGCAGACACAACTC | GGAGGCCAAACTGGCTTTGC |
| GAPDH | CATGTTCCAGTATGACTCCACTC | GGCCTCACCCCATTTGATGT |
Supplementary Material
Acknowledgments
This work was supported by Dutch Diabetes Foundation Grant 2009.60.003, the Netherlands Nutrigenomics Centre, and the Netherlands Organization of Scientific Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession nos. GSE41769, GSE18589, and GSE38590).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400889111/-/DCSupplemental.
References
- 1.Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006;86(1):205–243. doi: 10.1152/physrev.00023.2004. [DOI] [PubMed] [Google Scholar]
- 2.Keller P, et al. Using systems biology to define the essential biological networks responsible for adaptation to endurance exercise training. Biochem Soc Trans. 2007;35(Pt 5):1306–1309. doi: 10.1042/BST0351306. [DOI] [PubMed] [Google Scholar]
- 3.Buford TW, Cooke MB, Willoughby DS. Resistance exercise-induced changes of inflammatory gene expression within human skeletal muscle. Eur J Appl Physiol. 2009;107(4):463–471. doi: 10.1007/s00421-009-1145-z. [DOI] [PubMed] [Google Scholar]
- 4.Catoire M, et al. Pronounced effects of acute endurance exercise on gene expression in resting and exercising human skeletal muscle. PLoS ONE. 2012;7(11):e51066. doi: 10.1371/journal.pone.0051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmutz S, et al. Endurance training modulates the muscular transcriptome response to acute exercise. Pflugers Arch. 2006;451(5):678–687. doi: 10.1007/s00424-005-1497-0. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol (1985) 2005;98(5):1745–1752. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]
- 7.Buckley JD, Scroop GC, Catcheside PG. Lactate disposal in resting trained and untrained forearm skeletal muscle during high intensity leg exercise. Eur J Appl Physiol Occup Physiol. 1993;67(4):360–366. doi: 10.1007/BF00357636. [DOI] [PubMed] [Google Scholar]
- 8.Savard G, et al. Noradrenaline spillover during exercise in active versus resting skeletal muscle in man. Acta Physiol Scand. 1987;131(4):507–515. doi: 10.1111/j.1748-1716.1987.tb08270.x. [DOI] [PubMed] [Google Scholar]
- 9.Apró W, Blomstrand E. Influence of supplementation with branched-chain amino acids in combination with resistance exercise on p70S6 kinase phosphorylation in resting and exercising human skeletal muscle. Acta Physiol (Oxf) 2010;200(3):237–248. doi: 10.1111/j.1748-1708.2010.02151.x. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein L, et al. Angptl4 upregulates cholesterol synthesis in liver via inhibition of LPL- and HL-dependent hepatic cholesterol uptake. Arterioscler Thromb Vasc Biol. 2007;27(11):2420–2427. doi: 10.1161/ATVBAHA.107.151894. [DOI] [PubMed] [Google Scholar]
- 12.Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci USA. 2006;103(46):17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida K, Shimizugawa T, Ono M, Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J Lipid Res. 2002;43(11):1770–1772. doi: 10.1194/jlr.c200010-jlr200. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Eckel RH. Lipoprotein lipase: From gene to obesity. Am J Physiol Endocrinol Metab. 2009;297(2):E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 15.Georgiadi A, et al. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ Res. 2010;106(11):1712–1721. doi: 10.1161/CIRCRESAHA.110.217380. [DOI] [PubMed] [Google Scholar]
- 16.Kersten S, et al. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler Thromb Vasc Biol. 2009;29(6):969–974. doi: 10.1161/ATVBAHA.108.182147. [DOI] [PubMed] [Google Scholar]
