Significance
The relationship between the origin of animals and the oxygen content of the atmosphere is a pressing issue in historical geology. Here we challenge the widely held view that low levels of atmospheric oxygen delayed the origin of animals up until 850–542 million years ago. We provide experimental evidence suggesting that the last common ancestor of animals could have thrived in oxygen levels as low as 0.5% to 4% of present atmospheric levels, which were likely met on Earth well before animals evolved. This was achieved by observing the survival of sponges, basal animals similar to the earliest metazoans, under low-oxygen conditions in the laboratory. These results encourage us to reconsider the environmental constraints on the origin of animal life.
Keywords: hypoxia, Metazoa
Abstract
A rise in the oxygen content of the atmosphere and oceans is one of the most popular explanations for the relatively late and abrupt appearance of animal life on Earth. In this scenario, Earth’s surface environment failed to meet the high oxygen requirements of animals up until the middle to late Neoproterozoic Era (850–542 million years ago), when oxygen concentrations sufficiently rose to permit the existence of animal life for the first time. Although multiple lines of geochemical evidence support an oxygenation of the Ediacaran oceans (635–542 million years ago), roughly corresponding with the first appearance of metazoans in the fossil record, the oxygen requirements of basal animals remain unclear. Here we show that modern demosponges, serving as analogs for early animals, can survive under low-oxygen conditions of 0.5–4.0% present atmospheric levels. Because the last common ancestor of metazoans likely exhibited a physiology and morphology similar to that of a modern sponge, its oxygen demands may have been met well before the enhanced oxygenation of the Ediacaran Period. Therefore, the origin of animals may not have been triggered by a contemporaneous rise in the oxygen content of the atmosphere and oceans. Instead, other ecological and developmental processes are needed to adequately explain the origin and earliest evolution of animal life on Earth.
Geochemical evidence points to the general oxygenation of the oceans around 635 million years ago (Ma) (1), with an oxygenation of the deep oceans around 580 Ma, likely requiring a minimum of 10% present atmospheric levels (PAL) (2). This oxygenation of the deep ocean roughly corresponds with the conspicuous (up to 1 m in size) appearance of the so-called Ediacara macrobiota, which likely included metazoan stem lineages and other multicellular eukaryotes (3). This correlation is consistent with the notion that elevated atmospheric oxygen levels during this time finally permitted the evolution of metazoans, which have relatively high oxygen demands compared with the aerobic microbes that thrived earlier in the Proterozoic Eon (4–8). However, the relative timing between the origin of animals and the proposed Neoproterozoic rise of atmospheric oxygen remains ambiguous. Indeed, according to present evidence, the earliest proposed signs of animals in the rock record (9, 10), as well as molecular clock divergence estimates (11, 12), predate the earliest evidence for deep ocean oxygenation (1), potentially by tens of millions of years. Furthermore, to establish that low levels of atmospheric oxygen, in fact, delayed the origin of animal life on Earth, it is necessary to know the minimum oxygen requirements of early animals.
Although the oxygen requirements of early animals are not well established, there are multiple theoretical estimates (5, 6, 13–17). The first estimates suggest that 1% PAL was sufficient to initiate the evolution of metazoans (5), although later estimates suggest higher requirements, between 6% and 10% PAL (6, 17). The estimated minimum oxygen requirements for the sheet-like Dickinsonia, a prominent member of the Ediacara macrobiota and putative stem group metazoan (18), fall within 1–3% PAL (14). Similar estimates for the minimum oxygen requirements for the last common ancestor of bilaterians range from 0.14% to 0.36% PAL, depending primarily on the organism’s length, width, and possession of a vascular system (19). For example, although an animal limited by pure diffusion for its internal oxygen supply is estimated to require 10% PAL to reach millimeter width (15), a diffusion-limited, 600-μm-long by 25-μm-wide worm is estimated to require ∼0.36% PAL, with a 3-mm-long by 67-μm-wide worm with a circulatory system requiring only ∼0.14% PAL (19). Therefore, these estimates suggest that early animals, in general, may have had relatively low oxygen requirements. However, although these theoretical efforts provide some guidance, they are based on highly simplified, idealized organisms that do not reflect the complexity of living animals. Therefore, to more conclusively determine the minimum oxygen requirements of metazoans, direct experimental evidence is required. Such evidence can reasonably be obtained by studying modern animals that resemble the earliest metazoans.
Numerous phylogenetic analyses conclude that sponges are the earliest diverging extant metazoan group (11, 12, 20–22). The sponge body plan is characterized by a series of internal chambers and canals lined with flagellated feeding cells called choanocytes. These choanocytes morphologically resemble choanoflagellates, a group of single-celled microbial eukaryotes known to form colonies and feed like choanocytes (23). Choanoflagellates, in turn, are the closest living relatives of animals (24–28). This suggests that the similarities between choanocytes and choanoflagellates were inherited from a common ancestor and that the choanocyte-based feeding mode, as retained by sponges, is likely ancestral to all animals (29).
