Significance
Although the hippocampus has a well-documented role for spatial navigation across species, its role for spatial memory in nonnavigational tasks is uncertain. Thus, when monkeys are tested in tasks that do not require navigation through space, spatial memory seems unaffected by hippocampal lesions. However, the interpretation of these results is compromised by long-term compensatory adaptation occurring in the days and weeks after lesions. To preclude long-term compensation, we transiently inactivated hippocampus at the time of testing. Hippocampal inactivation reduced performance on a nonnavigational spatial memory task to chance levels. These data align the role of hippocampus for spatial memory in monkeys with the broader literature while simultaneously demonstrating the efficacy of inactivation of hippocampus in monkeys.
Keywords: self-ordered sequencing, muscimol, temporal lobe, cognition, nonhuman primate
Abstract
The hippocampus has a well-documented role for spatial navigation across species, but its role for spatial memory in nonnavigational tasks is uncertain. In particular, when monkeys are tested in tasks that do not require navigation, spatial memory seems unaffected by lesions of the hippocampus. However, the interpretation of these results is compromised by long-term compensatory adaptation occurring in the days and weeks after lesions. To test the hypothesis that hippocampus is necessary for nonnavigational spatial memory, we selected a technique that avoids long-term compensatory adaptation. We transiently disrupted hippocampal function acutely at the time of testing by microinfusion of the glutamate receptor antagonist kynurenate. Animals were tested on a self-ordered spatial memory task, the Hamilton Search Task. In the task, animals are presented with an array of eight boxes, each containing a food reinforcer; one box may be opened per trial, with trials separated by a delay. Only the spatial location of the boxes serves as a cue to solve the task. The optimal strategy is to open each box once without returning to previously visited locations. Transient inactivation of hippocampus reduced performance to chance levels in a delay-dependent manner. In contrast, no deficits were seen when boxes were marked with nonspatial cues (color). These results clearly document a role for hippocampus in nonnavigational spatial memory in macaques and demonstrate the efficacy of pharmacological inactivation of this structure in this species. Our data bring the role of the hippocampus in monkeys into alignment with the broader framework of hippocampal function.
Although a role for the hippocampus in navigational spatial memory has been well documented in many species, including humans (1–4), rodents (5, 6), and monkeys (7–9), a role for hippocampus in nonnavigational spatial memory is less certain. Because nonhuman primates have a close neuroanatomical and behavioral homology to humans, they offer a unique opportunity to assess the neural substrates of spatial memory using nonnavigational tasks. However, studies using nonnavigational spatial tasks with nonhuman primates have found little or no effect of hippocampal lesions (10–15).
The ability of hippocampal-lesioned monkeys to perform these tasks without impairment has been explained by the reliance on a strategy that is not dependent on hippocampus. Whereas the use of allocentric (world-centered) cues depends on the hippocampus, it is thought that the use of egocentric (body-centered) cues does not. Indeed, it has been suggested that the use of egocentric cues is supported by extrahippocampal substrates including striatum and parietal cortex (15–18).
Following a hippocampal lesion, subjects may learn to resolve a given task by developing a strategy distinct from that used in the presence of an intact hippocampus. Several factors may support this compensatory strategy, including (i) postlesion training or retraining and (ii) postlesion network reorganization (19–21). Thus, although lesion studies are useful in documenting altered function after injury, they cannot directly address the role of the hippocampus in the uninjured brain.
A technique frequently used in rodents to avoid the adaptations described above is the rapid and reversible inactivation of a brain region by focal intracerebral drug infusions (e.g., with drugs that block glutamatergic neurotransmission or enhance GABAergic neurotransmission). An important advantage of this approach is the ability to use each animal as its own control in a repeated-measures design. In monkeys, we (22–26) and others (27–29) have used this technique to probe functions of several brain regions, but it has yet to be applied to the hippocampus.
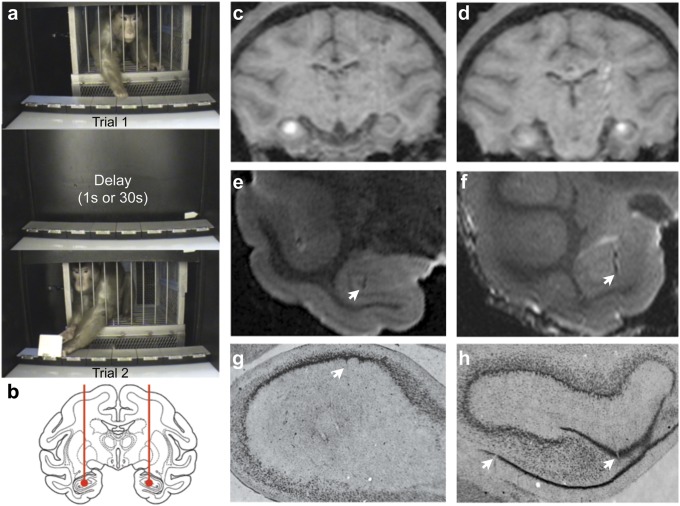
In the present experiments, we infused the glutamate receptor antagonist kynurenate [KYNA, 100 mM; 1–3 µL per infusion (30, 31)] bilaterally into the hippocampus of four macaques. These animals were pretrained to a stable level of performance on a self-ordered sequencing spatial memory task, the Hamilton Search Task (32–35), in which the monkey was in a fixed orientation relative to the stimuli within a Wisconsin General Testing Apparatus. Self-ordered tasks require subjects to monitor self-generated choices from a set of stimuli until each stimulus has been chosen (e.g., refs. 36 and 37). Here, the stimuli were an array of eight boxes arranged in a horizontal row; the boxes were identical, except for their spatial position within the row (Fig. 1 and Methods). As a contrast, we also tested the animals on a version of the task, in which each box was uniquely colored (color-cued version), thereby eliminating reliance on spatial position. At the start of a run, each box was baited with a single food reward; when the monkey collected all eight rewards the run was ended. The monkey was allowed to open one box at a time (boxes snapped shut after release). After each opening, an opaque screen in front of the monkey was lowered and then raised again after a delay (either 1 s or 30 s); each access to the box array constituted one trial. The optimal strategy, opening each box only once, allows all eight rewards to be retrieved within eight trials. Returning to a previously opened box constitutes an error.
