Significance
Adult onset neurodegenerative diseases are viewed as protein destabilization, misfolding, and aggregation diseases. TAR DNA binding protein-43 (TDP-43) protein is strongly associated with many neurological disorders, particularly amyotrophic lateral sclerosis and frontotemporal lobar degeneration. All of the disease-associated TDP-43 mutants tested have been shown to increase TDP-43 half-life and this correlates inversely with the age at which the sufferer first becomes aware of symptoms. Here we show that disease mutations in two TDP-43 nucleic acid binding domains also increase the protein’s half-life and this is commensurate with increased structural stability and resistance to aggregation. Our results are an unusual contrast to other neurodegenerative diseases and provide a potential link between the molecular characteristics of mutant TDP-43 and the symptoms of these debilitating diseases.
Keywords: motor neuron disease, oligemisation, SAXS, protein degradation
Abstract
Over the last two decades many secrets of the age-related human neural proteinopathies have been revealed. A common feature of these diseases is abnormal, and possibly pathogenic, aggregation of specific proteins in the effected tissue often resulting from inherent or decreased structural stability. An archetype example of this is superoxide dismutase-1, the first genetic factor to be linked with amyotrophic lateral sclerosis (ALS). Mutant or posttranslationally modified TAR DNA binding protein-32 (TDP-43) is also strongly associated with ALS and an increasingly large number of other neurodegenerative diseases, including frontotemporal lobar degeneration (FTLD). Cytoplasmic mislocalization and elevated half-life is a characteristic of mutant TDP-43. Furthermore, patient age at the onset of disease symptoms shows a good inverse correlation with mutant TDP-43 half-life. Here we show that ALS and FTLD-associated TDP-43 mutations in the central nucleic acid binding domains lead to elevated half-life and this is commensurate with increased thermal stability and inhibition of aggregation. It is achieved without impact on secondary, tertiary, or quaternary structure. We propose that tighter structural cohesion contributes to reduced protein turnover, increasingly abnormal proteostasis and, ultimately, faster onset of disease symptoms. These results contrast our perception of neurodegenerative diseases as misfolded proteinopathies and delineate a novel path from the molecular characteristics of mutant TDP-43 to aberrant cellular effects and patient phenotype.
The fatal course of age-related neurological disorders is often presaged and accompanied by protein destabilization leading to abnormal aggregation (1). A classic example is the protein superoxide dismutase-1 (SOD1); loss of structural stability (2) and increased aggregation propensity (3) are determinants of the disease course for people with amyotrophic lateral sclerosis (ALS) with mutations in the SOD1 gene (4). TAR DNA binding protein 43 (TDP-43) is associated with ALS (5, 6) but is also implicated in an increasingly large number of other neurological diseases, including frontotemporal lobar degeneration with ubiquitin-associated inclusions (FTLD-U) (7), Alzheimer’s disease (8), and Guam-Parkinsonism dementia (9). This justifies TDP-43’s position as a high value target but its involvement in familial and sporadic forms of disease doubly underscores this.
TDP-43 was first shown to bind and inhibit transcription of TAR DNA (10) but has since been linked with several other nucleic-acid–binding functions, such as transport (11), stress granule formation (12), and translation (13). Most importantly, TDP-43 is known to bind, stabilize, and regulate the splicing of a huge number of mRNAs, including its own (14–17). It is understandable therefore that targeted TDP-43 knockout or overexpression leads to motor neuron disease-like symptoms in mice (18, 19). These facts indicate that strict control of available TDP-43 is critical; however, ALS mutant TDP-43 proteins have been shown to have increased half-life in cell models (20, 21). Additionally, there is a good relationship between increased half-life and rapid disease onset, which links in vivo TDP-43 behavior with observable patient phenotypes (20).
Like FUS, TDP-43’s function is reliant on its ability to bind DNA/RNA targets (17, 22, 23). This is accomplished by two centrally located RNA recognition motif (RRM) domains (24), which are also important for normal localization of TDP-43 to nuclear Gems (25). Furthermore, whole RRM domains, or sections thereof, are important modifiers of TDP-43 toxicity and are involved in the formation of aggregated cytoplasmic C-terminal fragments (26–29). Two disease-associated mutations are found within TDP-43’s RRM domains: D169G and K263E. The D169G ALS mutant (6) has previously been used to assess mutant stress granule formation (30). This process requires functional nucleic acid binding and is unaffected by the D169G mutation (30, 31). It is situated in loop 5 of RRM1 between α-helix 2 and β-strand 4. The FTLD-U–associated K263E mutant (32) is found immediately after RRM2 and is part of the nucleic acid binding site (33).
In this study, we show that disease-related RRM domain mutants exhibit a significant increase in thermal stability in vitro, with D169G being the most stable. This correlates with increased resistance to in vitro aggregation and protraction of their half-life in a TDP-43 disease cell model. We also show that these mutants do not cause oligomerization, significant conformational changes, aberrant domain interactions, or unfolding, as is the case for the majority of neurodegenerative-disease–causing proteins (34). Our results add countenance to the notion that disease-related TDP-43 mutations increase the protein’s half-life regardless of location and pose the question, “Could increased TDP-43 half-life result from tighter structural cohesion and the resistance to degradation that it would confer?”
Results
TDP-43 RRM Mutant Proteins Have Abnormally Elevated Half-Life.