Because the last common ancestor of all living animals likely had a sponge-like body plan, the physiology and oxygen requirements of modern sponges should serve as a reasonable window into the oxygen requirements of the earliest animals. For the sponge body plan, most metabolically active cells, notably the choanocytes and pinacocytes, border and surround the sponge mesohyl, the collagen-rich, fibrous matrix that functions as an endoskeleton. Because these cells are always in direct contact with seawater, they do not rely on the diffusion of oxygen from the external environment into the interior of the animal, nor do they require oxygen delivery via a true circulatory system. Therefore, the oxygen requirements of sponges might be relatively low (13, 19). Indeed, sponges have been observed in modern low-oxygen environments, such as the Saanich Inlet on Vancouver Island, where Suberites simplex is abundant near the oxic–anoxic interface (30). Sponges were also observed on the Volcano 7 seamount in the eastern tropical Pacific, where oxygen concentrations ranged from 4.5 to 7.2 μM O2 (31). Despite these observations, however, the minimum oxygen requirements of modern sponges and their metabolic response to low oxygen have not, to our knowledge, been investigated.
In this study we explore the respiration kinetics and low-oxygen tolerance of the demosponge Halichondria panicea (Fig. 1).We show that although sensitive to oxygen, H. panicea can both tolerate and grow at low oxygen levels from 0.5% to 4% PAL. Because these levels may have been present in the environment well before animals originated, animal evolution may not have been prohibited by the presumably low atmospheric oxygen levels before the Neoproterozoic Era.
Fig. 1.

Photograph of H. panicea, a temperate marine demosponge collected in the Kerteminde Fjord, Denmark, at 1 m depth.
Methods
We collected specimens of H. panicea (32) from the well-oxygenated surface waters of Kerteminde Fjord, Denmark. After collection, our specimens were kept in aquaria with a constant and rapidly circulating supply of Keterminde Fjord seawater. To determine the respiration kinetics and oxygen tolerance of H. panicea, we carried out two sets of experiments. In the first, we monitored H. panicea’s respiration rate under a wide range of oxygen concentrations. In the second, we observed H. panicea’s long-term response to a continuous supply of seawater deoxygenated to 3–4% PAL.
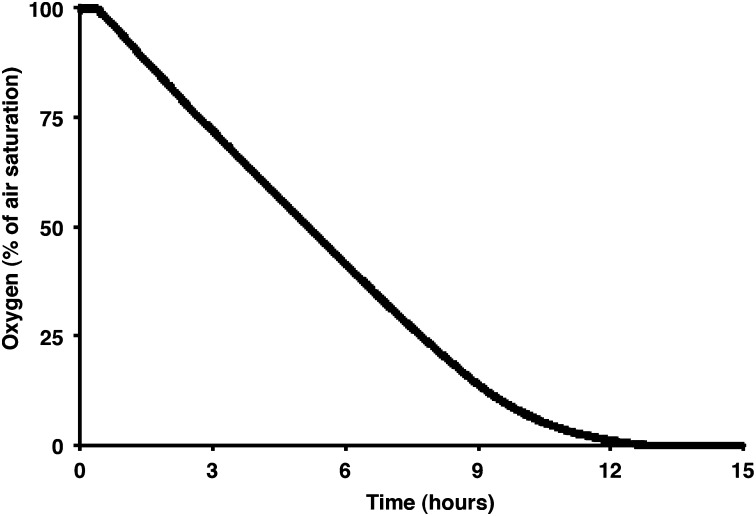
During experiment 1, we observed individual sponges in a tinfoil-covered, well-stirred, 1-L aquarium monitored with an oxygen microelectrode (33). The alga Rhodomonas sp., a unicellular phytoplankton, was used as a food source and was replenished periodically during the course of the experiment. Initially, we conducted a sponge-free control where we monitored the system’s background levels of oxygen consumption and Rhodomonas clearance. Next, the sponge was introduced, and the aquarium was sealed again. We then monitored the oxygen drawdown from full air saturation to anoxia (Fig. 2) and fit these results to a Michaelis–Menten kinetic model (Eq. 1):
Fig. 2.
Drawdown from 100% air saturation to anoxia, to calculate how respiration rate varies with oxygen concentration.
Here V represents the oxygen uptake rate, with Vmax signifying the maximum respiration rate, which occurs when the oxygen concentration ([O2]) is at its highest (air saturation). Km, which is of particular interest here, represents the oxygen concentration when the respiration rate is half of Vmax.