Fig. 1.
(A) Spatial version of the task. (B) Intended infusion sites in the anterior hippocampus. (C and D) Three- to four-millimeter region of diffusion of MR contrast agent (Methods) shown as hypersignal within hippocampus. (E and F) Postmortem MRI and (G and H) histological confirmation of infusion sites. Arrows indicate cannula tracks in E–H.
After training to criterion (Methods), cranial platforms were implanted to allow drug to be infused bilaterally into the hippocampus in awake animals (as described in refs. 22–26). Measures used to assess performance were number of trials needed to complete a run, number of correct openings in the first eight trials, and a repetition index (see Methods for calculations). These values were compared within subjects across treatments and task conditions.
Results
Performance after KYNA infusion was compared with (i) performance under control conditions (predrug baseline or saline infusions) and (ii) performance at chance levels (i.e., as if box selections were purely random; Methods and Table S1). The infusion sites were calculated based on at least one postsurgical MRI (described in Methods) and were verified by focal infusion of the MR contrast agent gadolinium (1.5 µL, 5 mM). We found that the contrast agent generated hypersignal visible in a 3- to 4-mm region of diffusion confined to hippocampus, which did not extend into structures dorsal or ventral to the hippocampus (Fig. 1 C and D).
Control Sessions.
Performance on control sessions (i.e., no drug infusion) did not differ as a function of session type (i.e., baseline, sham infusion, or saline infusion; Fig. S1A) for any performance measure. Similarly, under control conditions (no drug), performance did not differ as a function of task type or delay (i.e., spatial with 1-s or 30-s delay or color-cued with 30-s delay; Fig. S1B). Because performance did not differ across these different control session types, for each subject we calculated a single control value that was the mean of the animal’s performance on all control sessions. The mean of these control values across animals is shown as “control” in Fig. 2 (additional analyses were performed using control data without baseline values, limited to saline and sham infusions; as presented in Figs. S2 and S3, these analyses yielded patterns of deficits equivalent to those described below). The three experimental conditions (i.e., spatial with 1-s delay, spatial with 30-s delay, or color-cued with 30-s delay) were compared with the control condition by within-subject nonparametric Skillings–Mack test. These data were also analyzed using a parametric mixed-effects analysis that also included between-group comparisons (30-s spatial vs. 1-s spatial vs. color-cued; Fig. S3). These statistical evaluations were made for each of the three performance measures.
Fig. 2.
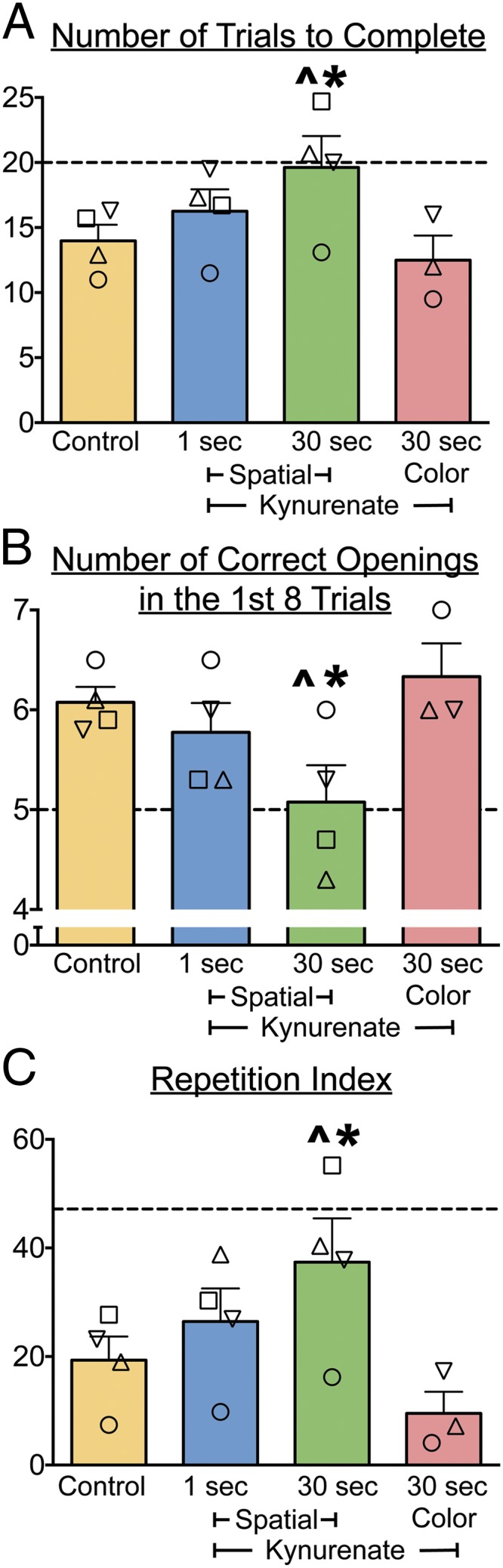
Intrahippocampal infusions of KYNA selectively impair spatial memory at 30-s delays. (A) Trials to complete a run (i.e., to open all eight boxes). (B) Number of openings before an error (reopening a box) in a run. (C) Repetition index (Methods). Each symbol represents an individual animal. Bars show means + SEM. Control is the average performance for each monkey collapsed across baseline, sham, and saline sessions. Chance levels for each measure are shown by the dotted line (see median values in Table S1). “Spatial” refers to the standard task with all eight boxes visually identical; “color” refers to the task version, in which each box is marked with a unique color. *Significantly different from control performance (P < 0.05); ^not different from chance. Symbols used to identify monkeys are as follows: □, OL; ▽, GZ; ○, TX; and △, LE.