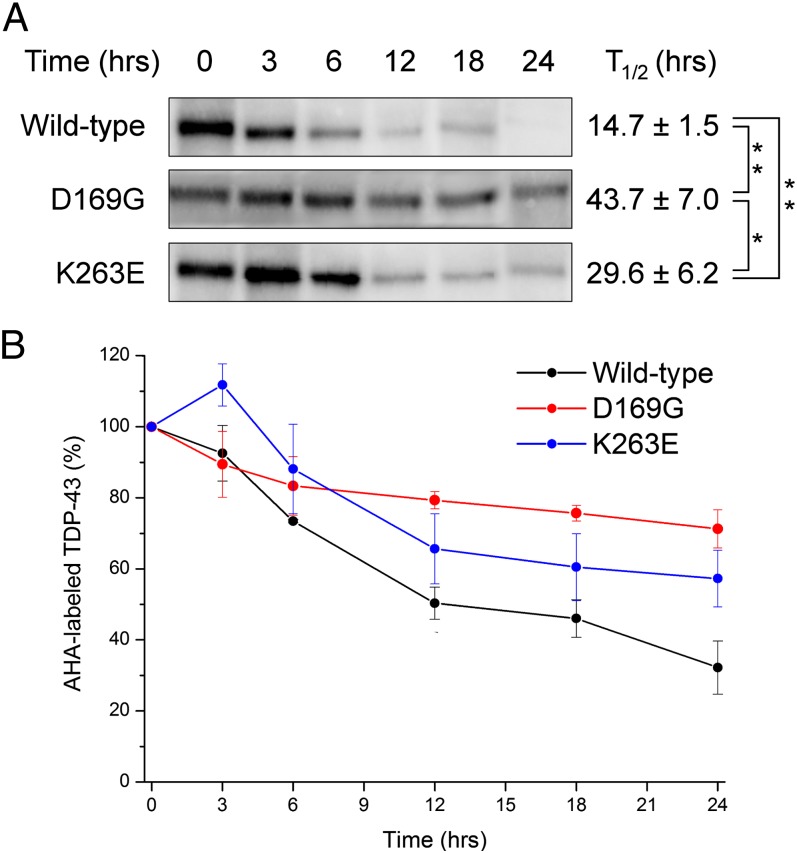
In a previous study, we revealed that increased stability of TDP-43 causes toxicity through abnormal proteostasis and RNA dysregulation (20). Several TDP-43 mutants have now been found to confer increased half-life in cell models (20, 21). However, to date, only mutants in the C-terminal glycine-rich tail have been analyzed. To examine whether RRM disease mutants are likely to affect TDP-43 stability in vivo, a pulse-chase labeling of D169G and K263E full-length human TDP-43 mutants was performed using the methionine analog l-azidohomoalanine in a cell culture model.The half-lives of these RRM disease mutants [T1/2 = 43.7 ± 7.0 h (D169G), 29.6 ± 6.2 h (K263E)] were distinctly longer than that of wild type (T1/2 = 14.7 ± 1.5 h) (Fig. 1). These results indicate that increased half-life is a common property of disease-causing TDP-43 mutations regardless of the location in the primary structure and suggest a common mechanism for their toxicity.
Fig. 1.
Increased half-lives of ALS/FTLD-linked RRM mutant full-length TDP-43 proteins in cultured cells. (A) Transfected Neuro2a cells were metabolically labeled with l-azidohomoalanine (AHA). AHA-labeled TDP-43 was immunoprecipitated and visualized as described in Materials and Methods. (B) The averages of three independent experiments are plotted. Error bars represent SE of mean (SEM). Half-lives of the proteins were calculated by curve fitting (A, Right) and expressed as means ± SEM from three independent experiments. The data were analyzed by one-way ANOVA followed by Tukey–Kramer post hoc tests. *P < 0.05, **P < 0.01.
Disease-Causing TDP-43 RRM Mutants Resist Thermal Unfolding.
In vitro study of TDP-43 RRM domains is now well established (24, 26, 35) and presents the opportunity to investigate potential reasons for the observed increase in cellular half-life at the macromolecular level using a variety of biophysical techniques. Mouse TDP-43 shares 93% sequence identity with human TDP-43 between residues 101 and 265 (TDP-43S) (Fig. S1). The majority of differences occur within the RRM2 domain (residues 192–265) and both Asp169 and Lys263 are conserved between species. Due to the high degree of sequence identity, structural changes are likely to strongly correlate between homologs and the mouse protein can be used as a representative model system.
Susceptibility to thermal and chemical unfolding is a common characteristic of proteins involved in neurodegeneration (36–39). This is exemplified by ALS SOD1 mutations, which are detrimental to protein stability, cause unfolding resulting in the exposure of hydrophobic regions, and increase the protein’s aggregation potential (37, 40). The unfolding temperature (Tm) of mTDP-43S has previously been determined by circular dichroism (CD) as 49.7 °C (24). To compare the thermal stability of wild-type mTDP-43S with the two point mutations described, differential scanning fluorimetry (DSF) and aromatic fluorescence were used to monitor their unfolding (41, 42).
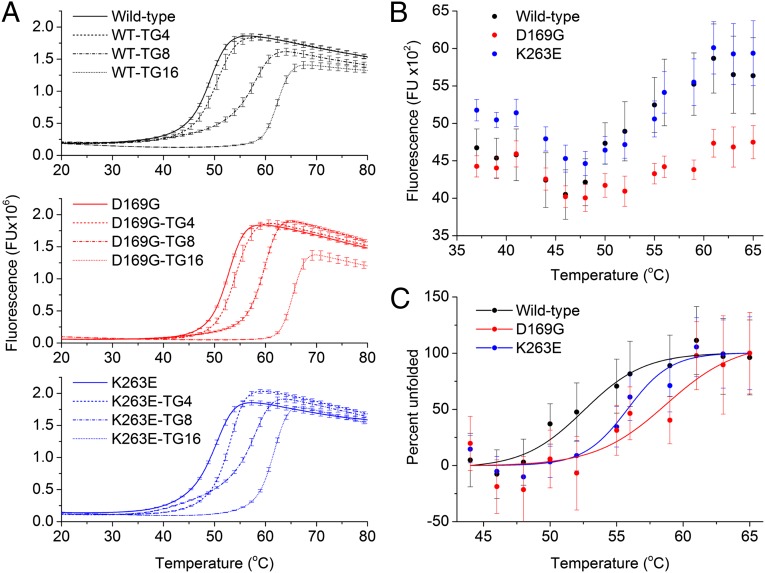
The changes in Sypro-orange fluorescence resulting from quenching and dequenching as the protein unfolds were measured for wild type, K263E, and D169G TDP-43S (Fig. 2A). Wild-type TDP-43S shows a melting transition (Tm) at 49.15 ± 0.16 °C, which is similar to the value obtained by CD spectroscopy (24). The melting transition was elevated by the K263E mutation to 50.2 ± 0.24 °C. However, an increase of 3.5 °C to a Tm of 52.64 ± 0.11 °C was observed for the D169G variant. In each case, a decline in the fluorescent signal at high temperature can be explained by aggregation of the protein (41). The increase in structural stability conferred by D169G and K263E mutations was confirmed using aromatic fluorescence over a temperature range of 37–65 °C (Fig. 2B). These data show two features, an increase in aromatic quenching up to 42 °C and subsequent dequenching to above the initial value. A Boltzmann fit was applied to the normalized unfolding values (Fig. 2C) giving melting transitions of 52.5 ± 0.9, 55.8 ± 0.8, and 58.7 ± 2.8 °C for wild type, K263E, and D169G, respectively.
