Significance
The safe, selective, and efficient delivery of siRNA is a key challenge to the broad application of siRNA therapeutics in humans. Motivated by the structure of lipoproteins, we developed lipopeptide nanomaterials for siRNA delivery. In vivo in mice, siRNA–lipopeptide particles provide the most potent delivery to hepatocytes (ED50 ∼ 0.002 mg/kg for FVII silencing), with the highest selectivity of delivery to hepatocytes over nontarget cell types (orders of magnitude), yet reported. These materials also show efficacy in nonhuman primates.
Keywords: lipopeptide nanomaterials, tissue and cell specificity, liver genetic disorders, translational research
Abstract
siRNA therapeutics have promise for the treatment of a wide range of genetic disorders. Motivated by lipoproteins, we report lipopeptide nanoparticles as potent and selective siRNA carriers with a wide therapeutic index. Lead material cKK-E12 showed potent silencing effects in mice (ED50 ∼ 0.002 mg/kg), rats (ED50 < 0.01 mg/kg), and nonhuman primates (over 95% silencing at 0.3 mg/kg). Apolipoprotein E plays a significant role in the potency of cKK-E12 both in vitro and in vivo. cKK-E12 was highly selective toward liver parenchymal cell in vivo, with orders of magnitude lower doses needed to silence in hepatocytes compared with endothelial cells and immune cells in different organs. Toxicity studies showed that cKK-E12 was well tolerated in rats at a dose of 1 mg/kg (over 100-fold higher than the ED50). To our knowledge, this is the most efficacious and selective nonviral siRNA delivery system for gene silencing in hepatocytes reported to date.
It is estimated that over 4,000 human diseases are caused by liver genetic disorders (1), with the majority of these diseases originating in hepatocytes (2). Since the discovery of RNA interference (RNAi) (3), there has been strong interest in the development of RNAi-based therapeutics for liver diseases in humans (4–8). Although several siRNA delivery materials have been developed with varying levels of success (4, 9–11), one notable approach involves the use of combinatorial synthesis for the development of lipids and lipid-like materials for siRNA delivery (12, 13). Many of these compounds have shown significant silencing effects in vivo (12, 13). Nonetheless, the efficient, selective, and safe delivery of siRNA to hepatocytes is still a key challenge to the broad application of siRNA therapeutics to liver diseases (6).
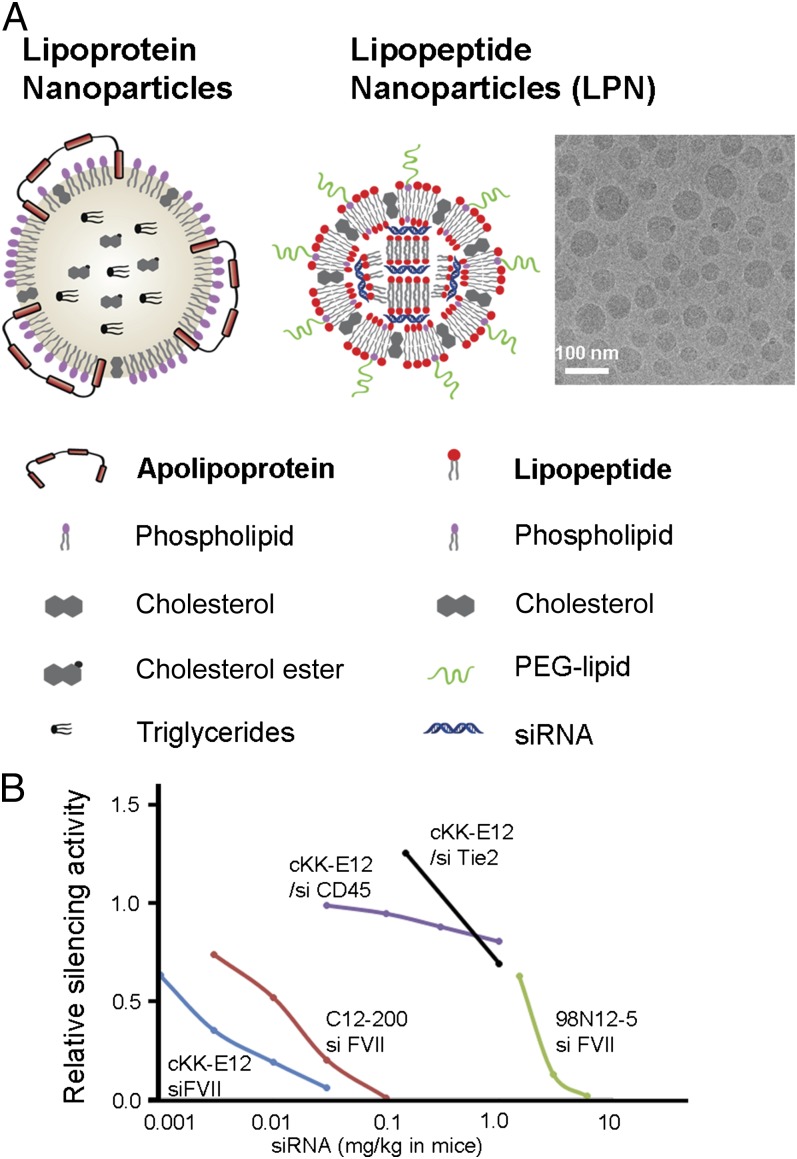
Biomimetic and bioinspired nanomaterials, such as exosomes and lipoprotein particles, have attracted extensive interest for a variety of applications including drug delivery (14, 15). Lipoproteins are particularly advantageous for some liver delivery applications due to their high delivery efficiency and specificity (16, 17). Recent reports have demonstrated delivery of cholesterol-modified siRNA via lipoprotein-based materials (15, 18). Amino acids and peptides are natural building blocks of apolipoproteins, and previous studies have examined the potential of amino acid derivatives for gene delivery or siRNA delivery (19–24). However, to our knowledge, none of these systems have shown potencies and/or specificities that rival traditional lipids or lipidoids. One approach to improve efficacy, tissue, and cell-type selectivity and tolerability of synthetic nanoparticles is to explore the broad chemical space of biomaterials. Natural lipoprotein particles are composed of apolipoproteins, phospholipids, cholesterol, cholesterol esters, and triglycerides (16). Inspired by the structure and activity of lipoprotein nanoparticles (Fig. 1A), we designed a class of lipopeptide nanoparticles (LPNs). We envisioned a modular design which enabled us to easily tune the chemical and physical properties of our materials while facilitating the entrapment of siRNA, and so we synthesized and evaluated a collection of synthetic lipopeptides that could serve as efficient siRNA carriers. These lipopeptides are a key component of our LPNs and are designed to mimic apolipoproteins by conjugating lipid tails to amino acids, peptides, and polypeptide head groups. Unlike lipoproteins, our lipopeptides can be produced on a large scale without using cells or animals.