- 17.Robciuc MR, et al. Angiopoietin-like 4 mediates PPAR delta effect on lipoprotein lipase-dependent fatty acid uptake but not on beta-oxidation in myotubes. PLoS ONE. 2012;7(10):e46212. doi: 10.1371/journal.pone.0046212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staiger H, et al. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes. 2009;58(3):579–589. doi: 10.2337/db07-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristensen JM, et al. Absence of humoral mediated 5’AMP-activated protein kinase activation in human skeletal muscle and adipose tissue during exercise. J Physiol. 2007;585(Pt 3):897–909. doi: 10.1113/jphysiol.2007.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mascher H, Ekblom B, Rooyackers O, Blomstrand E. Enhanced rates of muscle protein synthesis and elevated mTOR signalling following endurance exercise in human subjects. Acta Physiol (Oxf) 2011;202(2):175–184. doi: 10.1111/j.1748-1716.2011.02274.x. [DOI] [PubMed] [Google Scholar]
- 21.Koliwad SK, et al. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J Biol Chem. 2009;284(38):25593–25601. doi: 10.1074/jbc.M109.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson EC, et al. Opposite transcriptional regulation in skeletal muscle of AMP-activated protein kinase gamma3 R225Q transgenic versus knock-out mice. J Biol Chem. 2006;281(11):7244–7252. doi: 10.1074/jbc.M510461200. [DOI] [PubMed] [Google Scholar]
- 23.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7(5):1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 24.Mandard S, et al. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J Biol Chem. 2006;281(2):934–944. doi: 10.1074/jbc.M506519200. [DOI] [PubMed] [Google Scholar]
- 25.Kroupa O, et al. Linking nutritional regulation of Angptl4, Gpihbp1, and Lmf1 to lipoprotein lipase activity in rodent adipose tissue. BMC Physiol. 2012;12:13. doi: 10.1186/1472-6793-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartelt A, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17(2):200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 27.Schrauwen-Hinderling VB, et al. Intramyocellular lipid content is increased after exercise in nonexercising human skeletal muscle. J Appl Physiol (1985) 2003;95(6):2328–2332. doi: 10.1152/japplphysiol.00304.2003. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein L, et al. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab. 2010;12(6):580–592. doi: 10.1016/j.cmet.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergeron R, et al. Effect of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 2001;50(5):1076–1082. doi: 10.2337/diabetes.50.5.1076. [DOI] [PubMed] [Google Scholar]
- 30.Buhl ES, et al. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51(7):2199–2206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- 31.An D, et al. The metabolic “switch” AMPK regulates cardiac heparin-releasable lipoprotein lipase. Am J Physiol Endocrinol Metab. 2005;288(1):E246–E253. doi: 10.1152/ajpendo.00211.2004. [DOI] [PubMed] [Google Scholar]
- 32.Hauton D. Does long-term metformin treatment increase cardiac lipoprotein lipase? Metabolism. 2011;60(1):32–42. doi: 10.1016/j.metabol.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohira M, Miyashita Y, Murano T, Watanabe F, Shirai K. Metformin promotes induction of lipoprotein lipase in skeletal muscle through activation of adenosine monophosphate-activated protein kinase. Metabolism. 2009;58(10):1408–1414. doi: 10.1016/j.metabol.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 34.Enevoldsen LH, Simonsen L, Bülow J. Postprandial triacylglycerol uptake in the legs is increased during exercise and post-exercise recovery. J Physiol. 2005;568(Pt 3):941–950. doi: 10.1113/jphysiol.2005.091975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton MT, Etienne J, McClure WC, Pavey BS, Holloway AK. Role of local contractile activity and muscle fiber type on LPL regulation during exercise. Am J Physiol. 1998;275(6 Pt 1):E1016–E1022. doi: 10.1152/ajpendo.1998.275.6.E1016. [DOI] [PubMed] [Google Scholar]