Fig. 2.
Resistance of TDP-43 RRM mutants to heat-induced unfolding. (A) TDP-43S unfolding monitored by differential scanning fluorimetry. Sypro-orange fluorescence was monitored as a function of temperature in the absence and presence of TG repeat single-strand DNA for each TDP-43S variant. Error bars indicate SE calculated from eight replicates. Melting transitions for the apo protein, TG4-, TG8-, and TG16-bound complexes were calculated to be 49.15 ± 0.16, 50.65 ± 1.17, 57.74 ± 1.02, and 62.35 ± 0.32 °C for wild type; 52.64 ± 0.11, 54.18 ± 1.15, 59.65 ± 0.42, and 65.35 °C for D169G; and 50.20 ± 0.24, 53.05 ± 0.3, 57.78 ± 0.83, and 61.6 ± 0.23 for K263E °C. (B) Thermal unfolding of TDP-43S variants monitored by aromatic fluorescence. Two features are prominent: aromatic quenching beginning at 42 °C followed by dequenching at 48 °C. (C) Normalized unfolding curves. The melting temperature (Tm) of wild type, K263E, and D169G is 52.5 ± 0.9, 55.8 ± 0.8, and 58.7 ± 2.8 °C, respectively.
A central aspect of TDP-43 functionality is its ability to bind nucleic acid and it is known to have a strong preference for UG-rich mRNA (17). Fig. 2A, Top shows how the addition of increasing length TG repeat DNA affects the unfolding transition of wild-type TDP-43. The initial melting temperature of the apo protein rises to 50.65 ± 1.17, 57.74 ± 1.02, and 62.35 ± 0.32 °C when complexed with stoichiometric amounts of TG4, TG8, and TG16 DNA, respectively. Complex formation with DNA also consistently increased the stability of the D169G mutant with melting temperatures of 54.18 ± 1.15, 59.65 ± 0.42, and 65.35 ± 0.39 °C (Fig. 2A, Middle). The K263E–TG4 complex is found to be 2.4 °C more stable than the wild-type complex; however, the stability of this mutant does not increase in the same manner observed for wild type and D169G, with the addition of longer repeat DNA; the K263E–TG8 and –TG16 complexes have melting temperatures of 57.78 ± 0.83 and 61.60 ± 0.23 °C, respectively (Fig. 2A, Bottom). This indicates that the K263E mutant may have reduced affinity for long chain nucleic acids. In support of this, Lys263 has recently been shown to intercalate between bound RNA bases, and mutation to alanine reduces binding affinity (33).
RRM Mutant TDP-43S Is Resistant to Heat-Induced Aggregation.
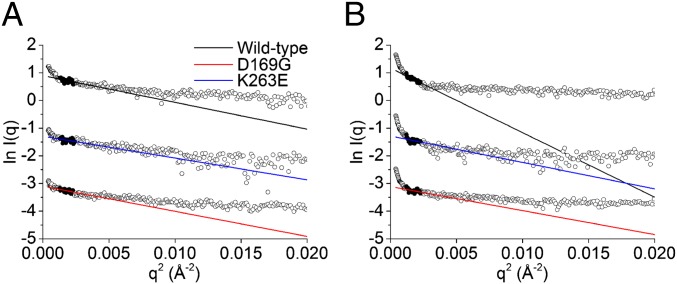
Small angle X-ray solution scattering (SAXS) is a powerful technique to probe the overall conformation and aggregation state of macromolecules in solution (43). Here, SAXS was used to assess differences in aggregation potential between mutant and wild-type TDP-43 at two temperatures: 20 °C and 40 °C. Guinier approximations yielded radii of gyration (Rg) values of 25.8 ± 5.3, 23.3 ± 5.2, and 25.0 ± 3.9 Å, respectively, for wild type, K263E, and D169G at 20 °C. At 40 °C, these were found to be 40.1 ± 1.9, 25.7 ± 5.5, and 24.5 ± 5.1 Å (Fig. 3). The Rg of the wild-type TDP-43S shows a large increase at 40 °C, suggestive of extensive aggregation, as opposed to essentially unchanged values for the mutants. Therefore, both mutants appear resistant to heat-mediated aggregation. An explanation can be found in our observation that wild-type TDP-43S unfolding occurs at a lower temperature and this leads to an increased aggregation propensity.
Fig. 3.
Inhibition of TDP-43S aggregation by RRM ALS/FTLD-U mutations. (A) Guinier plots of TDP-43S variants at 20 °C. Radii of gyration are 25.8 ± 5.3, 23.3 ± 5.2, and 25.0 ± 3.9 Å for wild type, K263E, and D169G, respectively. (B) Guinier plots of TDP-43S variants at 40 °C. The Rg of each variant increases to 40.1 ± 1.9, 25.7 ± 5.5, and 24.5 ± 5 Å. respectively.
RRM Mutations Do Not Engender Aberrant Oligomerization or Large Structural Changes.