Fig. 1.
A hypothetical description of lipoprotein nanoparticles, LPNs, and a Cryo-TEM image of cKK-E12 LPN. (A) Lipoprotein nanoparticles: surface components (apolipoprotein, phospholipid, and cholesterol) and core components (cholesterol ester and triglycerides). The LPNs are composed of synthetic lipopeptide (lipoamino acid or lipopolypeptide), phospholipid, cholesterol, PEG-lipid, and siRNA. (B) One lead material cKK-E12 significantly silenced Factor VII in mice with an ED50 of approximately 0.002 mg/kg. More importantly, it was 500-fold more specific for gene silencing in hepatocytes compared with endothelial cells and immune cells in different organs. Relative silencing activity is normalized with PBS-treated groups. cKK-E12/siRNA FVII (blue; silencing in hepatocytes), C12-200/siRNA FVII (red; silencing in hepatocytes with second-generation lipidoid) (13), 98N12-5 /siRNA FVII (green; silencing in hepatocytes with first-generation lipidoid) (12, 13), cKK-E12/siRNA CD45 (purple; silencing in spleen macrophages), and cKK-E12/siRNA Tie2 (black; silencing in liver endothelial cells).
Herein, we report the design, synthesis, and biological evaluation of LPNs (103 materials; SI Appendix, Table S1). Using this data set, we developed the structure–activity relationships (SARs) for four different sets of compounds. Our lead material cKK-E12 was more potent than other materials previously reported, with an ED50 approximate 0.002 mg/kg for FVII silencing in mice (Fig. 1B) (12, 13). More importantly, it was orders of magnitude more specific for gene silencing in hepatocytes compared with endothelial cells and immune cells in different organs (Fig. 1B). This lead material silenced multiple genes in three different animal species, inducing transthyretin (TTR) silencing of over 95% in cynomolgus monkeys.
Results
Structural Design, Synthesis, and in Vivo Delivery of siRNA to Hepatocytes in Mice.
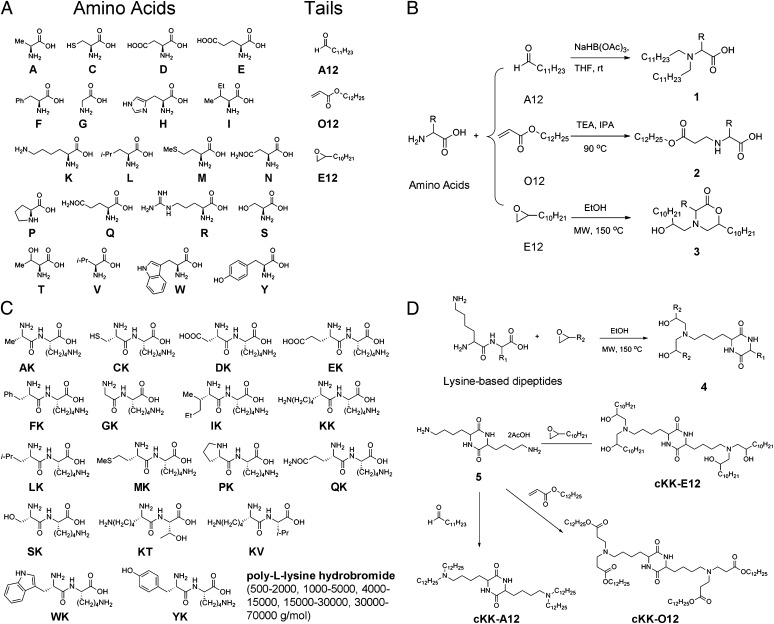
Single amino acids were reacted with aldehydes, acrylates, and epoxides to produce sets 1–3 (Fig. 2 and SI Appendix, Table S1; 60 compounds). Compounds were named by combination of the abbreviation of amino acids, aldehydes (A), acrylates (O), or epoxides (E) and the length of carbon chains. For example, K-E12 represents the reaction of lysine with 1,2-epoxydodecane (SI Appendix, Table S1).
Fig. 2.