After anoxia was reached, the system was reoxygenated, followed by a controlled stepwise decrease in oxygen concentration, where progressively lower oxygen levels were maintained for 1–3 d each, ultimately bringing the system down to the lowest oxygen range possible of regulation. These steps were as follows: 70%, 50%, 20%, 10%, 5%, and finally 0.5–4% of air saturation. Once this final oxygen range was achieved, it was maintained for up to 10 d. To regulate the oxygen concentration around a particular level, as the oxygen concentration fell below a critical threshold (slightly below the desired oxygen concentration), a peristaltic pump switched on to pump oxic water through the silicon tubing coiled at the base of the aquarium. This pumping allowed oxygen to diffuse back into the system (Fig. S1). During each of these steps, the oxygen concentration of the aquarium decreased as the sponge respired, which provided a measure of the sponge respiration rate. Additionally, the sponge’s feeding rate, based on the rate of clearance of Rhodomonas cells, was determined by measuring the fluorescence of the water over time with a fluorometer.
Finally, we removed the sponge and conducted a negative control to determine the water’s final background respiration and Rhodomonas clearance rates. Throughout the experiment, the vitality of the sponges was assessed by comparing the feeding and respiration rates to those obtained during the negative controls, coupled to qualitative observations of sponge health. Fresh seawater was exchanged once a week using a peristaltic pump to avoid the potentially lethal accumulation of waste products. See Supporting Information for further details of the experimental setup.
During experiment 2, we compared the long-term development, spanning multiple weeks, of sponges kept under a constant flow of oxygenated seawater with sponges receiving seawater degassed to 3–4% PAL oxygen. Our particular interest here was monitoring the maintenance of healthy tissue and the growth of new tissue during parallel experiments where the sponges were supplied with a continuous flow of new water at both low and high oxygen levels. Unlike the first setup, this system constantly supplied flowing water to the sponges, avoiding the potentially detrimental accumulation of metabolic waste products. We also used terminal restriction fragment length polymorphism (T-RFLP) and functional and 16S rRNA gene sequencing to investigate H. panicea’s microbiome. For the details of these procedures, see Supporting Information.
Results and Discussion
Oxygen Limits of Sponge Respiration.
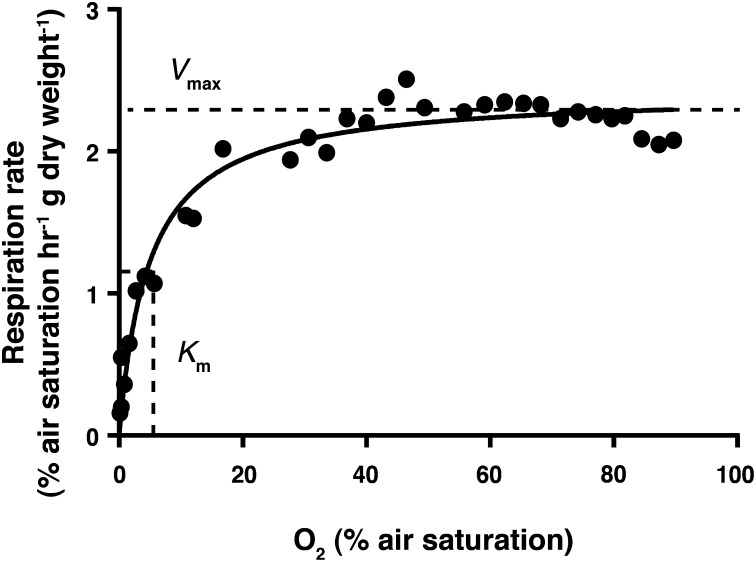
Overall, the patterns of oxygen uptake observed during the initial drawdown to anoxia followed Michaelis–Menten kinetics, with a Vmax of 2.42% air saturation/h⋅gdry weight, or 7.93 μM O2/h⋅gdry weight, and a Km of 4.86% of air saturation, or 15.9 μM O2, at the water salinity and temperature of our experiments (Fig. 3). This Km is less than half of the 10% PAL serving as the estimated minimum level of atmospheric oxygen thought to have permitted the oxygenation of the deep oceans around 580 Ma (2), as well as the level estimated to permit the existence of millimeter-width, diffusion-limited animals (15).
Fig. 3.
Michaelis–Menton kinetics: the maximum respiration rate, Vmax, was 2.42% air saturation/h⋅gdry weight or 7.93 μM O2/h⋅gdry weight, and Km, the oxygen level where the respiration rate is half of Vmax, was 4.86% of air saturation or 15.9 μM O2.