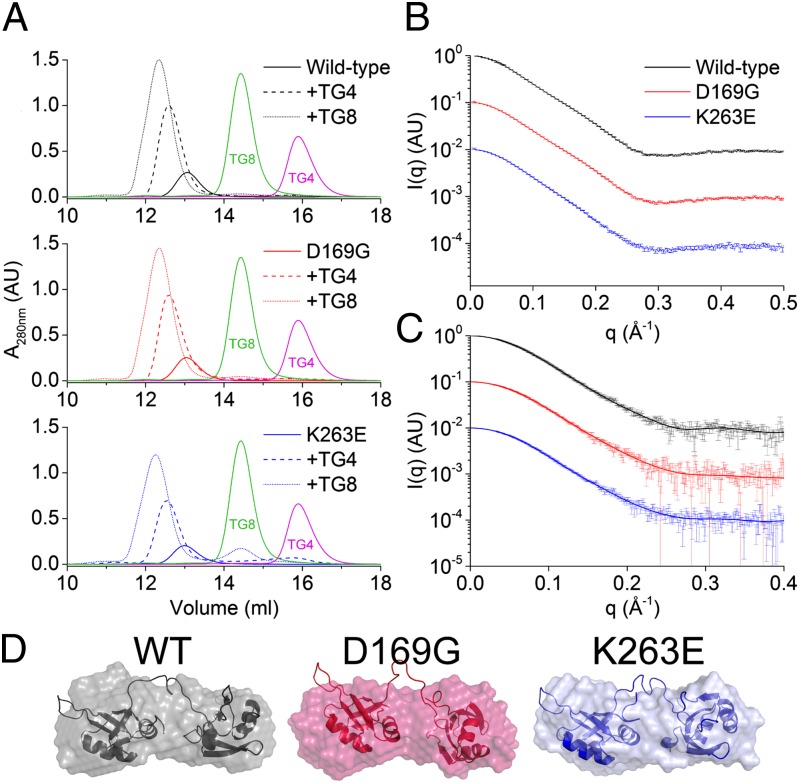
SAXS has previously been used to detect significant structural changes that occur due to disease-causing mutations in SOD1 (2, 44). Using SAXS, size exclusion chromatography (SEC), and CD, here we demonstrate that the secondary, tertiary, and quaternary structure of wild-type mTDP-43S is conserved when mutated. Mutant and wild-type TDP-43S elute concomitantly from a size exclusion chromatography column as single symmetrical peaks between myoglobin (17 kDa) and the SOD1 dimer (32 kDa) (Fig. 4A and Figs. S2 and S3). Binding of TG4 or TG8 repeat DNA causes an increase in hydrodynamic radius but the oligomeric state is maintained for each mutant (Fig. 4A). Fig. 4B shows the 1D scattering curves of TDP-43S variants obtained by SEC-SAXS. Radii of gyration of 21.9, 22.4, and 22.6 ± 0.5 Å for wild type, K263E, and D169G, respectively are slightly lower than those collected by static SAXS measurements and reflect the ability of SEC-coupled SAXS to isolate scattering from the species of interest (45). Molecular weight estimations from experimental SAXS data (46) predict a protein mass of 22 kDa, close to its theoretical monomer mass of 19 kDa. These data are consistent with TDP-43S being monomeric and that TDP-43 RRM mutations do not promote aberrant oligomerization. This correlates well with observations of human TDP-43 RRM truncation proteins (26) and as such no symmetry constraints were applied during subsequent 3D modeling.
Fig. 4.
Determination of the oligomeric state and conformation of TDP-43S variants. (A) Size exclusion chromatograms of TDP-43 variants in the absence or presence of TG4 or TG8 DNA. A total of 12.5 nmol of TDP-43 was loaded with or without a 1:1 molar ratio of DNA. TG4 and TG8 repeat single-strand DNA elute from the Superdex 75 10/300 column at 16 and 14.5 mL, respectively (purple and green). Standard elution is as follows: BSA 10.3 mL, SOD1 12.1 mL, and myoglobin 13.5. (B) SAXS curves for wild-type and mutant TDP-43S giving radii of gyration 21.9, 22.4, and 22.6 ± 0.5 Å for wild type, K263E, and D169G, respectively. (C) TG8-bound TDP-43S giving radii of gyration 21.3, 21.0, and 21.3 ± 0.5 Å, respectively. (D) Bead and rigid body models for TDP-43S variants. Models were generated with GASBOR (1.7 < χ2 < 8.2) and BUNCH (χ2 = 2.9, 5.1 and 2.3 respectively) without imposition of symmetry constraints.
The distance distribution function (p(r)) for mTDP-43S gives insight into the maximum dimension (Dmax) and the average electron distribution for both mutant and wild type (Fig. S4). The Dmax for wild-type mTDP-43S was calculated at 85.5 ± 5 Å which, when modeled, revealed two density-rich regions characteristic of tandem domains (Fig. 4D). Scattering curves and p(r) functions for each mutant are identical to those of the wild-type form, indicating conservation of protein structure. Similarly, when complexed with TG8 DNA the scattering curves for each TDP-43S variant can be superimposed on each other and closely resemble the computed scattering by human TDP43S with RNA bound (Fig. 4C and Fig. S5) (33). The radii of gyration for these protein–DNA complexes was found to be ∼21 Å and Dmax 70 Å (Fig. S6). These size parameters indicate that the DNA bound form is more compact than the apo protein and confirms observations by NMR that nucleic acid binding promotes interaction between the two RRM domains (33). To assess the arrangement of the individual RRM domains in solution in the absence of nucleic acid, a pseudoatomic model was built by rigid body refinement constrained by our experimental SAXS data (Fig. 4D). TDP-43 RRM domains have previously been suggested to behave as separate entities (35) and this appears to be the case for both disease mutants and wild type.
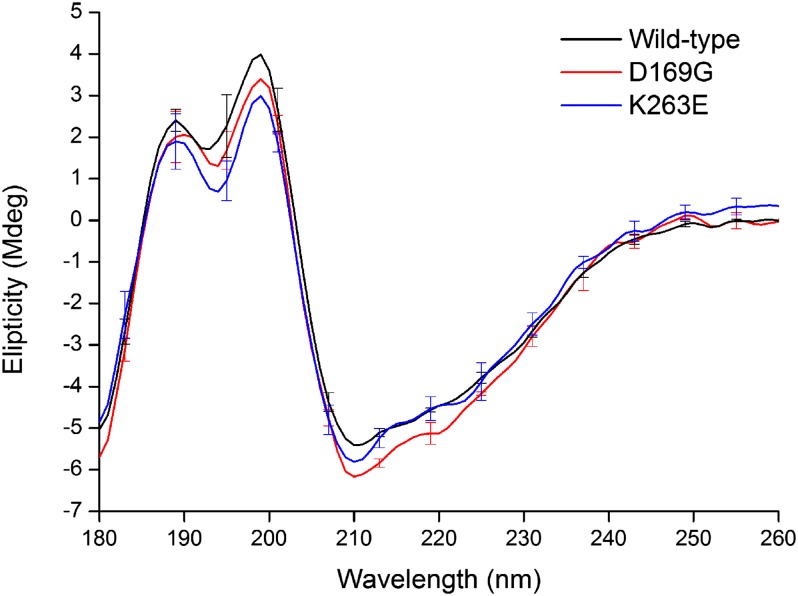
To substantiate our conclusion that the integrity and conformation of TDP-43S is maintained by mutation, the optical activity of each protein was determined at 20 °C by synchrotron radiation CD spectroscopy. Fig. 5 shows the CD spectra, which have good agreement with previously published work (24) but due to the higher intensity of synchrotron light, we are able to reliably show features in the vacuum UV range. The absorption spectra show little difference and the proportions of structural motifs for each variant indicates their secondary structure to be identical within the limits of error. Our results suggest these point mutations do not cause large structural changes and that differences in thermal stability, inhibition of aggregation, and protein turnover are more likely to occur through subtle structural changes at the atomic level.