Synthetic routes to 103 lipoamino acid, lipopeptide, and lipopolypeptide derivatives. (A) A list of amino acids and lipid tails as starting materials. (B) Single amino acids were reacted with aldehydes, acrylates, and epoxides to install lipid tails and produce 1–3. (C) Lysine-based dipeptides and polypeptides include KG, KT, YK, LK, DK, MK, KV, AK, CK, QK, PK, FK, WK, EK, IK, SK, and poly-l-lysine hydrobromide [molecular weight (g/mol): 500–2,000 (PK500), 1,000–5,000 (PK1K), 4,000–15,000 (PK4K), 15,000–30,000 (PK15K), and 30,000–70,000 (PK30K)]. (D) Microwave irradiation of epoxides and dipeptides dramatically reduced the reaction time from 3 d to 5 h. Reactions between lysine–lysine and poly-l-lysine and aldehydes and acrylates were similar to those of single amino acids in Fig. 2B. Diamine 5 was reacted with 1,2-epoxydodecane, dodecanal, or dodecyl acrylate to afford cKK-E12, cKK-A12, and cKK-O12.
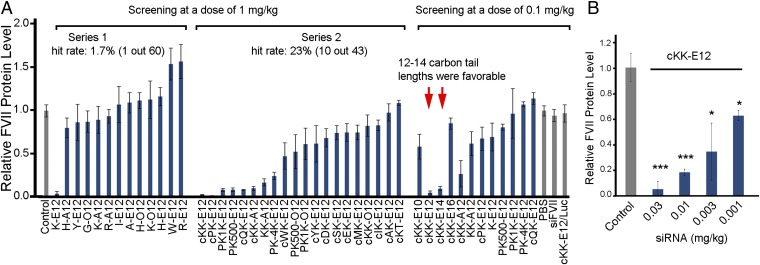
The newly synthesized lipoamino acid derivatives were evaluated for their capacity to silence hepatic genes in mice. Factor VII (a blood clotting factor) was selected as a silencing marker (12). Lipid derivatives from Fig. 2 were formulated with cholesterol, DSPC, PEG-lipid, and siRNA via a microfluidic-based mixing technology (25). Formulations that were unstable in solution or had no siRNA entrapment were not evaluated in vivo (SI Appendix, Table S1). Stable formulations were injected in mice i.v. at a dose of 1 mg/kg (Fig. 3A). In this initial study, K-E12 was the most potent. The hit rate (over 50% silencing) was 1 out of 60 compounds (i.e., 1.7%, including those compounds not screened due to particle instability or no entrapment of siRNA).
Fig. 3.
Identification of lead material cKK-E12 LPNs in mice. (A) LPNs were tested at a dose of 1 mg/kg in mice, which indicated that lysine was a favorable amino acid. Then, we investigated lysine-based peptide and polypeptide–lipid derivatives at the same dose. The hit rate was improved from 1.7% to 23% (including those compounds not screened due to particle instability or no entrapment of siRNA). The top hits and their analogs were explored at a lower dose of 0.1 mg/kg, which led to selection of cKK-E12 as the lead compound. Control, PBS; siFVII, FVII siRNA; cKK-E12/Luc, luciferase siRNA-formulated cKK-E12 LPNs. (B) Dose-dependent silencing of cKK-E12 in mice. K-E12, K, lysine; E, epoxide; A, aldehyde; O, acrylate; 12, carbon tail length. cKK-E12, c, cyclic. Data points represent group mean ± SD (n = 3 or 4; *P < 0.05; **P < 0.01; ***P < 0.005; t test, double-tailed).
The enhanced potency of K-E12 led to our design of a second set of lysine-based lipopeptide and lipopolypeptide derivatives (43 compounds). The synthetic routes used to prepare these compounds are summarized in Fig. 2 C and D (12, 13, 26). Lysine-based dipeptides were reacted with epoxides to give diketopiperizine 4. Compounds with a cyclic set 4 are designated c, such as cKK-E12. Microwave irradiation was used to produce sets 3–4, which dramatically reduced the reaction time from 3 d to 5 h. To further confirm the chemical structure and improve chemical availability for large-scale synthesis, an alternative synthetic route was developed for the synthesis of cKK-E12 (SI Appendix, Fig. S1A). Diamine 5 was synthesized according to the method reported previously (27), which reacted with 1,2-epoxydodecane to afford cKK-E12 (Fig. 2D). Compound 5 underwent reductive amination or Michael addition reactions with dodecanal or dodecyl acrylate to yield cKK-A12 and cKK-O12. Reactions between lysine–lysine and poly-l-lysine (molecular weight from 500 to 70,000 g/mol) and aldehydes and acrylates were similar to those of single amino acids as shown in Fig. 2B.
This second-generation library was again evaluated with Factor VII siRNA as described above. Ten out of 43 compounds showed 50% or greater silencing at a dose of 1 mg/kg (Fig. 3A). The hit rate of the second set of compounds was 23%, which was over 10-fold more efficient compared with the first set of materials. The results suggested that our iterative screening is an efficient approach for identifying lead compounds. Epoxide derivatives were consistently more potent than aldehyde and acrylate derivatives (such as cKK-E12 vs. cKK-A12 and cKK-O12). Hit materials were further evaluated at a lower dose of 0.1 mg/kg. The tail length significantly affected silencing, and materials with 12–14 carbon tail lengths were the most potent (cKK-E10, cKK-E12, cKK-E14, and cKK-E16). The structure of cKK-E12 is composed of a dilysine-derived diketopiperazine core and four amino alcohol-based lipid tails (Fig. 2D). The mean particle diameter of cKK-E12 LPNs is ∼70 nm as measured by dynamic light scattering (SI Appendix, Fig. S1B). The particle size of cKK-E12 LPNs was the same on day 1 and day 14. Particles were capable of silencing in vivo during the same time frame (SI Appendix, Fig. S1 B and C). Cryo-TEM images appear in Fig. 1 and SI Appendix, Fig. S1D. The cKK-E12 LPNs are spherical in morphology with diameters in the range of 35–85 nm and a textured interior. We further performed a dose-dependent study of cKK-E12 LPNs in mice with FVII as a gene target. As shown in Fig. 3B, cKK-E12 LPNs displayed significant dose-dependent silencing with high potency (ED50 ∼ 0.002 mg/kg, based on the concentration of entrapped siRNA). To our knowledge, this delivery system is the most efficacious yet reported in the literature.