Trials to Complete a Run.
Under control conditions, animals completed a run in a mean of 14 trials (i.e., six errors). This performance was significantly better than that expected by chance (21 trials; t = 3.87, df = 3, P < 0.05). Mean run duration (including response latencies) was 78 s for the 1-s delay condition and 455 s for the 30-s delay condition.
For the number of trials to complete a run, Skillings–Mack test revealed a significant difference across conditions (Q = 10.83, P < 0.001). Planned comparisons showed that KYNA infusions significantly increased the number of trials to complete a run when animals were tested on the spatial task with a 30-s delay (P < 0.05). In contrast, KYNA infusions did not affect performance when animals were tested on the spatial task with a 1-s delay or on the color-cued version with a 30-s delay (P > 0.05). Mixed-effects analysis also revealed a significant difference across conditions (F3,7.676 = 8.611, P < 0.01) with Sidak-corrected post hoc tests showing significant differences between the control and 30-s spatial condition, and between the 30-s spatial condition and the 30-s color-cued condition (P < 0.05).
Performance on the 30-s spatial task after KYNA infusion did not differ from chance (t = 0.71, P = 0.26); in contrast, performance remained significantly better than chance for the 1-s spatial and 30-s color-cued conditions (t = 3.87, df = 3, P < 0.05 and t = 3.46, df = 3, P < 0.05, respectively).
Number of Correct Openings in the First Eight Trials.
Under control conditions, animals made a mean number of 6.1 correct openings in the first eight trials. This level of performance was significantly better than that expected by chance (5.3; t = 3.87, df = 3, P < 0.05).
Skillings–Mack test revealed a significant difference across conditions (Q = 8.07, P < 0.0001), with post hoc comparisons indicating impaired performance on the spatial task with a 30-s delay (P < 0.05) but not with a 1-s delay or on the color-cued version (P > 0.05). Mixed-effects analysis also revealed a significant difference across conditions (F3,7.663 = 6.073, P < 0.05), with Sidak-corrected post hoc tests showing significant differences between the control and 30-s spatial conditions.
Performance following intrahippocampal KYNA infusion dropped to chance levels when animals were tested on the spatial task with a 30-s delay (t = 0, df = 3, P = 1.0).
Repetition Index.
The repetition index weighted errors more heavily when they occurred in close temporal proximity (e.g., returning to a box after one intervening trial is “worse” than returning to a box after two intervening trials). Random box openings (chance performance) yielded a repetition index of 51.4. Under control conditions the mean repetition index was 19.5, which was significantly lower than expected by chance (t = 3.87, df = 3, P < 0.05) and reflective of a low rate of revisiting boxes. Repetition errors in the 30-s spatial task occurred as little as 30 s apart (opening the same box on two consecutive trials) or as long as several minutes apart (e.g., separated by eight or more trials).
Intrahippocampal KYNA infusions caused the repetition index to reach chance levels, but only when animals were tested on the spatial task with a 30-s delay (t = 1.46, df = 3, P = 0.12). On the spatial task with a 1-s delay and on the color-cued version, animals performed significantly better than chance (t = 3.87, df = 3, P < 0.05 and t = 3.46, df = 3, P < 0.05, respectively). Skillings–Mack test revealed a significant difference across conditions (Q = 10.83, P < 0.0001) with KYNA treatment only on the spatial task with a 30-s delay differing significantly (P < 0.05) from control. Mixed-effects analysis also revealed a significant difference across conditions (F3,7.643 = 9.041, P < 0.01), with Sidak-corrected post hoc tests showing significant differences between the control and 30-s spatial condition and between the 30-s spatial condition and the color-cued condition (P < 0.05).
Other Observations.
Across individual control runs, for the first selection animals avoided boxes on the ends of the array, instead preferring boxes in the middle of the array; a similar pattern was obtained after KYNA infusion (Fig. S4). For openings across the first eight trials, the monkeys in the control condition showed fewer back-to-back errors compared with chance (t = 9.4, P < 0.005), reflecting the fact that they had learned not to go back to the same box (Fig. S5). After KYNA infusion, the mean rate of the back-to-back openings (across the first eight trials) on the spatial task with a 30-s delay was equivalent to control but was no longer different from chance because of increased variability. We also examined errors as a function of the number of openings that occurred between two successive visits to a given box. This showed that the overall error number was significantly increased after KYNA infusions on the spatial task with a 30-s delay. This increase in errors was evident with both short (including back-to-back errors) and long (separated by eight or more trials) intervals between two successive visits to a box (Fig. S6).
The overall pattern of openings under both conditions was similar to chance (Fig. S5). In addition, animals did not use a strategy of left–right alternation on successive trials, either under control or KYNA-infused conditions (Fig. S7). These data taken together indicate that the animals did not use a habit-like strategy, either under control or KYNA-infused condition.
To determine whether our manipulations altered motivation or motor function, we assessed the latency for animals to open a box at the start of a trial. KYNA had no effect on response latency on any of the task variants or delays (Fig. S8; F3,11 = 0.17, P = 0.9).