Fig. 5.
Circular dichroism spectra of wild-type, D169G, and K263E TDP-43S variants. Superimposition of the spectra indicates conserved secondary. This is confirmed by secondary structure content prediction using the DichroWeb server, which gives 6, 6, and 5% α-helix for wild type, K263E, and D169G, respectively. All three variants were found to have 40% β-sheet. Normalized rmsd is 0.092, 0.101, and 0.089, respectively.
Discussion
In recent years TDP-43 has been discovered to be the cause of several common neurodegenerative diseases (47). The number of conditions that are linked to TDP-43 malfunction increases on a seemingly monthly basis and this underscores the notion that neurological diseases often cannot be classified into discrete subcategories but manifest with a spectrum of symptoms. Whereas this seems daunting to those involved in biomedical research it may actually be a boon. Rather than addressing every disease state individually we may be able to effect changes in a single protein that are therapeutic across a whole disease spectrum. As such, finding unifying pathological themes is a worthy goal.
The results presented here indicate that ALS and FTLD-U mutations within the TDP-43 RRM domains do not affect the monomeric state or induce deleterious RRM interdomain interactions, global conformational changes, or unfolding. This is true in the apo or nucleic-acid–bound state. They do however confer resistance to temperature-induced unfolding, aggregation, and degradation. A correlation between increased in vitro thermal stability and increased cellular half-life has been observed for a wide range of proteins (48, 49) including point mutations in nucleic acid binding proteins (50). This indicates there may be a mechanistic link between the observed characteristics of TDP-43 mutants. The ALS-associated mutant, D169G, displayed the largest resistance to thermal unfolding, aggregation, and a half-life almost three times that of the control. In the presence of DNA, this mutant was consistently more stable than the wild type, indicating that its affinity for nucleic acid is unchanged. It is worth noting given its longer half-life, that this mutation caused a faster disease progression compared with other ALS mutations in the same study (6). Asp169 is located on loop 6 of the RRM1 structure, separating α-helix 2 and β-strand 4 with potential hydrogen bond interactions with the side chains of Lys114 and Thr115. The substitution of acidic aspartate for nonpolar glycine at this position would abrogate these bonds but may facilitate a tighter association of loop 6 with the hydrophobic inner core orchestrating the increase in stability. The K263E mutant was also shown to increase thermal stability by up to 3 °C in the apo state, which was unexpected due to its location outside of the RRM2 structure. This residue is part of the nucleic-acid–binding region (33) and the polarity change from negative to positive may aid in forming electrostatic interactions to local positive residues within the nucleic-acid–binding interface conferring a small change in stability. Like wild-type and D169G TDP-43S, K263E stability rises as the length of bound DNA increases. However, after 16 base pairs (TG8), the stability of the protein complex is less than that of the wild-type protein, indicating a reduced affinity for long nucleic acids.
Pathological posttranslational modifications such as phosphorylation (51) and cleavage (52) are currently the best explanation for those TDP-43 proteinopathy cases that occur without primary sequence mutation. These modifications predominate in the cytoplasmic inclusions found in affected cells and as a result were thought to initiate the aggregation cascade and lead to cell death (53). However, TDP-43 aggregation and nuclear depletion are not necessary for toxicity (54, 55). Phosphorylation of C-terminal fragments was found to increase their half-life from 14.2 to 22.1 h, indicating there may be a common toxic pathway for seemingly sporadic and familial disease forms (56). Interestingly the toxicity of some C-terminal mutations was shown to depend upon the presence and function of the RRM1 domain. When RRM1 was removed, an increase in Drosophila survival was observed (29), possibly by allowing more efficient degradation.
Protein unfolding and accumulation is now a well-characterized feature of neurodegenerative disease (57). A typical example of this is SOD1-related ALS where toxic SOD1 protein aggregation is thought to arise through mutation-induced protein instability and the exposure of hydrophobic regions buried within the structure (37, 40). The hypothesis that arises from the work presented here offers an interesting comparison; mutant TDP-43 becomes more stable, resists degradation, and its increased longevity changes the delicate balance of protein expression regulation, eventually leading to faster disease presentation. What remains to be established is whether other mutations throughout the TDP-43 primary structure elicit the same effect and if the posttranslational modifications thought to cause the largest subset of TDP-43 proteinopathies follow a similar route to toxicity. The finding that TDP-43 mutations promote complex formation with FUS (21) means we should not limit our search to stabilization of individual domains or even the TDP-43 monomer. The structural integrity of the dimer and complexes with RNA or other ribonucleoproteins, especially given the clustering of disease mutations in the C terminus, may all play important roles.
Materials and Methods
Pulse-Chase Assay by Chemical Labeling of Newly Synthesized Proteins.
Full-length human TDP-43 cDNA was cloned into a pF5K CMV-neo Flexi mammalian expression vector as described previously (20) and RRM mutations were incorporated by site-directed mutagenesis. Neuro2a cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (vol/vol) FBS, 100 units/mL penicillin, 100 µg/mL streptomycin, and 2 mM glutamine in a humidified atmosphere containing 5% (vol/vol) CO2 at 37 °C. Pulse-chase assay was performed as described previously (20) with slight modifications. Briefly, Neuro2a cells seeded at 2.0 × 105 /mL in poly-d-lysine–coated six-well plates were transiently transfected with pF5K-TDP-43 expression vectors by Lipofectamine 2000. After 6 h of incubation, the culture medium was replaced with methionine- and leucine-free DMEM supplemented with 2% (vol/vol) dialyzed FBS, 0.8 mM l-leucine, 2 mM glutamine, and 2 mM N6,2′-O-dibutyrylguanosine 3′,5′-cyclic monophosphate sodium salt. The cells were further incubated overnight to eliminate intracellular methionine. Newly synthesized TDP-43 proteins were labeled with AHA and reacted with PEG4 carboxamide-propargyl biotin in the presence of Tris-(2-Carboxyethyl)phosphine, Tris[(1-benzyl-1H-1, 2, 3-triazol-4-yl)methyl]amine, and CuSO4 at room temperature for 1 h. AHA-labeled TDP-43 proteins were immunoprecipitated and visualized as described elsewhere (20).