Selective Gene Silencing in Hepatocytes.
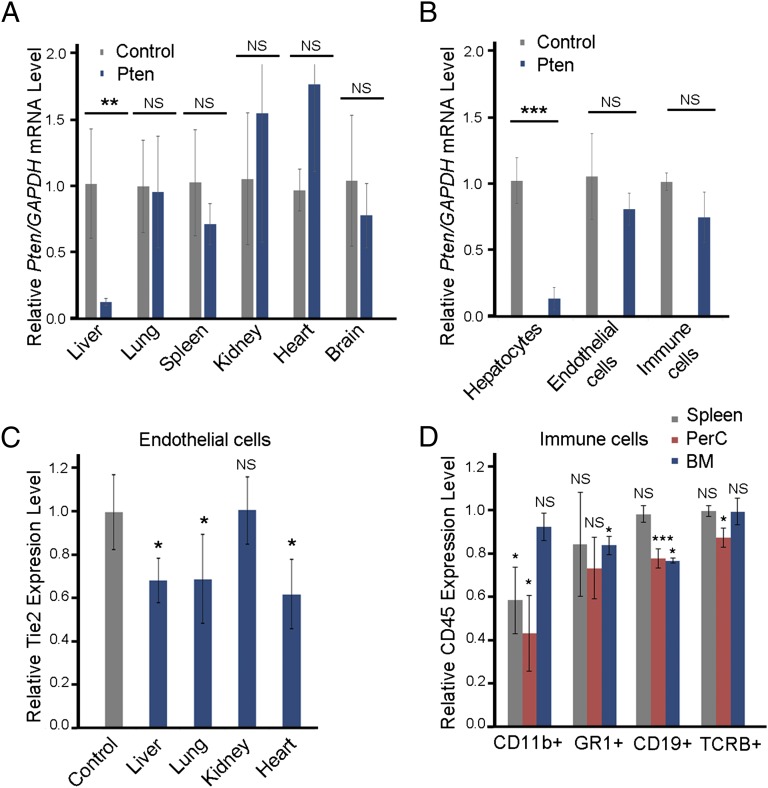
To evaluate the silencing activity of cKK-E12 LPNs in different organs, we formulated the nanoparticles with siRNA against Phosphatase and tensin homolog (Pten), a ubiquitously expressed protein in different cell types, and measured the expression level of Pten in different tissues after i.v. administration. cKK-E12 LPNs showed significant silencing in the liver over that seen in the lung, spleen, kidney, heart, and brain (Fig. 4A). In the liver tissue, we sorted hepatocytes, endothelial cells (CD31+), and leukocytes (CD45+) (28). cKK-E12 LPNs silenced over 80% of Pten in hepatocytes, whereas it showed no significant silencing in endothelial cells and leukocytes (dose = 0.1 mg/kg; Fig. 4B).
Fig. 4.
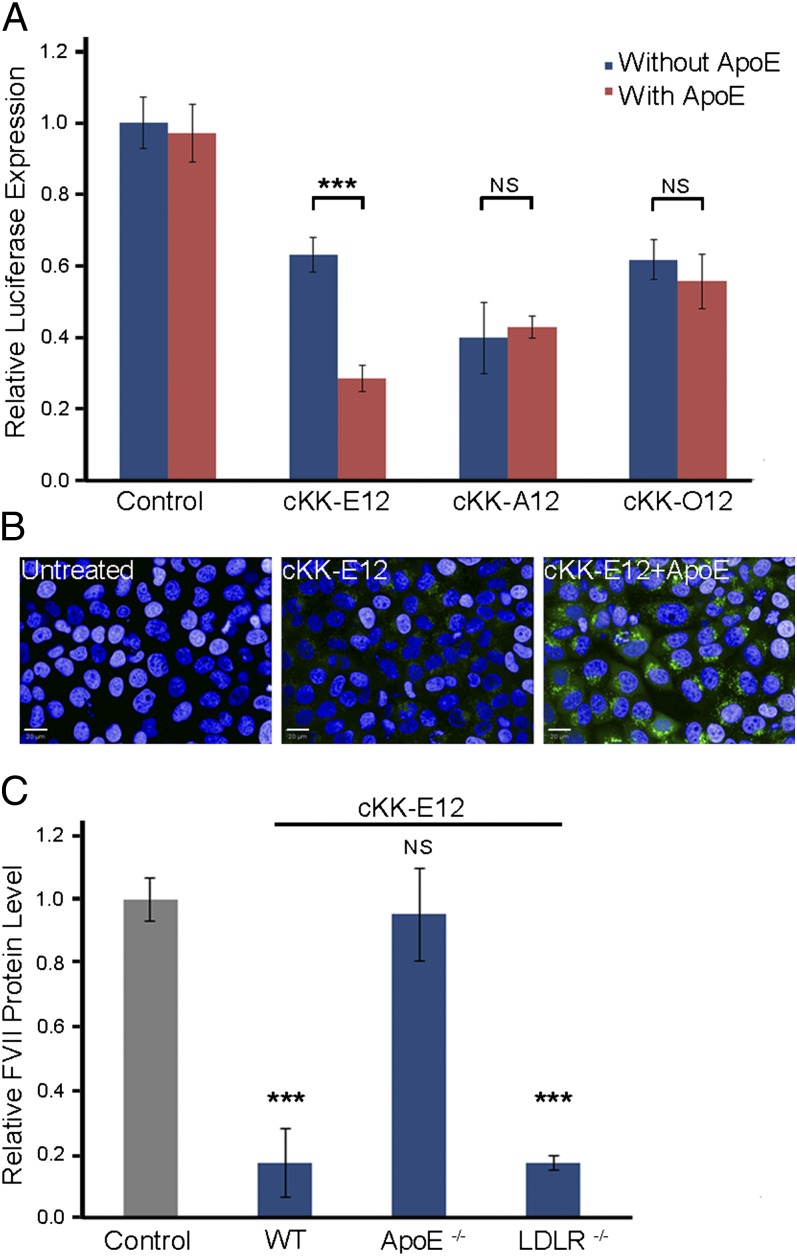
Effects of apoE on gene silencing and cell uptake. (A) Silencing effects of apoE on cKK-E12, cKK-A12, and cKK-O12 LPNs in HeLa cells (siRNA: 50 ng per well). In the presense of apoE, the order of silencing effects was cKK-E12 > cKK-A12 > cKK-O12, correlating well with in vivo activity. (B) Cellular internalization of cKK-E12 with Alex-647–labeled siRNA after 3 h of incubation is demonstrated by HT automated confocal microscopy (Alex-647 is pseudocolored green). ApoE enhanced cell uptake and endosomal escape of cKK-E12. (Scale bar: 20 μm.) (C) In vivo factor VII gene-silencing effects of cKK-E12 in wild-type apoE−/− and LDLR−/− mice. Data points are expressed as a percentage of PBS control animals and represent group mean ± SD (n = 3; ***P < 0.005; NS, not significant; t test, double-tailed). Control, PBS; WT, wild type.
We further evaluated the cell specificity of cKK-E12 LPNs by measuring silencing using siRNA against two additional target genes: Tie2 in endothelial cells and CD45 in immune cells (Fig. 4 C and D). These results indicate that cKK-E12 has much weaker silencing effects in endothelial cells of liver, heart, lung, and kidney at the dose of 1 mg/kg of anti-Tie2 siRNA compared with FVII silencing in hepatocytes (Fig. 4C). We also studied the silencing effects in four types of immune cells in spleen, peritoneal cavity, and bone marrow: macrophages (CD11b+), granulocytes (GR1+), B cells (CD19+), and T cells (TCRb) (29). cKK-E12 showed mild to medium silencing effects for all these immune cell lines in different organs at the dose of 1 mg/kg (Fig. 4D). Thus, this cKK-E12 LPN was orders of magnitude more selective toward hepatocyte silencing compared with other cell types in various organs, demonstrating high tissue and cell selectivity.
Effects of Apolipoproteins on Cell Uptake and Gene Silencing.