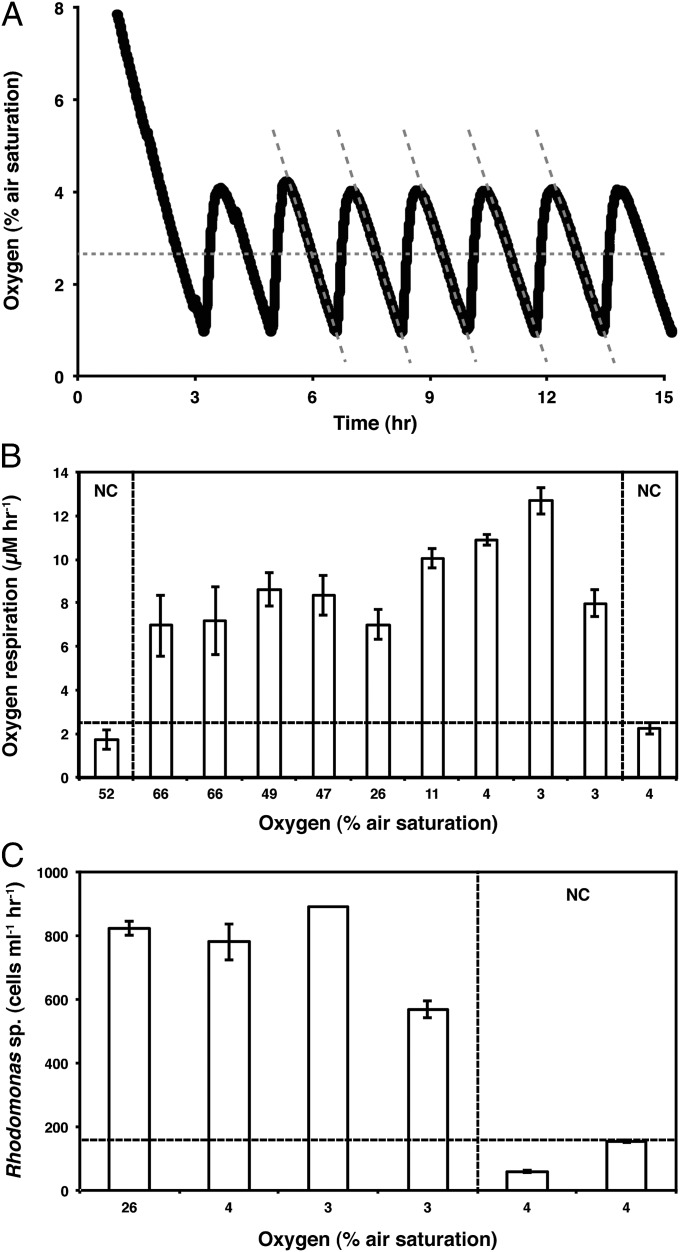
Characteristic examples of the respiration and feeding rates of H. panicea at the different oxygen levels maintained during active oxygen regulation are shown in Fig. 4. Some oxygen respiration was apparent during the sponge-free controls at the end of the experiment, amounting to 25% of the rates measured in the presence of living sponges (Fig. 4B). Some clearance of Rhodomonas sp. was also observed during postexperiment negative controls, at 14% of the rates observed while the sponges were active (Fig. 4C). Therefore, the majority of the oxygen uptake and Rhodomonas consumption observed during the experiments can be attributed to the sponges, with the background rates likely attributed to free-living microbes in our experimental system. Overall, the sponges were able to both respire and feed to oxygen levels cycling between 0.5% and 4% PAL. These were the lowest levels we could maintain without driving the sponges into anoxia during part of the oxygen control cycle.
Fig. 4.
Respiration and feeding under low oxygen. (A) Respiration curve showing oxygen regulation around a set point of 2.5% air saturation (indicated by the horizontal dashed line). The respiration rates are equal to the slopes of each downward curve (indicated by the diagonal dashed lines). (B) Oxygen consumption rates of a single sponge under different ambient oxygen levels. The areas marked with NC represent measurements taking during the sponge-free negative control. The horizontal line marks the maximum respiration rate observed under the negative control. (C) Clearance rates of Rhodomonas sp. cells under different oxygen concentrations. The area to right of the vertical dashed line designates the sponge-free controls. The horizontal dashed line marks the maximum clearance rate achieved during the negative controls.
While the sponges continued to feed and respire above background rates under the lowest oxygen range we could control, in many cases, the sponge showed visible signs of cell death (or “necrosis,” noted by dark patches of dead tissue) by the end of the experiment. This motivated us to assess whether this degradation of sponge health was (i) an experimental artifact due to the accumulation of sponge waste during the experiment, (ii) a problem with long-term sponge survival at low oxygen, or (iii) a general problem with keeping sponges in captivity within this particular setup for prolonged periods. We addressed this by constructing our second setup.