In addition to testing with KYNA, one animal was also tested using the GABAA receptor agonist muscimol (9 nmol) as another means of inactivation (to confirm that the effect we detected was not specific to the mechanism of pharmacological inactivation). Muscimol in the hippocampus produced an impairment comparable to that caused by KYNA (Fig. S9); no impairment was seen after injections of muscimol placed dorsal to hippocampus.
Discussion
Our data provide previously unidentified evidence for cognitive impairment in monkeys after transient inactivation of the hippocampus. In particular, we found an impairment in long-term spatial memory that was task-selective: It was not observed with a 1-s delay between trials (working memory) or in the presence of visual cues (colors) that eliminated the dependence on spatial position. The impairment was evident over delays ranging from 30 s (a single trial) to more than 8 min (an entire run). Importantly, the inactivation of hippocampus reduced performance to chance levels.
Unlike the chance-level performance we obtained with acute drug-induced inactivation of the hippocampus, hippocampal-lesioned animals typically perform nonnavigational tasks well above chance, if they exhibit deficits at all (10–13). It has been argued that egocentric cues allow animals with hippocampal damage to solve nonnavigational spatial tasks (15, 17). Indeed, in a study of hippocampal-lesioned animals that had deficits on a task (spatial memory span task) that most closely matches the behavior that we examined, the lesioned animals maintained performance significantly above chance (13). The fundamental difference between our current study and previous studies is the use of transient inactivation, which precludes animals from learning an alternate strategy.
The results we obtained across task variations demonstrate considerable specificity in the nature of the deficit caused by inactivation. The absence of deficits in the presence of additional visual (color) cues indicates that the impairment is selective to spatial memory, as opposed to a general problem with motivation, attention, inhibitory control, or memory for a sequence of objects or task rules. The few hippocampal lesion studies that detected deficits in nonnavigational spatial tasks have not consistently demonstrated such specificity, with some deficits extending to nonspatial tasks (13). Moreover, the fact that performance of our animals at the 1-s delay in the presence of drug was well above chance indicates that substrates other than the hippocampus, such as prefrontal cortex, can support spatial memory over short delays (<30 s). Likewise, the intact performance in the color-cued version of the task may be mediated by extrahippocampal substrates that subserve object recognition memory, such as perirhinal cortex. This is consistent with studies reporting that damage within perirhinal cortex impairs object recognition memory (38–40).
An important feature of the experimental design in our study is that the animals were pretrained to criterion on the task before introducing any intervention in the hippocampus. This is a critical feature because it has been well documented that the integrity of the hippocampus is essential for efficient learning in a variety of cognitive tasks. For example, ibotenic acid lesions within the monkey hippocampus markedly impaired acquisition of the delayed non-match-to-sample recognition memory task as evidenced by a significant increase in both errors and trials to criterion during training (13), an impairment also observed after radiofrequency lesions of hippocampus (40); similarly, monkeys with excitotoxic hippocampal lesions displayed deficits in object reversal learning and spatial scene learning (41). Learning of repeated visual sequences has also been found to be impaired in hippocampectomized monkeys (42), with similar deficits reported after excitotoxic hippocampal lesions in animals tested in a delayed recognition span task (13). Interestingly, this impairment was observed across spatial, color, and object versions of the task (13). The latter deficit resembles that described after unilateral medial temporal lobe damage in human subjects tested using the Corsi Block-tapping task and the Hebb Digit Sequence task, in which the ability to learn a repeating sequence that is either spatially cued (Corsi) or verbally cued (Hebb) was impaired by right and left hemisphere lesions, respectively (43).
Our experiments avoided these types of learning deficits by pretraining the animals to criterion in the presence of an intact hippocampus. Pretraining has also been suggested (44, 45) to render monkeys resistant to hippocampal lesion-induced deficits in visual recognition memory, thereby providing one plausible explanation for the lack of impairment after hippocampal lesions in some laboratories (10, 46) and significant impairment in others (44, 45, 47). The former is consistent with the lack of impairment we observed on the color-cued version of the Hamilton Search Task after hippocampal inactivation. This makes the lack of spatial memory we observed after hippocampal inactivation all the more striking.
The fact that we found that spatial memory performance with 30-s delays fell to chance levels after hippocampal inactivation indicates that the task performance normally relies fully on a hippocampal-dependent (presumably allocentric) strategy, and that other mechanisms for spatial processing, such as parietal cortex (for review see ref. 18), are not sufficient to support performance without hippocampal processing. However, because parietal spatial representations seem to function over relatively short delays (48, 49), it is possible that this mechanism may support performance in parallel with the hippocampus on the 1-s delay version of the Hamilton Search Task, which was not impaired in our study.
The Hamilton Search Task was originally used without the use of intertrial delays, to assess search strategy (32, 35), a function that has been associated with frontal cortex (36, 37). The first experimental manipulations in the Hamilton Search Task were in monkeys with large frontal lesions, which showed impairment in applying searching strategy compared with control animals (35). This is similar to the self-ordered pointing task used in humans by Petrides and Milner (36), where participants with either large temporal or frontal lesions displayed impairment in self-ordered sequencing within an array of visual stimuli. The results of our present study, which included 30-s delays, indicate that this task is also effective for assessing long-term spatial memory. This is consistent with the report of Levin and Bowman (33), who found that systemically administered scopolamine impaired the performance of monkeys on the Hamilton Search Task with 20-s delays. Thus, the Hamilton Search Task seems to be an especially sensitive probe for hippocampal function.