Apolipoproteins are natural components of lipoproteins and exist endogenously in the blood stream (16). Previous studies have reported that apolipoprotein E (apoE) is able to enhance cell uptake and gene silencing for ionizable lipid nanoparticles; however, apoE showed little effect on cationic lipid-like materials (30). To study the interactions between our LPNs and diverse apolipoproteins on cell uptake and gene silencing, we performed experiments with cKK-E12 LPNs and 11 isoforms of apoA, apoB, apoC, apoE, and apoH. We transfected the mixture of cKK-E12 LPNs and apolipoproteins in HeLa cells (30), which were used to study the binding of lipoproteins previously (31). Results in HeLa cells showed that most apolipoproteins did not affect cell viability with the exception of apoB (SI Appendix, Fig. S2). ApoA, apoC, and apoH did not show significant effects on silencing compared with free cKK-E12 LPNs (SI Appendix, Fig. S2). However, four different apoE isoforms significantly improved luciferase silencing. We also compared the activity of cKK-E12, cKK-A12, and cKK-O12 LPNs with and without addition of apoE3 (apoE3 is the dominant isoform in humans; Fig. 5A). In the absence of apoE3, cKK-A12 was more potent than cKK-E12 and cKK-O12; the addition of apoE3 reversed the order of silencing effects (cKK-E12 > cKK-A12 > cKK-O12), which correlated well with in vivo activity. These results suggested that a cell assay with addition of apoE might be a practical and an effective model for preliminary screening for hepatocyte silencing. We further studied the impact of apoE on the cell uptake of cKK-E12, cKK-A12, and cKK-O12 LPNs. Cellular uptake of cKK-E12 formulated with an Alexa-Fluor 647 labeled siRNA was examined using automated confocal microscopy (Fig. 5B). These results indicate that apoE3 increased the cell uptake of cKK-E12 over ninefold compared with fourfold for cKK-O12 and no significant increase for cKK-A12 (SI Appendix, Fig. S4C). ApoE not only increased cell uptake of cKK-E12 LPNs but also improved the escape of siRNA from endosomes to cytosol. To characterize the endocytic mechanism of LPNs in the presence of apoE, we incubated cKK-E12 LPNs with apoE in the presence of labeled endocytic markers: dextran (a fluid phase marker), transferrin (clathrin-mediated endocytosis), and Cholera toxin B (caveolae-mediated endocytosis). The labeled LPNs colocalized mainly with dextran other than transferrin or Cholera toxin B (SI Appendix, Fig. S3). We further explored the cell uptake of cKK-E12 LPNs by inhibiting dynamin and macropinocytosis with dynasore and 5-N-ethyl-N-isoproamiloride (EIPA) in the presence of apoE (SI Appendix, Fig. S4 A and SB). Both dyansore and EIPA significantly blocked the internalization of the LPNs. These results indicated a dynamin-dependent macropinocytosis for cKK-E12 LPNs, which was a similar entry mechanism for Bluetongue Virus-1 reported previously (32).