With our second experimental setup (Fig. S2), a relatively small sponge with numerous branching structures completely retained its healthy appearance under incubation at 3–4% PAL oxygen for a full 24 d, after which the experiment was terminated. Indeed, this individual even displayed signs of growth, evidenced by the elongation of several of its smaller protrusions (Fig. S3) and by the growth of new biomass on the walls of the incubation container (Fig. S4). Given the complete absence of necrosis in the sponges maintained at both low and high oxygen levels during this run, the necrosis observed during experiment 1 was likely due to the infrequent exchange of fresh seawater and not necessarily the low oxygen levels.
However, during another run of experiment 2, the sponge kept at 3–4% PAL developed necrosis after its water became inadvertently anoxic for a period of 4 h on the ninth day of incubation (Figs. S3 and S5). This condition persisted for the next 5 d of the experiment under 3–4% PAL oxygen. The necrosis, however, disappeared when the sponge was reintroduced to fully oxygenated water (Fig. S5). This suggests that although 3–4% PAL oxygen may not compromise H. panicea’s growth or maintenance of healthy tissue, anoxia still does.
Our results suggest that H. panicea is sensitive to both anoxia and prolonged exposure to relatively stagnant seawater. The sensitivity of sponges to water stagnation is not too surprising because sponges are notoriously difficult to maintain in aquaria, possibly due to the accumulation of metabolic products that interfere with their metabolism. The sensitivity to anoxia is also not surprising because H. panicea is an aerobic organism. Moreover, like many other sponges, H. panicea hosts a diversity of symbiotic microbes, and in the case of our sponges, functional gene and 16S rRNA analyses reveal a significant population of ammonium-oxidizing Archaea and betaproteobacteria, as well as other aerobic organisms (Fig. S6 and Table S1). Internal anoxia, however, does appear to be a common feature of sponge physiology (34, 35). This suggests that sponges, in general, are adapted to endure internal low-oxygen conditions.
H. panicea is classified as a low-microbial abundance (LMA) sponge (36, 37). LMA sponges are known to have shorter and wider water canals (38), as well as larger choanoflagellate chambers (39), than high-microbial abundance (HMA) sponges. Presumably, this allows LMA sponges to better maintain oxic conditions within their interiors because the surface area to volume ratio of their water canals is relatively low. Because of this, as water pumps through the water canal system, less oxygen diffuses out of the canals. In contrast, narrower water canals, as found in HMA sponges, have higher surface area to volume ratios, allowing relatively more oxygen to diffuse out of the canals during seawater pumping. It would appear, then, that LMA sponges, such as H. panicea, may be better adapted to low-oxygen conditions than HMA sponges. Although we did not test for this explicitly, our observations are also consistent with the idea that smaller sponges have an increased tolerance to low-oxygen conditions relative to larger sponges. This idea makes sense as smaller sponges have shorter water canals, which should result in less oxygen drawdown as water is pumped through the sponge. Taken together, it seems plausible that even smaller sponges, especially those with a low microbial abundance, could metabolize and grow under even lower oxygen concentrations than the 0.5–4% PAL range explored in our experiments.
Sponge Evolution and Environmental Oxygen Levels.
Recent molecular clock estimates place the last common ancestor of all sponges at nearly 800 Ma (12), whereas putative sponge-like body fossils are found in rocks dated between 660 and 635 Ma (10) in the Cryogenian Period (850–635 Ma). Elevated levels of possible demosponge biomarkers are also reported from rocks of similar age (9), but there is ambiguity as to whether these biomarkers truly originate from sponges (40). Unequivocal sponge body fossils are only first described in the lower Cambrian Period (41, 42). Nevertheless, if the body fossil and/or biomarker evidence are substantiated as true sponge fossils, they would reinforce the molecular clock divergence estimates and suggest that sponges evolved sometime during the Cryogenian Period. Such an early evolution for sponges would be well before the general oxygenation of the deep oceans in the Ediacaran Period.
Although levels of atmospheric oxygen may have reached 10% PAL by 580 Ma, the geochemical record does not preclude oxygen levels of 0.5–4% PAL during the Cryogenian Period (43, 44). Indeed, oxygen levels this high may have been reached as early as the Great Oxidation Event some 2.3–2.4 billion years ago (43, 45) and may have persisted at these levels through much of the intervening time (43, 44), with some likely swings to lower levels, however, around 1.8 billion years ago (46). Oxygen levels supportive of sponges may have been met even earlier, at least locally, in Archean oxygen oases (47).
Therefore, it is possible that the oxygen content of the atmosphere was completely permissive to the origin and early evolution of sponge-grade metazoans well before their evolutionary first appearance. This would mean, in turn, that the origin of animal life did not require a concurrent oxygenation event. Indeed, biological developments, such as the evolution of sophisticated gene regulatory networks, may have controlled the timing of animal origins more so than environmental parameters (12, 19, 48). Overall, animal-grade multicellularity likely required the accumulation and integration of specific traits, including cell adhesion molecules necessary for multicellular body plans, signaling proteins for cell–cell communication, and transcription factors essential for coordinated development (49). The accumulation of these traits and not the enhanced availability of oxygen may have controlled the onset of animal evolution.