TDP-43S Protein Production.
Mouse TDP-43 DNA was used to PCR base pairs corresponding to residues 101–265 and cloned into the pGEX3x expression vector. D169G and K263E mutations were generated from the wild-type mTDP-43S pGEX3x plasmid using site-directed mutagenesis. The mTDP-43S fusion protein, which contained a cleavable N-terminal GST protein tag was expressed in Escherichia coli BL21 (DE3) by induction with 1.0 mM Isopropyl β-D-1-thiogalactopyranoside and cultured at 30 °C for 5 h. The GST-mTDP-43S protein was purified on a glutathione Sepharose column equilibrated with 50 mM Tris⋅HCl pH 7.4, 150 mM NaCl, 2 mM CaCl2. A 500-mM NaCl wash in the same buffer was performed on-column to remove any DNA or RNA bound to GST-TDP-43S. GST was cleaved on-column with addition of Factor Xa and incubation overnight at 4 °C. Factor Xa was removed by elution through a benzamidine column. Size exclusion chromatography was performed on a Superdex 200 16/600 or Superdex 75 10/300. DNA binding was achieved by addition of equimolar amounts of TG repeat oligonucleotides.
TDP-43S Unfolding Experiments.
The temperature dependence of Sypro-orange (10×) fluorescence in the presence of TDP-43S variants (25 μM) was measured over a temperature range of 14–95 °C, increasing at a rate of 1.2 °C/min. Fluorescence was measured at ∼470 nm and ∼610 nm for excitation and emission, respectively. A total of 8 melt curves for wild-type, K263E, and D169G constructs with 4×, 8×, and 16× TG repeat DNA were obtained and averaged and their first derivatives calculated to obtain the melting temperatures.
Aromatic fluorescence of the samples was tested at a concentration of 0.45 mg/mL using excitation and emission wavelengths of 280 nm and 340 nm, respectively. A total of six repeats for each variant and buffer were performed. Samples and buffer were preheated for 5 min over 2 °C increments from 37 to 65 °C. The samples were buffer subtracted and averaged to produce the data points and SD. Boltzmann curve fitting was calculated using Origin 8.6 (OriginLab).
SAXS Data Collection, Processing, and Interpretation.
SAXS data collection for modeling purposes was performed at beamline SWING (58) at Synchrotron Soleil, which is suited to the characterization of aggregation prone proteins (45). Concentrated TDP-43S (40 µL, 5–10 mg/mL) was separated from high molecular weight aggregates on a Shodex KW402.5–4F SEC column with 300 µL/min flow. A total of 250 exposures spanning the protein elution were collected on an Aviex 170 × 170 charged coupled detector, over an angular momentum transfer range (q) of 0.01–0.57, where q = 4πsinθλ−1 (λ is the wavelength of the incident radiation and θ is half the angle between the incident and scattered radiation). Data averaging, reduction, and preliminary Rg and I(0) calculations were performed using FoxTrot software, developed in-house at Soleil, but scrutinized in more detail with PRIMUS (59). Distance distribution functions P(r) were calculated with Gnom (60). Ten-bead models, generated with GASBOR (61), were averaged using DAMAVER to yield an average electron density model. Rigid body refinement using human RRM1 [Protein Data Bank (PDB): 2CQG, residues 106–177] and mouse RRM2 (PDB: 3D2W, residues 193–259) was performed against experimental SAXS data using BUNCH (62). Residues including N-terminal Gly, Ile and Leu (remaining after factor Xa cleavage) and 101–105, 178–192 and 260–265 were modelled from the Cα trace and reconstituted using SABBAC (63). Rigid-body and bead models were visualized and aligned with PyMOL (www.pymol.org). All curve fittings and 1D profile estimations mentioned were performed using CRYSOL (64). Molecular weight estimations were determined using SAXS MoW (46).
Radii of gyration at 20 °C and 40 °C were calculated by the Guinier approximation using data collected at the Barkla X-ray Laboratory of Biophysics at the University of Liverpool (65). TDP-43S samples were concentrated to 3 mg/mL in the buffer previously described but with the addition of 20 mM DTT. Scattering was collected on a MAR300 image plate at a distance of 1.25 m from the sample. Protein and buffer scattering was recorded over three separate 20-min exposures, which were then averaged. Data integration and analysis was performed using Fit2D and PRIMUS. The bounds of the Guinier region (qRg) all lay below 1.3 for each construct except the wild type at 40 °C, which, due to extensive aggregation, was 2.0.
Circular Dichroism.
The optical activity of TDP-43S variants was determined at beamline DISCO also at Synchrotron Soleil. Following dialysis against 50 mM phosphate buffer pH 7.2, 50 mM NaF and concentration to 0.8 mg/mL, measurements were taken using a 0.02-cm quartz cell and 9-s acquisition time in range 180–260 nm. Data acquisition was repeated three times for each construct before buffer subtraction, averaging, and smoothing by averaging two consecutive points. CDSSTR secondary structure prediction was performed using the DichroWeb on-line server, using the SP175 reference set.
Supplementary Material
Acknowledgments
We acknowledge the interest and help of Prof. Bob Eady, Dr. Richard Strange, and Dr. Hyun Chul Lee. We are grateful to the staff of SWING and DISCO beamlines at Synchrotron SOLEIL for provision of SAXS and CD facilities and their helpful guidance. This work was funded by the Motor Neurone Disease Association (Grant Hasnain/Apr11/6076 to S.S.H. and S.V.A.); the Science and Technologies Funding Council (J.G.G. and S.S.H.); the University of Liverpool, Grants-in-Aid for Scientific Research [Grants 23111006 (to K.Y.) and 22700404 (to S.W.)]; the Ministry for Education, Culture and Sports, Science, and Technology of Japan; the Research Committee of CNS Degenerative Diseases, the Ministry of Health, Labour, and Welfare of Japan; and Japan Science Technology, CREST. Access to SOLEIL was partly funded by the European Community’s Seventh Framework Programme (FP7/2007-2013) under BioStruct-X (Grant Agreement 283570 and Proposal 2370).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317317111/-/DCSupplemental.