During inactivation of the hippocampus, the impairments we have observed are most likely a result of the loss of the ability to keep track of the relative position of a given box with respect to the other boxes in the array. This is supported by the observation that specific neurons in the monkey hippocampus selectively respond to rewards located in particular locations within a scene (50, 51) and by the finding that neurons in the human hippocampus show object-place coding (52). This process may subserve the mapping of allocentric cues with respect to each other and/or with respect to egocentric space. However, the particular spatial cues used by the monkeys to resolve the task cannot be deduced from our results. In humans, the hippocampus has been shown to be engaged when subjects make a sequence of remembered turns in a virtual starmaze (53). This response was localized to the left hippocampus, whereas the right hippocampus was engaged by navigating based on relative position within the maze. This suggests that the hippocampus in humans may contribute to both egocentric and allocentric spatial processing in a hemisphere-specific manner (53). Although compelling, these observations do not offer insight into whether or not the integrity of hippocampus is necessary for performing both types of spatial processing. The lesion data in both humans and animal models clearly point to allocentric spatial orientation requiring hippocampus (2, 4, 5, 7, 54), but there is no similar evidence for egocentric spatial orientation. The pharmacological inactivation approach that we have used will be an effective way to directly assess questions about the role of hippocampus for egocentric vs. allocentric spatial processing using tasks in which egocentric and allocentric representations can be individually manipulated.
Our data provide compelling evidence that the role of the hippocampus in primates is not limited to spatial processing in tasks that require navigation, but is also a critical mediator of spatial memory in nonnavigational tasks. Although preferred behavioral strategies may differ across taxa (55), the fundamental processing and functions of a structure seem to be highly conserved. Our data bring the role of the hippocampus with respect to spatial memory processing in the nonhuman primate into alignment with observations of hippocampal function in spatial memory in other species, including humans. The robust deficits in spatial memory we obtained by blocking glutamate-mediated transmission in a circumscribed region within the hippocampus demonstrate that pharmacological inactivation of the nonhuman primate hippocampus is a viable technique for assessing the function of this structure. These data open the door to further explore the role of the hippocampus in the intact primate brain for processing spatial relationships in nonnavigational settings.
Methods
Animals.
Four adult macaques aged 3–7 y (two rhesus, OL and GZ, and two pigtail, TX and LE) were used. Monkeys were pair-housed in a room with regulated lighting (12 h light/dark cycle) and maintained on primate Lab Diet (5049; PMI Nutrition International) supplemented with fruit and vegetables. Water was available for ad libitum consumption. This study was conducted with approval of the Georgetown University Animal Care and Use Committee.
Animal History.
All monkeys were previously tested on object-based tasks. OL had received microinjections in substantia nigra, superior colliculus, and orbitofrontal cortex (23, 24, 26). GZ had received microinjections in amygdala and superior colliculus. TX had received microinjections in orbitofrontal cortex (23), piriform cortex, amygdala, and substantia nigra. In the present study, TX was first tested with two injection sites per hemisphere based on our experience in another brain region (perirhinal cortex). After achieving a behavioral effect, we examined the efficacy of a single injection site to determine whether this would be sufficient to achieve the same effect in this animal. Because the behavioral effect with a single site was comparable to that with two sites, all subsequent experiments with this animal (and all experiments in the other animals) were conducted using a single site per hemisphere.
Materials.
Apparatus.
The testing board (Fig. 1A) consisted of a linear array of eight boxes attached to a base plate (33). Each box measured 87 × 63 × 32 mm and was made of black plastic with gray metal lids for the spatial-only version. The lids were painted red, blue, white, green, orange, purple, yellow, or black for the cued version. A spring hinge connected the lid to the box, such that the box only remained open while held open by the subject. The apparatus was produced to our specifications by Elmeco Engineering.
Reinforcers.
At the start of each session, all eight boxes were baited with a food reward (e.g., a fruit snack, a piece of dried fruit or nut, or a small piece of candy), and the choice of reinforcer was consistent within a session.
Training.
Animals were trained by approximation to open boxes. On initial test days, animals were allowed to remove fruit snacks from the tops of the boxes, and next from boxes held open by the experimenter. The box openings were made progressively smaller until animals began spontaneously opening them and retrieving rewards. After animals reliably retrieved a reward within 30 s of being granted access to the test board, daily testing sessions commenced.
During each session, animals were typically tested on two back-to-back runs separated by at least 5 min. Delay and task version were counterbalanced within a session to avoid any order effect. During each run animals were presented with the testing board (either spatial-only or color-cued versions) and tested at one of two delays (1 s or 30 s). Only the 30-s delay was used for the color-cued version of the task. One animal (OL) was not tested on the cued version of the task because it was introduced only after this animal completed testing and was no longer available for further testing. After animals completed the test session (by opening all eight boxes) they were rewarded with several additional fruit snacks. All animals were first tested on the spatial-only version of the task. After several weeks of testing, the cued version was introduced. Animals were tested daily (up to five times per week) until they reached criterion. Criterion performance was assessed by taking the average (for three runs) of the inverse of trials to complete, using a sliding window over a 10-run period. All task types were used when evaluating criterion performance. Criterion was defined as more than 87% of these values exceeding chance, with coefficients of variation below 0.3 over the 10-run period. Criterion was met after a mean of 35 sessions.
Surgical Procedures.
Each monkey was implanted with a stereotaxically positioned chronic infusion chamber, which allowed a removable injector, fitted with an infusion cannula of adjustable length, to be inserted into predetermined sites in the brain. We have previously described in detail the approach, the chamber, and the surgery (22–26). Briefly, a chamber made of polyoxymethylene (Helm Tech Machining Inc.) was implanted during aseptic surgery. A removable grid allowed injections spaced at 2-mm intervals in the anteroposterior and mediolateral planes. Adjustments to the length of the injection cannula set the dorsoventral position of the infusions. The cannula was inserted using a custom-built telescoping injector (22), allowing for the 27-gauge stainless steel infusion cannula to be positioned at the desired intracerebral sites.
Postoperative MRI.