Is there, then, any causal relationship between the geochemical record of increased ocean oxygenation during the late Neoproterozoic Era and animal evolution? It is argued that before the advent of suspension-feeding metazoans and large export-prone eukaryotic phytoplankton, the relatively slow sinking of organic detritus in the oceans resulted in higher water turbidity, with the decomposition of this slowly settling organic matter enhancing water column anoxia at shallow depths (48, 50–52). The evolution of large planktonic eukaryotes and the evolution of animals themselves may have changed this dynamic by producing large, rapidly settling particles, such as the tests of large plankton and the fecal pellets of pelagic animals. This rapidly settling organic debris would have promoted the decomposition of surface-derived organic matter at greater depths, producing less oxygen demand in the upper regions of the ocean and allowing, in turn, for the greater oxygenation of these waters (51, 52). In this way, the geochemical proxies used to track ancient marine redox conditions may be revealing a biologically induced ventilation of the Neoproterozoic oceans, as opposed to an oxygenation of the atmosphere (52). Under this scenario, the origin of animal life was not a response to atmospheric oxygenation but part of the ecological reworking of marine biogeochemical cycles.
Conclusion
This study shows that sponges can survive, and even thrive, under oxygen levels as low as 0.5–4% of present-day levels. However, sponges are sensitive to anoxia, and anecdotal evidence points to the reasonable conclusion that small body size facilitates survival under low-oxygen conditions (53). Therefore, and not surprisingly, smaller sponges are likely more tolerant of low oxygen levels than larger sponges. Although the precise history of the oxygen content of the atmosphere remains uncertain, it seems likely that the levels supportive of sponge respiration and growth were environmentally attained well before sponges themselves evolved. In this view, the origin and earliest evolution of animal life on Earth was not triggered by a rise of atmospheric and marine oxygen in the Neoproterozoic Era.
Supplementary Material
Acknowledgments
We thank A. Glud for constructing and repairing the microelectrodes; D. Mathiesen, S. Ballo, and J. Laurenborg for collecting the sponges; and K. Lundgreen for technical support. We also acknowledge the very helpful comments of E. Sperling and N. Butterfield. Funding from the European Research Council (“Oxygen” Grant), the Danish National Research Foundation (Grant DNRF53), and the Agouron Institute supported this research.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 3907.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400547111/-/DCSupplemental.
References
- 1.Sahoo SK, et al. Ocean oxygenation in the wake of the Marinoan glaciation. Nature. 2012;489(7417):546–549. doi: 10.1038/nature11445. [DOI] [PubMed] [Google Scholar]
- 2.Canfield DE, Poulton SW, Narbonne GM. Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science. 2007;315(5808):92–95. doi: 10.1126/science.1135013. [DOI] [PubMed] [Google Scholar]
- 3.Laflamme M, Darroch SAF, Tweedt SM, Peterson KJ, Erwin DH. The end of the Ediacara biota: Extinction, biotic replacement, or Cheshire Cat? Gondwana Res. 2013;23(2):558–573. [Google Scholar]
- 4.Nursall JR. Oxygen as a prerequisite to the origin of the Metazoa. Nature. 1959;183(4669):1170–1172. [Google Scholar]
- 5.Berkner LV, Marshall LC. On the origin and rise of oxygen concentration in the Earth’s atmosphere. J Atmos Sci. 1965;22(3):225–261. [Google Scholar]
- 6.Cloud PE. Beginnings of biospheric evolution and their biogeochemical consequences. Paleobiology. 1976;2(4):351–387. [Google Scholar]
- 7.Knoll AH. The early evolution of eukaryotes: A geological perspective. Science. 1992;256(5057):622–627. doi: 10.1126/science.1585174. [DOI] [PubMed] [Google Scholar]
- 8.Kasting JF. Earth’s early atmosphere. Science. 1993;259(5097):920–926. doi: 10.1126/science.11536547. [DOI] [PubMed] [Google Scholar]
- 9.Love GD, et al. Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature. 2009;457(7230):718–721. doi: 10.1038/nature07673. [DOI] [PubMed] [Google Scholar]
