In the annals of Earth history, few associations have proven more iconic or durable than that between animals and oxygen. As early as 1919, the experimental physiologist August Krogh (1) explored the relationship between O2 and animal anatomy, and 40 y later J. R. Nursall (2) posited explicitly that metazoans appear as fossils only in uppermost Proterozoic rocks because pO2 was insufficient to support animal physiology before this time. Preston Cloud, the first great historical geobiologist, championed this idea (3), and in recent years the hypothesis has been bolstered by geochemical data that place redox transition in broad synchrony with the first animal body fossils (4). Now, however, Mills et al. (5) challenge this view of life, at least in its simplest form.
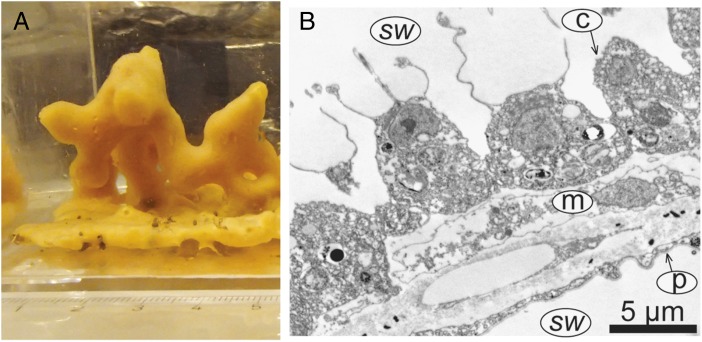
The phylogenetic relationships of basal metazoans have become more clouded in recent years, but analyses least associated with apparent systematic errors place sponges as the sister group or grade to all other animals (6, 7). For this reason, as well as the clear antiquity of many poriferan lineages (8), living sponges may provide our best physiological guide to ancestral animals. Experiments by Mills et al. (5) on the temperate demosponge Halichondria panicea (Fig. 1A) indicate that these animals can live at oxygen levels as low as 0.5–4% of present-day levels (PAL), a condition likely to have characterized surface oceans long before the Ediacaran Period. Importantly, this species grows in shallow, well-oxygenated environments and apparently has no special adaptations to low oxygen. The experimental data therefore support previous theoretical suggestions that—by virtue of their basic body plan, with essentially every cell in contact with seawater (Fig. 1B)—ancestral sponges and early diploblastic animals would have had only modest oxygen requirements (9, 10). Thus, oxygen availability probably provided little impediment to the origin of animal multicellularity.
Fig. 1.
(A) The marine demosponge Halichondria panicea investigated by Mills et al. (5); note centimeter scale in the foreground. (B) Transmission electron microscopy through the body of the calcareous sponge Sycon coactum. The ability of sponges to tolerate low oxygen is probably related to their basic body design, with only two cell layers, the external pinacoderm (p) and the internal choanoderm (c), separated by a largely inert mesohyl (m). Both cell layers are in direct contact with seawater (sw), and diffusion distances for oxygen to any cell are short. (Scale bar, 5 μm.) Thus, sponges and other small thin animals may have been able to tolerate low Proterozoic oxygen levels; however, larger, metabolically active animals, particularly carnivores, would have been excluded. Images courtesy of D. B. Mills (A) and S. Leys (B).
The hypothesis that animals originated in a low-oxygen world gains further support from two independent sources. The first source is ecological. The full range of oxygen tensions likely to have characterized Neoproterozoic oceans can be found today, including oxygen-minimum zones (OMZs) where pO2 can be exceedingly low. Even where oxygen falls to 1–3% PAL, however, animals, mostly tiny and unmineralized, thrive (11) (because of substrate effects, sponges are often absent from the soupy sediments that characterize OMZs). Second, increasing geochemical data support the view that oxygen levels remained low—perhaps only a few percent PAL—in the mid-Neoproterozoic oceans where animals are thought to have originated (ref. 10, and references therein).
Thus, as Mills et al. argue (5), explanations for animal origins must be sought elsewhere. Complex multicellular organisms in multiple clades share key features of genetics and cell biology (12), and these illuminate the mechanisms by which animals evolved complex structures. By themselves, however, these characters do not address selection pressures that may have favored simple multicellularity in Neoproterozoic oceans; these must lie elsewhere, for example, in advantages of feeding or defense against protistan predators (13). Both fossils and molecular clocks suggest that eukaryovorus protists (protists that feed by ingesting other eukaryotic cells) radiated during the Neoproterozoic Era (14). Just as carnivory is thought to have provided an ecological driver for Cambrian animal evolution, this change in the biological environment of Neoproterozoic oceans might have facilitated the evolution of multicellarity in stem group metazoans.