Fig. 5.
Selective gene silencing in hepatocytes. (A) Silencing activity of Pten siRNA-formulated cKK-E12 LPNs (0.1 mg/kg) in the liver, lung, spleen, kidney, heart, and brain using luciferase siRNA-formulated cKK-E12 LPNs as a control in mice. (B) Silencing activity of Pten siRNA-formulated cKK-E12 LPNs (0.1 mg/kg) in hepatocytes, endothelial cells, and immune cells isolated from the mouse liver tissue using luciferase siRNA-formulated cKK-E12 LPNs as a control. (C) Silencing effects in endothelial cells in mice treated with cKK-E12 LPNs at 1 mg/kg. (D) Silencing activity in immune cells from spleen, peritoneal cavity, and bone marrow with anti-CD45 siRNA in mice at 1 mg/kg. Macrophages (CD11b+), granulocytes (GR1+), B cells (CD19+), and T cells (TCRB+). Data points represent group mean ± SD (n = 3 or 4; *P < 0.05; **P < 0.01; ***P < 0.005; NS, not significant; t test, double-tailed).
To study the impact of apoE on cKK-E12 LPNs in vivo, we evaluated the silencing activity in apoE knockout mice (apoE−/−). Because the low-density lipoprotein receptor (LDLR) is one of the major hepatic receptors for apoE in vivo (30), we also investigated the impact of LDLR using LDLR knockout mice (LDLR−/−). cKK-E12 was formulated with FVII-targeting siRNA and injected at a dose of 0.03 mg/kg via tail vein in wild-type, apoE−/−, and LDLR−/− mice (Fig. 5C). Silencing activity of cKK-E12 was dramatically reduced in the apoE−/− group. However, the potency remained for LDLR−/− group. The results were consistent with the effects in vitro and demonstrated that apoE was able to significantly enhance the silencing potency of cKK-E12, whereas the efficacy of cKK-E12 is independent of LDLR.
Silencing Activity of cKK-E12 in Nonhuman Primates.
Current mouse models are not ideal to mimic the pathological lesions and the symptoms of TTR-related amyloidosis in humans (33). We evaluated cKK-E12 efficacy using siRNA targeting TTR in nonhuman primates, which is more relevant to humans. TTR, primarily synthesized in hepatocytes, plays significant roles in transporting thyroxine and retinol (10). TTR mutations can cause fatal amyloidosis, including familial amyloidotic polyneuropathy and familial amyloid cardiomyopathy. In this study, cynomolgus monkeys were systemically administrated with cKK-E12 formulations via a single i.v. injection. At a dose of 0.3 mg/kg, cKK-E12 silenced TTR mRNA levels over 95% (Fig. 6).
Fig. 6.
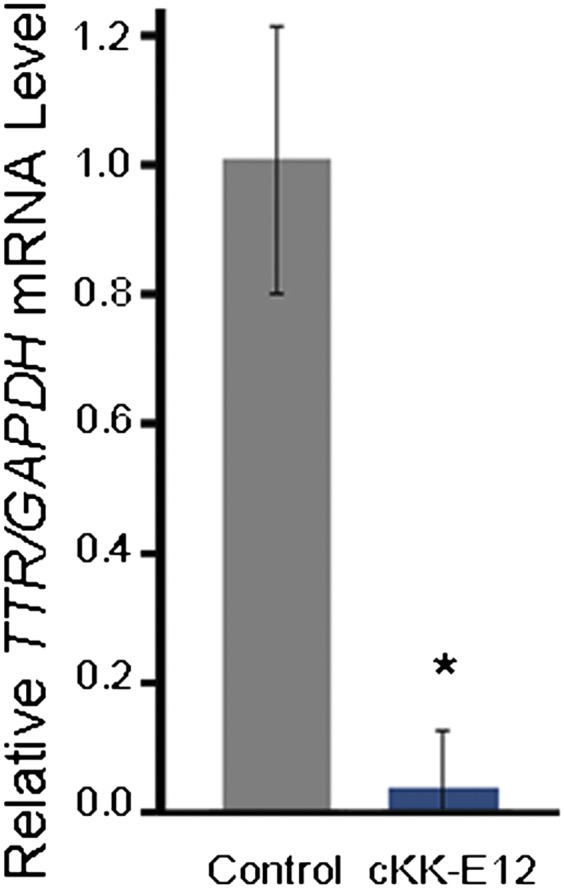
Silencing effects of cKK-E12 LPNs in nonhuman primates. Cynomolgus monkeys received a total dose of 0.3 mg/kg siTTR LPNs as 15-min i.v. infusions (5 mL/kg) through the cephalic vein. A liver biopsy was collected from each animal at 48 h postadministration. TTR mRNA levels relative to GAPDH mRNA levels were determined in liver samples. Control, Naive; TTR, transthyretin. Data points represent group mean ± SD (n = 3; *P < 0.05; t test, double-tailed).
Discussion
Previously, a library of lipid-like molecules, termed lipidoids, for siRNA delivery was developed (12). The ED50 for FVII silencing is at the range of single-digit mg/kg in mice. Further optimization of lipidoids led to the development of a lead material C12-200 (ED50 ∼ 0.01 mg/kg in mice; the potency is in the context of FVII silencing) (13). Improvements in specificity and efficacy offer the potential for even broader therapeutic application for siRNA therapeutics.