- 10.Maloof AC, et al. Possible animal-body fossils in pre-Marinoan limestones from South Australia. Nat Geosci. 2010;3(9):653–659. [Google Scholar]
- 11.Peterson KJ, Butterfield NJ. Origin of the Eumetazoa: Testing ecological predictions of molecular clocks against the Proterozoic fossil record. Proc Natl Acad Sci USA. 2005;102(27):9547–9552. doi: 10.1073/pnas.0503660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erwin DH, et al. The Cambrian conundrum: Early divergence and later ecological success in the early history of animals. Science. 2011;334(6059):1091–1097. doi: 10.1126/science.1206375. [DOI] [PubMed] [Google Scholar]
- 13.Runnegar B. Oxygen requirements, biology and phylogenetic significance of the late Precambrian wormDickinsonia, and the evolution of the burrowing habit. Alcheringa Australasian J Palaeontol. 1982;6(3):223–239. [Google Scholar]
- 14.Runnegar B. Precambrian oxygen levels estimated from the biochemistry and physiology of early eukaryotes. Palaeogeogr Palaeoclimatol Palaeoecol. 1991;97(1-2):97–111. [Google Scholar]
- 15.Catling DC, Glein CR, Zahnle KJ, McKay CP. Why O2 is required by complex life on habitable planets and the concept of planetary “oxygenation time”. Astrobiology. 2005;5(3):415–438. doi: 10.1089/ast.2005.5.415. [DOI] [PubMed] [Google Scholar]
- 16.Sperling EA, et al. Oxygen, ecology, and the Cambrian radiation of animals. Proc Natl Acad Sci USA. 2013;110(33):13446–13451. doi: 10.1073/pnas.1312778110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhoads DC, Morse JW. Evolutionary and ecological significance of oxygen-deficient marine basins. Lethaia. 1971;4(4):413–428. [Google Scholar]
- 18.Sperling EA, Vinther J. A placozoan affinity for Dickinsonia and the evolution of late Proterozoic metazoan feeding modes. Evol Dev. 2010;12(2):201–209. doi: 10.1111/j.1525-142X.2010.00404.x. [DOI] [PubMed] [Google Scholar]
- 19.Sperling EA, Halverson GP, Knoll AH, Macdonald FA, Johnston DT. A basin redox transect at the dawn of animal life. Earth Planet Sci Lett. 2013;371–372:143–155. [Google Scholar]
- 20.Sperling EA, Pisani D, Peterson KJ. Poriferan paraphyly and its implications for Precambrian palaeobiology. Geol Soc Lond Spec Publ. 2007;286(1):355–368. [Google Scholar]
- 21.Nosenko T, et al. Deep metazoan phylogeny: When different genes tell different stories. Mol Phylogenet Evol. 2013;67(1):223–233. doi: 10.1016/j.ympev.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Philippe H, et al. Resolving difficult phylogenetic questions: Why more sequences are not enough. PLoS Biol. 2011;9(3):e1000602. doi: 10.1371/journal.pbio.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James-Clark H. Conclusive proofs on the animality of the ciliate sponges, and their affinities with the Infusoria Flagellata. Am J Sci. 1866;2(42):320–325. [Google Scholar]
- 24.Medina M, et al. Phylogeny of Opisthokonta and the evolution of multicellularity and complexity in Fungi and Metazoa. Int J Astrobiol. 2003;2(3):203–211. [Google Scholar]
- 25.Steenkamp ET, Wright J, Baldauf SL. The protistan origins of animals and fungi. Mol Biol Evol. 2006;23(1):93–106. doi: 10.1093/molbev/msj011. [DOI] [PubMed] [Google Scholar]
- 26.King N, et al. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451(7180):783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz-Trillo I, Roger AJ, Burger G, Gray MW, Lang BF. A phylogenomic investigation into the origin of metazoa. Mol Biol Evol. 2008;25(4):664–672. doi: 10.1093/molbev/msn006. [DOI] [PubMed] [Google Scholar]
- 28.Carr M, Leadbeater BS, Hassan R, Nelson M, Baldauf SL. Molecular phylogeny of choanoflagellates, the sister group to Metazoa. Proc Natl Acad Sci USA. 2008;105(43):16641–16646. doi: 10.1073/pnas.0801667105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols SA, Dayel MJ, King N. Genomic, phylogenetic, and cell biological insights into metazoan origins. In: Telford MJ, Littlewood DTJ, editors. Animal Evolution: Genes, Genomes, Fossils and Trees. Oxford: Oxford University Press; 2009. pp. 24–32. [Google Scholar]
- 30.Tunnicliffe V. High species diversity and abundance of the epibenthic community in an oxygen-deficient basin. Nature. 1981;294(26):354–356. [Google Scholar]