Postoperatively, each monkey received one or more T1-weighted scans to determine and/or verify the coordinates for the infusion sites. Scans were conducted as previously described (23) with an effective resolution of 1.0 × 1.0 × 1.0 mm. To calculate infusion site coordinates, tungsten microelectrodes were inserted through the infusion grid with the tip of the electrode placed ∼2–10 mm above the intended site. Based on the position of the tip of the electrode on the MRI scan, the final coordinates for the drug infusions were adjusted with respect to the coordinates of the infusion grid.
MRI Verification of Tissue Volume Reached by Infusion.
To verify the volume of diffusion of the infused solution, we infused 1.5 μL of an MRI contrast agent, gadolinium (5 mM gadopentetic acid; Magnevist; Fig. 1 C and D), as we have previously described (24).
Intracerebral Microinfusions.
As shown in Fig. 1B, hippocampus was targeted bilaterally for microinfusion. KYNA (glutamate antagonist, 100 mM), muscimol (GABAA agonist, 9.0 nmol in 1.0 μL), or sterile saline (1.0 μL of 0.9% NaCl solution) was infused into the brain of the awake animal while it was seated in a nonhuman primate chair (Crist Instrument Company).
Sterile drug solutions (1.5–2.0 µL) were infused at a rate of 0.2 µL/min using a pump-driven syringe, connected by polyethylene tubing to the cannula, as previously described (22–26). One or two sites per hemisphere were infused, producing a radius of inactivation covering at least the 3–4 mm of diffusion seen after gadolinium infusion.
Sham sessions involved cleaning the cranial implant, placing the grid into the chamber, and activating the pump without inserting cannulae.
At least 48 h were allowed to elapse between experimental sessions in an individual subject.
Perfusion and Histological Confirmation of Infusion Site.
Animals (TX, GZ, and LE) were anesthetized with ketamine (5–10 mg/kg, i.m.) followed by sodium pentobarbital (25–50 mg/kg, i.v.) and perfused according to the methods we have previously used (22). The formaldehyde-fixed brain was removed from the skull and postfixed overnight in 4% (wt/vol) paraformaldehyde, 10% (vol/vol) glycerol, and 2% (vol/vol) DMSO in 0.1 M phosphate buffer then transferred for up to 6 d into 20% (vol/vol) glycerol and 2% (vol/vol) DMSO in 0.1 M phosphate buffer. After cryoprotection, the brain was scanned (described below) then frozen and sectioned (40-µm sections) on a freezing-stage sliding microtome (860; American Optical). Sections were processed and stained with thionin as previously described (22). Examples of cannula tracks in the hippocampus are presented in Fig. 1 G and H.
Postmortem MRI Confirmation of Infusion Sites.
Cryoprotected brains were wrapped loosely in paper towel moistened with cryoprotectant and placed into a plastic bag. The brain was centered in a 72-mm transmit/receive volume coil within a 7-Tesla Bruker Biospin magnet running Paravision 4.0. The brain was imaged using a TURBO-RARE sequence (TE = 12 ms, rare factor = 8, TR =1,400 ms) with four averages. The in-plane resolution was 0.25 mm with 0.5-mm slice thickness. Examples of cannula tracks visible on the scan are presented in Fig. 1 E and F.
Data Analysis.
Chance performance.
Chance performance (Table S1) was calculated by Monte Carlo simulations with the following parameters: Each box had an equal and random probability of being selected on a given trial and data were generated for a maximum of 40 trials regardless of the state of task completion. The sequences of box openings were scored for the same measures above and chance performance was defined as the mean response from 2,000 simulations.
Primary measures.
Data were analyzed as described by Levin and Bowman (33) using three measures of behavior: the number of trials needed to complete the task, the number of correct openings in the first eight trials, and a repetition index. The last measure (modified from Levin) was calculated according to the following formula: sum of the inverse of the number of trials that had elapsed between successive openings of a box, multiplied by 10. This temporally weighted index thus penalized errors more severely if they occurred closer together. For example, opening box 1, then box 2, then returning to box 1 (one box opening before returning to box 1) is a “worse” error than opening box 1, then box 2, then box 3, then returning to box 1 (two box openings before returning to box 1). Whereas the number of errors is reflected in each of these measures, each measure also offers additional information. Trials to complete provided an overall index of performance, number of correct openings in the first eight trials reflects performance early in a session (at a point when the task is easier owing to less interference from within-session openings), and repetition index reflects the number of errors and the severity of the errors (i.e., errors occurring at short delays are “worse” because they should suffer from less interference and less “forgetting”). Error rate as a function of severity is also examined in Fig. S6.
Supplementary behavioral measures.
To determine whether the animals used a specific habit-like strategy to solve the task under control conditions, we assessed the distribution of box openings on the first trial (Fig. S4) and the distance between successive box choices across all trials (Fig. S5). For these purposes, boxes were assigned numbers 1 through 8 (left to right, from the experimenter’s point of view). The distance between choices was calculated according to the formula
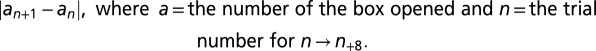 |
We also assessed the degree to which animals adopted a strategy of alternation or nonalternation. This was calculated according to the formula
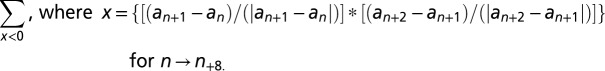 |
Analysis of search strategy under control conditions indicated that animals did not adopt a nonspatial habit strategy (Figs. S4–S7).
Statistics.