Does this mean, then, that oxygen was irrelevant to early animal evolution? Not at all. There is a serious disconnect between molecular clock and biomarker evidence for the origin of sponges in Cryogenian oceans and their widespread appearance as fossils in Cambrian rocks (8). Mills et al.’s (5) experiments offer tantalizing evidence that although sponges may be able to tolerate very low oxygen conditions, they are sensitive to fluctuating anoxia, and that, as in bilaterian animals, smaller forms may cope better with low oxygen than larger ones. Thus, if the trends exhibited by Halichondria are substantiated in other sponges, it may be that sponges were able to evolve in Cryogenian seaways, but remained rare, limited to small size, and difficult to fossilize. As molecular divergence estimates simply inform us about the temporal origins of a group, and not its paleo-abundance or ecological dominance, the experiments of Mills et al. may help to explain some of the discordance between molecular and fossil records.
Equally important, at the minimum oxygen levels capable of supporting sponges, most familiar animals would die, so there must be more to the story. Oxygen may not have lit the fuse for the Cambrian Explosion, but it might have supplied some fuel. Oxygen requirements reflect size, transport mechanisms within tissues, and metabolic demand, and the metazoans found in modern dysoxic waters (O2 present but in low amounts) tend to be tiny (11). The famous Ediacaran macrofossils may not reflect the earliest animals, but along with a number of forms with controversial affinities, they do record the oldest large animals capable of widespread preservation (15). Moreover, in modern dysoxic environments, one functional class of animals is notably rare or absent: carnivores, the postulated ecological drivers of Cambrian diversification (16). Thus, although the redox transformation of global oceans may postdate the origin of animals by more than 100 My, it does approximate the emergence of large animals capable of fossilization and carnivores capable of fomenting biological revolution within the metazoan ecological landscape. The threshold values needed to sustain such animals were perhaps not especially higher than the minimal requirements for animal life (10, 11, 16, 17), but there are clear differences between oxygen levels permitting animal life and those permitting large, diverse, and ecologically important animals.
It is possible, then, that modestly rising oxygen levels facilitated Ediacaran and Cambrian animal evolution, as envisaged by Cloud and other pioneers. Following Butterfield (18), however, Mills et al. (5) suggest the intriguing alternative that Ediacaran oxygen transition was a reflection rather than cause of animal diversification. In this view, filter-feeding animals cleared surface oceans of dense bacterial populations, while planktonic bilaterians expedited export from surface waters via rapidly sinking fecal pellets, lessening the oxygen demand of surface waters and, thereby, promoting oxygen enrichment. In no small part, this hypothesis depends on the view that Proterozoic oceans maintained high bacterial concentrations, determined more by the absence of filter feeders than by alternative controls, such as
The experiments of Mills et al. may help to explain some of the discordance between molecular and fossil records.
nutrient supply and viral lysis. It also requires types of animals little observed before the Cambrian. Nonetheless, this is a hypothesis worth considering as Earth scientists strive to build a more complete picture of Ediacaran life and environments. Even if we accept the premise that animal diversification helped to ventilate Ediacaran oceans, however, we needn’t view the relationship between oxygen and animal evolution as one-way. Insofar as we are not making the parallel error of assuming that insufficient oxygen was present beforehand, increasing oxygenation would have made new types of animal life possible: metazoans with the greater size, mineralized armor, and higher oxygen demands first recorded in Cambrian rocks.
Thus, the coupling of oxygen and early animal evolution, so central to generations of geobiological thought, is not dead, although, following Mills et al., we can bid adieu to simplistic textbook versions for the origin of animals. In its place, new hypotheses are taking shape, reflecting novel approaches from paleontology, geochemistry, and comparative physiology. In this telling, the focus is not on animals per se but rather on specific anatomies, physiologies, and functions that collectively result in high oxygen demand. Nor is our sense of Ediacaran environmental history likely to persevere as one in which low Proterozoic oxygen tensions gave way rapidly to an essentially modern world. Rather, as predicted by some models of Phanerozoic environmental history (19), the rise of oxygen to present-day levels is beginning to look protracted, with relatively low pO2 in Cambrian oceans (20) giving way over many millions of years to oxygen tensions equal to or greater than the present. Continuing research, then, will increasingly focus on Paleozoic evolution and environments, with the sequential appearances of large and heavily skeletonized invertebrates, enormous predatory fish, and even giant dragonflies, interpreted in the context of protracted atmospheric evolution. New geochemical proxies will be needed to provide a more nuanced accounting of environmental history, and they must be complemented by expanding physiological research on animals in low-oxygen environments. With their novel experiments on sponges, Mills et al. (5) help to show the way forward.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4168.