- 31.Levin LA, Huggett CL, Wishner KF. Control of deep-sea benthic community structure by oxygen and organic matter gradients in the eastern Pacific Ocean. J Mar Res. 1991;49(4):763–800. [Google Scholar]
- 32.Schönberg CHL, Barthel D. Inorganic skeleton of the demosponge Halichondria panicea. Mar Biol. 1997;130(2):133–140. [Google Scholar]
- 33.Revsbech NP, Jorgensen BB. Microelectrodes: Their use in microbial ecology. Adv Microb Ecol. 1986;9:293–352. [Google Scholar]
- 34.Hoffmann F, et al. An anaerobic world in sponges. Geomicrobiol J. 2005;22(1-2):1–10. [Google Scholar]
- 35.Hoffmann F, et al. Oxygen dynamics and transport in the Mediterranean sponge Aplysina aerophoba. Mar Biol. 2008;153(6):1257–1264. doi: 10.1007/s00227-008-0905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisz JB, Lindquist N, Martens CS. Do associated microbial abundances impact marine demosponge pumping rates and tissue densities? Oecologia. 2008;155(2):367–376. doi: 10.1007/s00442-007-0910-0. [DOI] [PubMed] [Google Scholar]
- 37.Maldonado M, Ribes M, van Duyl FC. Nutrient fluxes through sponges: Biology, budgets, and ecological implications. Adv Mar Biol. 2012;62:113–182. doi: 10.1016/B978-0-12-394283-8.00003-5. [DOI] [PubMed] [Google Scholar]
- 38.Vacelet J, Donadey C. Electron-microscope study of association between some sponges and bacteria. J Exp Mar Biol Ecol. 1977;30(3):301–314. [Google Scholar]
- 39.Boury-Esnault N, de Vos L, Donadey C, Vacelet J. Ultrastructure of Choanosome and Sponge Classification. Washington, DC: Smithsonian Institution Press; 1990. pp. 237–244. [Google Scholar]
- 40.Antcliffe JB. Questioning the evidence of organic compounds called sponge biomarkers. Palaeontology. 2013;56(5):917–925. [Google Scholar]
- 41.Xiao S, Hu J, Yuan X, Parsley RL, Cao R. Articulated sponges from the Lower Cambrian Hetang Formation in southern Anhui, South China: Their age and implications for the early evolution of sponges. Palaeogeogr Palaeoclimatol Palaeoecol. 2005;220(1-2):89–117. [Google Scholar]
- 42.Sperling EA, Robinson JM, Pisani D, Peterson KJ. Where’s the glass? Biomarkers, molecular clocks, and microRNAs suggest a 200-Myr missing Precambrian fossil record of siliceous sponge spicules. Geobiology. 2010;8(1):24–36. doi: 10.1111/j.1472-4669.2009.00225.x. [DOI] [PubMed] [Google Scholar]
- 43.Kump LR. The rise of atmospheric oxygen. Nature. 2008;451(7176):277–278. doi: 10.1038/nature06587. [DOI] [PubMed] [Google Scholar]
- 44.Canfield DE. Proterozoic Atmospheric Oxygen. In: Holland HD, Turekian KK, editors. Treatise on Geochemistry. 2nd Ed. Elsevier, Oxford; 2014. pp. 197–216. [Google Scholar]
- 45.Bekker A, Holland HD. Oxygen overshoot and recovery during the early Paleoproterozoic. Earth Planet Sci Lett. 2012;317–318:295–304. [Google Scholar]
- 46.Canfield DE, et al. Oxygen dynamics in the aftermath of the Great Oxidation of Earth’s atmosphere. Proc Natl Acad Sci USA. 2013;110(42):16736–16741. doi: 10.1073/pnas.1315570110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olson SL, Kump LR, Kasting JF. Quantifying the areal extent and dissolved oxygen concentrations of Archean oxygen oases. Chem Geol. 2013;362(Special issue):35–43. [Google Scholar]
- 48.Butterfield NJ. Oxygen, animals and oceanic ventilation: An alternative view. Geobiology. 2009;7(1):1–7. doi: 10.1111/j.1472-4669.2009.00188.x. [DOI] [PubMed] [Google Scholar]
- 49.Knoll AH. The multiple origins of complex multicellularity. Annu Rev Earth Planet Sci. 2011;39(1):217–239. [Google Scholar]
- 50.Butterfield NJ. Animals and the invention of the Phanerozoic Earth system. Trends Ecol Evol. 2011;26(2):81–87. doi: 10.1016/j.tree.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Logan GA, Hayes JM, Hieshima GB, Summons RE. Terminal Proterozoic reorganization of biogeochemical cycles. Nature. 1995;376(6535):53–56. doi: 10.1038/376053a0. [DOI] [PubMed] [Google Scholar]
- 52.Canfield DE. Oxygen: A Four Billion Year History. Princeton: Princeton University Press; 2014. p. 224. [Google Scholar]
- 53.Payne JL, et al. The evolutionary consequences of oxygenic photosynthesis: A body size perspective. Photosynth Res. 2011;107(1):37–57. doi: 10.1007/s11120-010-9593-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.