To further improve the efficacy, specificity, and safety, bioinspired and biomimetic nanomaterials offer one paradigm for the design of siRNA delivery systems. Inspired by the lipoprotein nanoparticles (Fig. 1), we developed a class of LPNs, which were composed of lipopeptides, phospholipids, PEG-lipid, cholesterol, and siRNA. This modular design offers the ability to optimize the properties of the LPN and improve siRNA delivery efficiency. We applied an iterative screening process for structural optimization and SAR study. Based on the iterative learning process, the hit rate was 10-fold more efficient for the second set of materials compared with the first set. The key structural features of effective materials revealed the following SAR and design criteria for next-generation siRNA delivery systems: (i) lysine-derived lipopeptides; (ii) lysine-based ring structures as a core, such as lysine-derived morpholin-2-one and dilysine-based diketopiperazine; (iii) epoxide and aldehyde-derived lipid tails; and (iv) carbon tail length between 12 and 14.
Our rational design and iterative screening approach led to the discovery of a lead material, which we term cKK-E12. The efficacious dose of the cKK-E12 siRNA formulation described here is in the single-digit μg/kg range in mice (ED50 ∼ 0.002 mg/kg), which translates to a 40-ng dose for a 20-g mouse and is comparable to the amount used for treating cells in a 96-well plate (Fig. 3B). Moreover, the potency of FVII and Tie2 are comparable in vitro (SI Appendix). However, cKK-E12 was able to silence hepatocytes at low doses of administered FVII siRNA relative to endothelium (Tie2). Furthermore, in vivo comparison of cKK-E12 to the C12-200 formulation (ED50 < 0.3 mg/kg for gene silencing of CD45) (29) shows that cKK-E12 was more selective toward liver parenchymal cell, with orders of magnitude lower doses needed to silence in hepatocytes compared with endothelial cells and immune cells in different organs (Fig. 4). The well-tolerated dose is over 100-fold higher than the efficacious dose (SI Appendix, Table S2). To our knowledge, this is the most efficacious and selective siRNA delivery system silencing hepatocytes yet reported.
To study the mechanism of action for cKK-E12 LPNs, we performed a series of experiments both in vitro and in vivo. Our results showed that siRNA delivery by cKK-E12 was significantly enhanced by association with apoE. The addition of apoE increased the cell uptake of LPNs and also enhanced luciferase silencing in dual-luciferase–expressing HeLa cells (Fig. 5). Furthermore, in vitro colocalization and studies suggest that macropinocytosis is the main route of cell internalization compared with clathrin- or caveolae-mediated endocytosis (SI Appendix, Fig. S3). Both dynamin and macropinocytosis inhibitors significantly reduced the uptake of cKK-E12 LPNs (SI Appendix, Fig. S4). These results indicate that cKK-E12 LPNs appear to use a noncanonical pathway by which they use dynamin-dependent macropinocytosis. This pathway is similar to the entry mechanism for Bluetongue Virus-1, which stands in contrast with the classical clathrin-meditated endocytosis (32). Consistent with in vitro results, the efficacy of cKK-E12 LPNs was dramatically reduced in apoE knockout mice compared with wild-type mice (Fig. 5C). As such, apoE plays a key role in mediating potent and selective silencing activity in hepatocytes, which would be instructive for future design of new siRNA delivery materials. Moreover, the potency remained in LDLR knockout mice, suggesting that in vivo hepatic uptake is independent of LDLR. These results indicate that the mechanism of action for cKK-E12 LPNs is different from stable nucleic acid lipid particles, which are both apoE- and LDLR-dependent (30).
In summary, our bioinspired design of lipopeptide nanoparticles leads to cKK-E12 siRNA LPNs with high potency, high specificity, and a broad therapeutic window. Given the performance in nonhuman primates (>95% silencing at a dose of 0.3 mg/kg), we believe that cKK-E12 has significant potential for use as a therapeutic delivery system. We further hypothesize that the bioinspired approach to biomaterial design may prove a useful strategy in other areas of biomedical research.
Materials and Methods
General Procedures for Reactions of Amino Acids, Peptides, Polypeptides with Aldehydes, Acrylates, and Epoxides.
To a solution of amino acids, peptides or polypeptides, and aldehydes (a ratio of 3:1 aldehydes/amine) in THF was added sodium triacetoxyborohydride at room temperature (rt). The reaction mixture was stirred for 3 d at rt. The reaction solution was concentrated with silica gel and purified using flash column chromatography. A mixture of amino acids, peptides or polypeptides, and acrylates (a ratio of 3:1 acrylates/amine) with the presence of triethylamine (TEA) in isopropanol alcohol or acetonitrile was heated to 90 °C and stirred for 2 h. The reaction solution was concentrated with silica gel and purified using flash chromatography. A mixture of amino acids, peptides or polypeptides, and epoxides (a ratio of 3:1 epoxides/amine) in EtOH was irradiated in a microwave oven at 150 °C for 5 h. The reaction mixture was purified by flash chromatography. If amino acids, peptides, or polypeptides were in salt form, TEA was added to the solution and stirred for 30 min at rt before irradiation.
Supplementary Material
Acknowledgments
The authors thank Dr. Yuan Xue and Philip Chang for their help. The authors thank NanoImaging Services for their help with the Cryo-TEM imaging. This work was supported by Alnylam Pharmaceuticals and National Institutes of Health (NIH) Grants R01-EB000244-27 and 5-R01-CA132091-04. Y.D. acknowledges the NIH for his Postdoctoral Fellowship.
Footnotes
Conflict of interest statement: R.L. is a shareholder and member of the Scientific Advisory Board of Alnylam. D.G.A. is a consultant with Alnylam. R.L and D.G.A have sponsored research grants from Alnylam. Alnylam also has a license to certain intellectual property invented at Massachusetts Institute of Technology. A.A., S.A.B., M.C., K.F., J.H., V. Kumar, J.Q., and W.Q. are employed by Alnylam.