Data were analyzed using a repeated-measures ANOVA (Greenhouse–Geisser corrected for violations of sphericity) in SPSS or GraphPad Prism, by the Skilling–Mack test in Stata, and by a mixed-effects analysis in SPSS. The Skillings–Mack test is an extension of the nonparametric Friedman’s test that allows for missing values. This was chosen because one animal (OL) was not available for testing on the cued version. In all cases, planned comparisons were corrected for multiple comparisons using the method of Sidak. Sidak-corrected planned comparisons (control vs. each of the infused conditions) were performed as recommended by Conover (56). For analyses presented in Fig. S3 (comparing performance on each task type to the task-type-specific control values), we used a mixed-effects approach (task type within treatment as a repeated measure and monkey as a random effect) with Sidak-corrected post hocs. To evaluate performance relative to chance, data were assessed using rank-transformed (56), one-sample, one-tailed t tests, against chance level for a given parameter. P < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
Carrie Silver, Colin Soper, and Jenna McClane assisted with surgery, histology, and data analysis. Drs. Jocelyne Bachevalier, Elizabeth West, and Michael Ullman provided critical feedback on drafts of this manuscript. P.A.F. received support from Grant HD046388. P.A.F. and L.M. received support from the Department of Pharmacology and Physiology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320562111/-/DCSupplemental.
References
- 1.Parslow DM, et al. Allocentric spatial memory activation of the hippocampal formation measured with fMRI. Neuropsychology. 2004;18(3):450–461. doi: 10.1037/0894-4105.18.3.450. [DOI] [PubMed] [Google Scholar]
- 2.Bartsch T, et al. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2010;328(5984):1412–1415. doi: 10.1126/science.1188160. [DOI] [PubMed] [Google Scholar]
- 3.Maguire EA, Nannery R, Spiers HJ. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain. 2006;129(Pt 11):2894–2907. doi: 10.1093/brain/awl286. [DOI] [PubMed] [Google Scholar]
- 4.Parslow DM, et al. Allocentric spatial memory in humans with hippocampal lesions. Acta Psychol (Amst) 2005;118(1-2):123–147. doi: 10.1016/j.actpsy.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Morris RG, Hagan JJ, Rawlins JN. Allocentric spatial learning by hippocampectomised rats: A further test of the “spatial mapping” and “working memory” theories of hippocampal function. Q J Exp Psychol B. 1986;38(4):365–395. [PubMed] [Google Scholar]
- 6.Vann SD, Brown MW, Erichsen JT, Aggleton JP. Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. J Neurosci. 2000;20(7):2711–2718. doi: 10.1523/JNEUROSCI.20-07-02711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavenex PB, Amaral DG, Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J Neurosci. 2006;26(17):4546–4558. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glavis-Bloom C, Alvarado MC, Bachevalier J. Neonatal hippocampal damage impairs specific food/place associations in adult macaques. Behav Neurosci. 2013;127(1):9–22. doi: 10.1037/a0031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hampton RR, Hampstead BM, Murray EA. Selective hippocampal damage in rhesus monkeys impairs spatial memory in an open-field test. Hippocampus. 2004;14(7):808–818. doi: 10.1002/hipo.10217. [DOI] [PubMed] [Google Scholar]
- 10.Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci. 1998;18(16):6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malkova L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J Neurosci. 2003;23(5):1956–1965. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belcher AM, Harrington RA, Malkova L, Mishkin M. Effects of hippocampal lesions on the monkey’s ability to learn large sets of object-place associations. Hippocampus. 2006;16(4):361–367. doi: 10.1002/hipo.20147. [DOI] [PubMed] [Google Scholar]
- 13.Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9(5):562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Heuer E, Bachevalier J. Effects of selective neonatal hippocampal lesions on tests of object and spatial recognition memory in monkeys. Behav Neurosci. 2011;125(2):137–149. doi: 10.1037/a0022539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banta Lavenex P, Lavenex P. Spatial memory and the monkey hippocampus: Not all space is created equal. Hippocampus. 2009;19(1):8–19. doi: 10.1002/hipo.20485. [DOI] [PubMed] [Google Scholar]
- 16.O’Keefe J, Nadel L. 1978. The Hippocampus As a Cognitive Map (Clarendon, Oxford)
- 17.Nadel L, Hardt O. The spatial brain. Neuropsychology. 2004;18(3):473–476. doi: 10.1037/0894-4105.18.3.473. [DOI] [PubMed] [Google Scholar]
- 18.Kravitz DJ, Saleem KS, Baker CI, Mishkin M. A new neural framework for visuospatial processing. Nat Rev Neurosci. 2011;12(4):217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shamy JL, et al. Alterations of white matter tracts following neurotoxic hippocampal lesions in macaque monkeys: A diffusion tensor imaging study. Hippocampus. 2010;20(8):906–910. doi: 10.1002/hipo.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox AS, et al. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30(20):7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Reilly JX, et al. Causal effect of disconnection lesions on interhemispheric functional connectivity in rhesus monkeys. Proc Natl Acad Sci USA. 2013;110(34):13982–13987. doi: 10.1073/pnas.1305062110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wellman LL, Gale K, Malkova L. GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. J Neurosci. 2005;25(18):4577–4586. doi: 10.1523/JNEUROSCI.2257-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West EA, DesJardin JT, Gale K, Malkova L. Transient inactivation of orbitofrontal cortex blocks reinforcer devaluation in macaques. J Neurosci. 2011;31(42):15128–15135. doi: 10.1523/JNEUROSCI.3295-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DesJardin JT, et al. Defense-like behaviors evoked by pharmacological disinhibition of the superior colliculus in the primate. J Neurosci. 2013;33(1):150–155. doi: 10.1523/JNEUROSCI.2924-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dybdal D, et al. Topography of dyskinesias and torticollis evoked by inhibition of substantia nigra pars reticulata. Mov Disord. 2013;28(4):460–468. doi: 10.1002/mds.25215. [DOI] [PubMed] [Google Scholar]