References
- 1.Koo EH, Lansbury PT, Jr, Kelly JW. Amyloid diseases: Abnormal protein aggregation in neurodegeneration. Proc Natl Acad Sci USA. 1999;96(18):9989–9990. doi: 10.1073/pnas.96.18.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hough MA, et al. Dimer destabilization in superoxide dismutase may result in disease-causing properties: Structures of motor neuron disease mutants. Proc Natl Acad Sci USA. 2004;101(16):5976–5981. doi: 10.1073/pnas.0305143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruijn LI, et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281(5384):1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Johnson JL, Agar NYR, Agar JN. Protein aggregation and protein instability govern familial amyotrophic lateral sclerosis patient survival. PLoS Biol. 2008;6(7):e170. doi: 10.1371/journal.pbio.0060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sreedharan J, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kabashi E, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40(5):572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 7.Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 8.Higashi S, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa M, et al. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain. 2007;130(Pt 5):1386–1394. doi: 10.1093/brain/awm065. [DOI] [PubMed] [Google Scholar]
- 10.Ou SH, Wu F, Harrich D, García-Martínez LF, Gaynor RB. Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol. 1995;69(6):3584–3596. doi: 10.1128/jvi.69.6.3584-3596.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallini C, Bassell GJ, Rossoll W. The ALS disease protein TDP-43 is actively transported in motor neuron axons and regulates axon outgrowth. Hum Mol Genet. 2012;21(16):3703–3718. doi: 10.1093/hmg/dds205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombrita C, et al. TDP-43 is recruited to stress granules in conditions of oxidative insult. J Neurochem. 2009;111(4):1051–1061. doi: 10.1111/j.1471-4159.2009.06383.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang I-F, Wu L-S, Chang H-Y, Shen C-KJ. TDP-43, the signature protein of FTLD-U, is a neuronal activity-responsive factor. J Neurochem. 2008;105(3):797–806. doi: 10.1111/j.1471-4159.2007.05190.x. [DOI] [PubMed] [Google Scholar]
- 14.Buratti E, et al. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 2001;20(7):1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayala YM, et al. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J. 2011;30(2):277–288. doi: 10.1038/emboj.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polymenidou M, et al. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011;14(4):459–468. doi: 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tollervey JR, et al. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci. 2011;14(4):452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iguchi Y, et al. Loss of TDP-43 causes age-dependent progressive motor neuron degeneration. Brain. 2013;136(Pt 5):1371–1382. doi: 10.1093/brain/awt029. [DOI] [PubMed] [Google Scholar]
- 19.Wils H, et al. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci USA. 2010;107(8):3858–3863. doi: 10.1073/pnas.0912417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe S, Kaneko K, Yamanaka K. Accelerated disease onset with stabilized familial amyotrophic lateral sclerosis (ALS)-linked mutant TDP-43 proteins. J Biol Chem. 2013;288(5):3641–3654. doi: 10.1074/jbc.M112.433615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling S-C, et al. ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS. Proc Natl Acad Sci USA. 2010;107(30):13318–13323. doi: 10.1073/pnas.1008227107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhardwaj A, Myers MP, Buratti E, Baralle FE. Characterizing TDP-43 interaction with its RNA targets. Nucleic Acids Res. 2013;41(9):5062–5074. doi: 10.1093/nar/gkt189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, et al. The RRM domain of human fused in sarcoma protein reveals a non-canonical nucleic acid binding site. Biochim Biophys Acta. 2013;1832(2):375–385. doi: 10.1016/j.bbadis.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo P-H, Doudeva LG, Wang Y-T, Shen C-KJ, Yuan HS. Structural insights into TDP-43 in nucleic-acid binding and domain interactions. Nucleic Acids Res. 2009;37(6):1799–1808. doi: 10.1093/nar/gkp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuiji H, et al. Spliceosome integrity is defective in the motor neuron diseases ALS and SMA. EMBO Mol Med. 2013;5(2):221–234. doi: 10.1002/emmm.201202303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y-T, et al. The truncated C-terminal RNA recognition motif of TDP-43 protein plays a key role in forming proteinaceous aggregates. J Biol Chem. 2013;288(13):9049–9057. doi: 10.1074/jbc.M112.438564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo W, et al. An ALS-associated mutation affecting TDP-43 enhances protein aggregation, fibril formation and neurotoxicity. Nat Struct Mol Biol. 2011;18(7):822–830. doi: 10.1038/nsmb.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson BS, McCaffery JM, Lindquist S, Gitler AD. A yeast TDP-43 proteinopathy model: Exploring the molecular determinants of TDP-43 aggregation and cellular toxicity. Proc Natl Acad Sci USA. 2008;105(17):6439–6444. doi: 10.1073/pnas.0802082105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ihara R, et al. RNA binding mediates neurotoxicity in the transgenic Drosophila model of TDP-43 proteinopathy. Hum Mol Genet. 2013;22(22):4474–4484. doi: 10.1093/hmg/ddt296. [DOI] [PubMed] [Google Scholar]
- 30.Bentmann E, et al. Requirements for stress granule recruitment of fused in sarcoma (FUS) and TAR DNA-binding protein of 43 kDa (TDP-43) J Biol Chem. 2012;287(27):23079–23094. doi: 10.1074/jbc.M111.328757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald KK, et al. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum Mol Genet. 2011;20(7):1400–1410. doi: 10.1093/hmg/ddr021. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs GG, et al. TARDBP variation associated with frontotemporal dementia, supranuclear gaze palsy, and chorea. Mov Disord. 2009;24(12):1843–1847. doi: 10.1002/mds.22697. [DOI] [PubMed] [Google Scholar]
- 33.Lukavsky PJ, et al. Molecular basis of UG-rich RNA recognition by the human splicing factor TDP-43. Nat Struct Mol Biol. 2013;20(12):1443–1449. doi: 10.1038/nsmb.2698. [DOI] [PubMed] [Google Scholar]