*This Direct Submission article had a prearranged editor.
See Commentary on page 3903.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322937111/-/DCSupplemental.
References
- 1.McClellan J, King MC. Genetic heterogeneity in human disease. Cell. 2010;141(2):210–217. doi: 10.1016/j.cell.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Krawczyk M, Müllenbach R, Weber SN, Zimmer V, Lammert F. Genome-wide association studies and genetic risk assessment of liver diseases. Nat Rev Gastroenterol Hepatol. 2010;7(12):669–681. doi: 10.1038/nrgastro.2010.170. [DOI] [PubMed] [Google Scholar]
- 3.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 4.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 5.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: Advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Huang L. Tumor-targeted delivery of siRNA by non-viral vector: Safe and effective cancer therapy. Expert Opin Drug Deliv. 2008;5(12):1301–1311. doi: 10.1517/17425240802568505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenske DB, Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv. 2008;5(1):25–44. doi: 10.1517/17425247.5.1.25. [DOI] [PubMed] [Google Scholar]
- 9.Rozema DB, et al. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Natl Acad Sci USA. 2007;104(32):12982–12987. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semple SC, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28(2):172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 11.Woodrow KA, et al. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8(6):526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akinc A, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26(5):561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Love KT, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA. 2010;107(5):1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10(7):521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 15.Yang M, et al. Efficient cytosolic delivery of siRNA using HDL-mimicking nanoparticles. Small. 2011;7(5):568–573. doi: 10.1002/smll.201001589. [DOI] [PubMed] [Google Scholar]
- 16.Marcovina SM, Morrisett JD. Structure and metabolism of lipoprotein (a) Curr Opin Lipidol. 1995;6(3):136–145. doi: 10.1097/00041433-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 18.Ng KK, Lovell JF, Zheng G. Lipoprotein-inspired nanoparticles for cancer theranostics. Acc Chem Res. 2011;44(10):1105–1113. doi: 10.1021/ar200017e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prata CA, et al. Lipophilic peptides for gene delivery. Bioconjug Chem. 2008;19(2):418–420. doi: 10.1021/bc700451b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adami RC, et al. An amino acid-based amphoteric liposomal delivery system for systemic administration of siRNA. Mol Ther. 2011;19(6):1141–1151. doi: 10.1038/mt.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margus H, Padari K, Pooga M. Cell-penetrating peptides as versatile vehicles for oligonucleotide delivery. Mol Ther. 2012;20(3):525–533. doi: 10.1038/mt.2011.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Futaki S, et al. Stearylated arginine-rich peptides: A new class of transfection systems. Bioconjug Chem. 2001;12(6):1005–1011. doi: 10.1021/bc015508l. [DOI] [PubMed] [Google Scholar]
- 23.McKenzie DL, Collard WT, Rice KG. Comparative gene transfer efficiency of low molecular weight polylysine DNA-condensing peptides. J Pept Res. 1999;54(4):311–318. doi: 10.1034/j.1399-3011.1999.00104.x. [DOI] [PubMed] [Google Scholar]
- 24.Wadhwa MS, Collard WT, Adami RC, McKenzie DL, Rice KG. Peptide-mediated gene delivery: Influence of peptide structure on gene expression. Bioconjug Chem. 1997;8(1):81–88. doi: 10.1021/bc960079q. [DOI] [PubMed] [Google Scholar]
- 25.Chen D, et al. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J Am Chem Soc. 2012;134(16):6948–6951. doi: 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Magid AF, Carson KG, Harris BD, Maryanoff CA, Shah RD. Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride. Studies on direct and indirect reductive amination procedures (1) J Org Chem. 1996;61(11):3849–3862. doi: 10.1021/jo960057x. [DOI] [PubMed] [Google Scholar]
- 27.Bergeron RJ, Phanstiel OIV, Yao GW, Milstein S, Weimar WR. Macromolecular self-assembly of diketopiperazine tetrapeptides. J Am Chem Soc. 1994;116(19):8479–8484. [Google Scholar]
- 28.Yin H, Cheng L, Langenbach R, Ju C. Prostaglandin I(2) and E(2) mediate the protective effects of cyclooxygenase-2 in a mouse model of immune-mediated liver injury. Hepatology. 2007;45(1):159–169. doi: 10.1002/hep.21493. [DOI] [PubMed] [Google Scholar]
- 29.Novobrantseva TI, et al. Systemic RNAi-mediated gene silencing in nonhuman primate and rodent myeloid cells. Mol Ther Nucleic Acids. 2012;1:e4. doi: 10.1038/mtna.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akinc A, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18(7):1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agnani G, et al. Interaction of LpB, LpB:E, LpB:C-III, and LpB:C-III:E lipoproteins with the low density lipoprotein receptor of HeLa cells. Arterioscler Thromb. 1991;11(4):1021–1029. doi: 10.1161/01.atv.11.4.1021. [DOI] [PubMed] [Google Scholar]
- 32.Gold S, Monaghan P, Mertens P, Jackson TA. A clathrin independent macropinocytosis-like entry mechanism used by bluetongue virus-1 during infection of BHK cells. PLoS ONE. 2010;5(6):e11360. doi: 10.1371/journal.pone.0011360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito S, Maeda S. 2009. Mouse models of transthyretin amyloidosis. Recent Advances in Transthyretin Evolution, Structure and Biological Functions, eds Richardson SJ, Cody V (Springer, Berlin), pp 261–280.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.