References
- 1.Krogh A. The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion. J Physiol. 1919;52(6):391–408. doi: 10.1113/jphysiol.1919.sp001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nursall JR. Oxygen as a prerequisite to the origin of the metazoa. Nature. 1959;183(4669):1170–1172. [Google Scholar]
- 3.Cloud PE., Jr Atmospheric and hydrospheric evolution on the primitive earth. Both secular accretion and biological and geochemical processes have affected earth’s volatile envelope. Science. 1968;160(3829):729–736. doi: 10.1126/science.160.3829.729. [DOI] [PubMed] [Google Scholar]
- 4.Lyons TW, Reinhard CT, Planavsky NJ. The rise of oxygen in Earth's early ocean and atmosphere. Nature. 2014;506(7488):307–315. doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- 5.Mills DB, et al. Oxygen requirements of the earliest animals. Proc Natl Acad Sci USA. 2014;111:4168–4172. doi: 10.1073/pnas.1400547111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philippe H, et al. Resolving difficult phylogenetic questions: Why more sequences are not enough. PLoS Biol. 2011;9(3):e1000602. doi: 10.1371/journal.pbio.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nosenko T, et al. Deep metazoan phylogeny: When different genes tell different stories. Mol Phylogenet Evol. 2013;67(1):223–233. doi: 10.1016/j.ympev.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Sperling EA, Robinson JM, Pisani D, Peterson KJ. Where’s the glass? Biomarkers, molecular clocks, and microRNAs suggest a 200-Myr missing Precambrian fossil record of siliceous sponge spicules. Geobiology. 2010;8(1):24–36. doi: 10.1111/j.1472-4669.2009.00225.x. [DOI] [PubMed] [Google Scholar]
- 9.Runnegar B. The Cambrian explosion: Animals or fossils? J Geol Soc Aust. 1982;29(3-4):395–411. [Google Scholar]
- 10.Sperling EA, et al. A basin redox transect at the dawn of animal life. Earth Planet Sci Lett. 2013;371–372:143–155. [Google Scholar]
- 11.Levin LA. Oxygen minimum zone benthos: Adaptation and community response to hypoxia. Oceanogr Mar Biol Annu Rev. 2003;41:1–45. [Google Scholar]
- 12.Knoll AH. The multiple origins of complex multicellularity. Annu Rev Earth Planet Sci. 2011;39:217–239. [Google Scholar]
- 13.Boraas ME, Seale DB, Boxhorn JE. Phagotrophy by a flagellate selects for colonial prey: A possible origin of multicellularity. Evol Ecol. 1998;12(2):153–164. [Google Scholar]
- 14.Knoll AH. Paleobiological perspectives on early eukaryotic evolution. Cold Spring Harb Perspect Biol. 2014;6(1):pii, a016121. doi: 10.1101/cshperspect.a016121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao S, Laflamme M. On the eve of animal radiation: Phylogeny, ecology and evolution of the Ediacara biota. Trends Ecol Evol. 2009;24(1):31–40. doi: 10.1016/j.tree.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Sperling EA, et al. Oxygen, ecology, and the Cambrian radiation of animals. Proc Natl Acad Sci USA. 2013;110(33):13446–13451. doi: 10.1073/pnas.1312778110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin LA, Gage JD. Relationship between oxygen, organic matter and the diversity of bathyal macrofauna. Deep Sea Res Part II Top Stud Oceanogr. 1998;45(1-3):129–163. [Google Scholar]
- 18.Butterfield NJ. Oxygen, animals and oceanic ventilation: An alternative view. Geobiology. 2009;7(1):1–7. doi: 10.1111/j.1472-4669.2009.00188.x. [DOI] [PubMed] [Google Scholar]
- 19.Bergman NM, Lenton TM, Watson AJ. COPSE: A new model of biogeochemical cycling over Phanerozoic time. Am J Sci. 2004;304(5):397–437. [Google Scholar]
- 20.Dahl TW, et al. Devonian rise in atmospheric oxygen correlated to the radiations of terrestrial plants and large predatory fish. Proc Natl Acad Sci USA. 2010;107(42):17911–17915. doi: 10.1073/pnas.1011287107. [DOI] [PMC free article] [PubMed] [Google Scholar]