- 34.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4(1):49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 35.Chang CK, et al. The N-terminus of TDP-43 promotes its oligomerization and enhances DNA binding affinity. Biochem Biophys Res Commun. 2012;425(2):219–224. doi: 10.1016/j.bbrc.2012.07.071. [DOI] [PubMed] [Google Scholar]
- 36.Tam S, et al. The chaperonin TRiC blocks a huntingtin sequence element that promotes the conformational switch to aggregation. Nat Struct Mol Biol. 2009;16(12):1279–1285. doi: 10.1038/nsmb.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vassall KA, et al. Decreased stability and increased formation of soluble aggregates by immature superoxide dismutase do not account for disease severity in ALS. Proc Natl Acad Sci USA. 2011;108(6):2210–2215. doi: 10.1073/pnas.0913021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersson FI, et al. The effect of Parkinson’s-disease-associated mutations on the deubiquitinating enzyme UCH-L1. J Mol Biol. 2011;407(2):261–272. doi: 10.1016/j.jmb.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 39.Ni C-L, Shi H-P, Yu H-M, Chang Y-C, Chen Y-R. Folding stability of amyloid-beta 40 monomer is an important determinant of the nucleation kinetics in fibrillization. FASEB J. 2011;25(4):1390–1401. doi: 10.1096/fj.10-175539. [DOI] [PubMed] [Google Scholar]
- 40.Münch C, Bertolotti A. Exposure of hydrophobic surfaces initiates aggregation of diverse ALS-causing superoxide dismutase-1 mutants. J Mol Biol. 2010;399(3):512–525. doi: 10.1016/j.jmb.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niesen FH, Berglund H, Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc. 2007;2(9):2212–2221. doi: 10.1038/nprot.2007.321. [DOI] [PubMed] [Google Scholar]
- 42.Eftink MR. The use of fluorescence methods to monitor unfolding transitions in proteins. Biophys J. 1994;66(2 Pt 1):482–501. doi: 10.1016/s0006-3495(94)80799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacques DA, Trewhella J. Small-angle scattering for structural biology—expanding the frontier while avoiding the pitfalls. Protein Sci. 2010;19(4):642–657. doi: 10.1002/pro.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright GSA, Antonyuk SV, Kershaw NM, Strange RW, Samar Hasnain S. Ligand binding and aggregation of pathogenic SOD1. Nat Commun. 2013;4:1758. doi: 10.1038/ncomms2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright GSA, Hasnain SS, Grossmann JG. The structural plasticity of the human copper chaperone for SOD1: Insights from combined size-exclusion chromatographic and solution X-ray scattering studies. Biochem J. 2011;439(1):39–44. doi: 10.1042/BJ20110948. [DOI] [PubMed] [Google Scholar]
- 46.Fischer H, de Oliveira Neto M, Napolitano HB, Polikarpov I, Craievich AF. Determination of the molecular weight of proteins in solution from a single small-angle X-ray scattering measurement on a relative scale. J Appl Cryst. 2009;43:101–109. [Google Scholar]
- 47.Chen-Plotkin AS, Lee VM-Y, Trojanowski JQ. TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol. 2010;6(4):211–220. doi: 10.1038/nrneurol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLendon G, Radany E. Is protein turnover thermodynamically controlled? J Biol Chem. 1978;253(18):6335–6337. [PubMed] [Google Scholar]
- 49.Inoue I, Rechsteiner M. On the relationship between the metabolic and thermodynamic stabilities of T4 lysozymes. Measurements in eukaryotic cells. J Biol Chem. 1994;269(46):29247–29251. [PubMed] [Google Scholar]
- 50.Parsell DA, Sauer RT. The structural stability of a protein is an important determinant of its proteolytic susceptibility in Escherichia coli. J Biol Chem. 1989;264(13):7590–7595. [PubMed] [Google Scholar]
- 51.Hasegawa M, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64(1):60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y-J, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci USA. 2009;106(18):7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arai T, et al. Phosphorylated and cleaved TDP-43 in ALS, FTLD and other neurodegenerative disorders and in cellular models of TDP-43 proteinopathy. Neuropathology. 2010;30(2):170–181. doi: 10.1111/j.1440-1789.2009.01089.x. [DOI] [PubMed] [Google Scholar]
- 54.Arnold ES, et al. ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc Natl Acad Sci USA. 2013;110(8):E736–E745. doi: 10.1073/pnas.1222809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu R, et al. Reducing TDP-43 aggregation does not prevent its cytotoxicity. Acta Neuropathol Commun. 2013;1(1):49. doi: 10.1186/2051-5960-1-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y-J, et al. Phosphorylation regulates proteasomal-mediated degradation and solubility of TAR DNA binding protein-43 C-terminal fragments. Mol Neurodegener. 2010;5:33. doi: 10.1186/1750-1326-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 58.David G, Pérez J. Combined sampler robot and high-performance liquid chromatography: A fully automated system for biological small-angle X-ray scattering experiments at the Synchrotron SOLEIL SWING beamline. J Appl Cryst. 2009;42:892–900. [Google Scholar]
- 59.Konarev PV, Volkov VV, Sokolova AV, Koch MHJ, Svergun DI. PRIMUS: A Windows PC-based system for small-angle scattering data analysis. J Appl Cryst. 2003;36:1277–1282. [Google Scholar]
- 60.Svergun DI. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J Appl Cryst. 1992;25:495–503. [Google Scholar]
- 61.Svergun DI, Petoukhov MV, Koch MH. Determination of domain structure of proteins from X-ray solution scattering. Biophys J. 2001;80(6):2946–2953. doi: 10.1016/S0006-3495(01)76260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petoukhov MV, Svergun DI. Global rigid body modeling of macromolecular complexes against small-angle scattering data. Biophys J. 2005;89(2):1237–1250. doi: 10.1529/biophysj.105.064154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maupetit J, Gautier R, Tufféry P. SABBAC: Online structural alphabet-based protein backbone reconstruction from alpha-carbon trace. Nucleic Acids Res. 2006;34(Web Server issue):W147–151. doi: 10.1093/nar/gkl289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Svergun D, Barberato C, Koch MHJ. CRYSOL: A program to evaluate x-ray solution scattering of biological macromolecules from atomic coordinates. J Appl Cryst. 1995;28:768–773. [Google Scholar]
- 65.Wright GSA, et al. The application of hybrid pixel detectors for in-house SAXS instrumentation with a view to combined chromatographic operation. J Synchrotron Radiat. 2013;20(Pt 2):383–385. doi: 10.1107/S0909049513001866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.