- 36.Vissing K, Andersen JL, Schjerling P. Are exercise-induced genes induced by exercise? FASEB J. 2005;19(1):94–96. doi: 10.1096/fj.04-2084fje. [DOI] [PubMed] [Google Scholar]
- 37.Hardman AE. The influence of exercise on postprandial triacylglycerol metabolism. Atherosclerosis. 1998;141(Suppl 1):S93–S100. doi: 10.1016/s0021-9150(98)00225-1. [DOI] [PubMed] [Google Scholar]
- 38.Seip RL, Semenkovich CF. Skeletal muscle lipoprotein lipase: Molecular regulation and physiological effects in relation to exercise. Exerc Sport Sci Rev. 1998;26:191–218. [PubMed] [Google Scholar]
- 39.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 40.Pillon NJ, Bilan PJ, Fink LN, Klip A. Cross-talk between skeletal muscle and immune cells: Muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab. 2013;304(5):E453–E465. doi: 10.1152/ajpendo.00553.2012. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson SK, et al. Triacylglycerol-rich lipoproteins protect lipoprotein lipase from inactivation by ANGPTL3 and ANGPTL4. Biochim Biophys Acta. 2012;1821(10):1370–1378. doi: 10.1016/j.bbalip.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Schrauwen-Hinderling VB, et al. The increase in intramyocellular lipid content is a very early response to training. J Clin Endocrinol Metab. 2003;88(4):1610–1616. doi: 10.1210/jc.2002-021464. [DOI] [PubMed] [Google Scholar]
- 43.Schrauwen P, et al. The effect of a 3-month low-intensity endurance training program on fat oxidation and acetyl-CoA carboxylase-2 expression. Diabetes. 2002;51(7):2220–2226. doi: 10.2337/diabetes.51.7.2220. [DOI] [PubMed] [Google Scholar]
- 44.Schrauwen P, et al. Effect of acute exercise on uncoupling protein 3 is a fat metabolism-mediated effect. Am J Physiol Endocrinol Metab. 2002;282(1):E11–E17. doi: 10.1152/ajpendo.2002.282.1.E11. [DOI] [PubMed] [Google Scholar]
- 45.Hoeks J, et al. Prolonged fasting identifies skeletal muscle mitochondrial dysfunction as consequence rather than cause of human insulin resistance. Diabetes. 2010;59(9):2117–2125. doi: 10.2337/db10-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoeks J, et al. Effect of beta1- and beta2-adrenergic stimulation on energy expenditure, substrate oxidation, and UCP3 expression in humans. Am J Physiol Endocrinol Metab. 2003;285(4):E775–E782. doi: 10.1152/ajpendo.00175.2003. [DOI] [PubMed] [Google Scholar]
- 47.Alex S, et al. ANGPTL4 is produced by entero-endocrine cells in the human intestinal tract. Histochem Cell Biol. 2013 doi: 10.1007/s00418-013-1157-y. [DOI] [PubMed] [Google Scholar]
- 48.Goh YY, et al. Angiopoietin-like 4 interacts with integrins beta1 and beta5 to modulate keratinocyte migration. Am J Pathol. 2010;177(6):2791–2803. doi: 10.2353/ajpath.2010.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jong MC, et al. Reduced very-low-density lipoprotein fractional catabolic rate in apolipoprotein C1-deficient mice. Biochem J. 1997;321(Pt 2):445–450. doi: 10.1042/bj3210445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rensen PC, et al. Particle size determines the specificity of apolipoprotein E-containing triglyceride-rich emulsions for the LDL receptor versus hepatic remnant receptor in vivo. J Lipid Res. 1997;38(6):1070–1084. [PubMed] [Google Scholar]
- 51.Teusink B, et al. Contribution of fatty acids released from lipolysis of plasma triglycerides to total plasma fatty acid flux and tissue-specific fatty acid uptake. Diabetes. 2003;52(3):614–620. doi: 10.2337/diabetes.52.3.614. [DOI] [PubMed] [Google Scholar]
- 52.Sparks LM, et al. Remodeling lipid metabolism and improving insulin responsiveness in human primary myotubes. PLoS ONE. 2011;6(7):e21068. doi: 10.1371/journal.pone.0021068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.