- 26.Holmes AL, et al. Superior colliculus mediates cervical dystonia evoked by inhibition of the substantia nigra pars reticulata. J Neurosci. 2012;32(38):13326–13332. doi: 10.1523/JNEUROSCI.2295-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Y, Mishkin M, Aigner TG. Effects of muscarinic blockade in perirhinal cortex during visual recognition. Proc Natl Acad Sci USA. 1997;94(23):12667–12669. doi: 10.1073/pnas.94.23.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilke M, Turchi J, Smith K, Mishkin M, Leopold DA. Pulvinar inactivation disrupts selection of movement plans. J Neurosci. 2010;30(25):8650–8659. doi: 10.1523/JNEUROSCI.0953-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu W, Saunders RC, Mishkin M, Turchi J. Differential effects of m1 and m2 receptor antagonists in perirhinal cortex on visual recognition memory in monkeys. Neurobiol Learn Mem. 2012;98(1):41–46. doi: 10.1016/j.nlm.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247(1):184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 31.Schwarcz R. Metabolism and function of brain kynurenines. Biochem Soc Trans. 1993;21(1):77–82. doi: 10.1042/bst0210077. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton G. A study of trial and error reactions in mammals. J Anim Behav. 1911;1:33–66. [Google Scholar]
- 33.Levin ED, Bowman RE. Scopolamine effects on Hamilton search task performance in monkeys. Pharmacol Biochem Behav. 1986;24(4):819–821. doi: 10.1016/0091-3057(86)90417-x. [DOI] [PubMed] [Google Scholar]
- 34.Levin ED, Bowman RE. Long-term lead effects on the Hamilton Search Task and delayed alternation in monkeys. Neurobehav Toxicol Teratol. 1986;8(3):219–224. [PubMed] [Google Scholar]
- 35.Meyer DR, Settlage PH. Analysis of simple searching behavior in the frontal monkey. J Comp Physiol Psychol. 1958;51(4):408–410. doi: 10.1037/h0048236. [DOI] [PubMed] [Google Scholar]
- 36.Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20(3):249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 37.Petrides M. Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. J Neurosci. 1995;15(1 Pt 1):359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Málková L, Bachevalier J, Mishkin M, Saunders RC. Neurotoxic lesions of perirhinal cortex impair visual recognition memory in rhesus monkeys. Neuroreport. 2001;12(9):1913–1917. doi: 10.1097/00001756-200107030-00029. [DOI] [PubMed] [Google Scholar]
- 39.Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13(12):5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci. 1989;9(12):4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray EA, Baxter MG, Gaffan D. Monkeys with rhinal cortex damage or neurotoxic hippocampal lesions are impaired on spatial scene learning and object reversals. Behav Neurosci. 1998;112(6):1291–1303. doi: 10.1037//0735-7044.112.6.1291. [DOI] [PubMed] [Google Scholar]
- 42.Kimble DP, Pribram KH. Hippocampectomy and behavior sequences. Science. 1963;139(3557):824–825. doi: 10.1126/science.139.3557.824. [DOI] [PubMed] [Google Scholar]
- 43.Milner B. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull. 1971;27(3):272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- 44.Alvarez P, Zola-Morgan S, Squire LR. The animal model of human amnesia: Long-term memory impaired and short-term memory intact. Proc Natl Acad Sci USA. 1994;91(12):5637–5641. doi: 10.1073/pnas.91.12.5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zola-Morgan S, Squire LR. Memory impairment in monkeys following lesions limited to the hippocampus. Behav Neurosci. 1986;100(2):155–160. doi: 10.1037//0735-7044.100.2.155. [DOI] [PubMed] [Google Scholar]
- 46.Baxter MG, Murray EA. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus. 2001;11(1):61–71. doi: 10.1002/1098-1063(2001)11:1<61::AID-HIPO1021>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 47.Zola SM, et al. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20(1):451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishida S, et al. 2013. Discharge-rate persistence of baseline activity during fixation reflects maintenance of memory-period activity in the macaque posterior parietal cortex. Cereb Cortex. Available at www.cercor.oxfordjournals.org/cgi/doi/10.1093/cercor/bht031. Accessed December 30, 2013.
- 49.Rossetti Y, et al. Visually guided reaching: Bilateral posterior parietal lesions cause a switch from fast visuomotor to slow cognitive control. Neuropsychologia. 2005;43(2):162–177. doi: 10.1016/j.neuropsychologia.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Rolls ET, Xiang J-Z. Reward-spatial view representations and learning in the primate hippocampus. J Neurosci. 2005;25(26):6167–6174. doi: 10.1523/JNEUROSCI.1481-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rolls ET, Xiang JZ. Spatial view cells in the primate hippocampus and memory recall. Rev Neurosci. 2006;17(1-2):175–200. doi: 10.1515/revneuro.2006.17.1-2.175. [DOI] [PubMed] [Google Scholar]
- 52.Miller JF, et al. Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science. 2013;342(6162):1111–1114. doi: 10.1126/science.1244056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iglói K, Doeller CF, Berthoz A, Rondi-Reig L, Burgess N. Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc Natl Acad Sci USA. 2010;107(32):14466–14471. doi: 10.1073/pnas.1004243107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holdstock JS, et al. A comparison of egocentric and allocentric spatial memory in a patient with selective hippocampal damage. Neuropsychologia. 2000;38(4):410–425. doi: 10.1016/s0028-3932(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 55.Clark RE, Squire LR. Similarity in form and function of the hippocampus in rodents, monkeys, and humans. Proc Natl Acad Sci USA. 2013;110(Suppl 2):10365–10370. doi: 10.1073/pnas.1301225110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conover W. Practical Nonparametric Statistics. 3rd Ed. New York: Wiley; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.