Abstract
INTRODUCTION
Class III malocclusion is characterized by a composite of dento-skeletal patterns that lead to the forward positioning of the mandibular teeth in relation to the maxillary teeth and a concave profile. Environmental and genetic factors are associated with this condition, which affects 1% of the US population and imposes significant esthetic and functional burdens on affected individuals. The purpose of this study was to capture the phenotypic variation present in a large sample of white adults with Class III malocclusion by using multivariate reduction methods.
METHODS
Sixty-three lateral cephalometric variables were measured from pre-treatment records of 292 Class II Caucasian adults (126 males, 166 females; ages 16-57 years). Principal component analysis and cluster analysis were used to capture the phenotypic variation and identify the most homogeneous groups of individuals to reduce genetic heterogeneity.
RESULTS
Principal component analysis resulted in 6 principal components that accounted for 81.2% of the variation. The first three components represented variations in mandibular horizontal and vertical position, maxillary horizontal position, and mandibular incisor angulation, respectively. The cluster model identified 5 distinct subphenotypes of Class III malocclusion.
CONCLUSIONS
A spectrum of phenotypic definitions was obtained replicating results of previous studies and supporting the validity of these phenotypic measures in future research of genetic and environmental etiology of Class III malocclusion.
INTRODUCTION
A disproportionate facial appearance often accompanies a severe Class III malocclusion and can result in a significant burden on the quality of life for those affected. Current therapies for this condition are aimed at treatment rather than prevention; thus, patients undergo years of orthodontic and/or orthopedic treatment with many requiring surgical correction in adulthood. Studies since the 1970s have provided evidence that Class III skeletal characteristics have a strong genetic component.1-3 In order to elucidate preventive strategies and improve treatment modalities for these patients, studies identifying the genetic etiology of Class III malocclusion are warranted. However, detection of human susceptibility genes for Class III malocclusion is in its initial stages since no etiologic mutations have yet been identified.
The few genetic mapping studies of class III malocclusion thus far have found genetic linkage of mandibular prognathism to chromosome loci 1p22.1, 1p36, 3q26.2, 4p16, 6q25, 11q22, 12q13.13, 12q23, 14q 24.3 and 19p13.2 4-7 and positive association signals of mandibular height and prognathism to genes GHR, Matrilin-1, EPB41, TGFB3 LTBP and MYO1H 7-10 indicating that molecular pathways implicated in bone (TGFB3, LTBP) and cartilage (GHR, Matrilin-1) development are plausible candidates for mandibular size discrepancies and should be considered in future research.
While informative, the few genetic studies to date have limitations including modest sample sizes, exclusion of environmental effects, unknown generalization of results to other ancestries; and finally and perhaps more importantly, limited phenotypes that cannot capture the complexities present in the Class III malocclusion. The success of genetic studies aimed at identifying causative genes for complex traits such as malocclusion depends greatly on a well-characterized phenotype to reduce heterogeneity.11 Studies of cross sectional and retrospective longitudinal samples utilizing conventional cephalometry or shape analysis methods have attempted to characterize the dento-skeletal morphology present in Class III children and adults of different ethnicities.12-23 In general, the majority of these studies have shown a great variation in dento-skeletal morphology, yet the most common features in Class III individuals include a short anterior cranial base with an acute saddle angle, maxillary retrusion with a normal or protruded mandible, mandibular protrusion with a normal maxilla and combinations of these antero-posterior discrepancies with a normal, excessive or deficient vertical facial dimension along with protrusive maxillary incisors and retrusive mandibular incisors. Most of these components are present in the majority of class III individuals regardless of ethnic background, appear early on in development and tend to worsen with age.13, 14, 16, 23, 24
Recently, studies using multivariable methods such as principal component analysis (PCA) and cluster analysis applied to data from cephalometric radiographs have provided further insight into the characterization of Class III malocclusion phenotypes beyond traditional cephalometric methods 24-27. PCA essentially decomposes the correlations of a set of variables into orthogonal linear combinations of these variables (called components) 28. The information captured by the components decreases with the component order. Each component has scoring coefficients or weights for the included variables that allow for constructing a linear index that reflects a phenotypic axis of variation in the variables. In other words, PCA accounts for the overall morphological variation in the craniofacial complex 29, 30. On the other hand, cluster analysis complements PCA by identifying groups of individuals of similar phenotypes and allowing for traditional case-control comparisons.
Mackay et al. (1992) studied morphologic variation in craniofacial form using cluster analysis in 50 severe, non-growing Class III cases requiring surgical correction and identified 5 subgroups.25 These findings provided good evidence that different forms of Class III malocclusion exist and can successfully be divided into groups based on similar phenotypes. Hong and Yi (2001) used cluster analysis to illustrate that different patterns of skeletal architecture - beyond the current simple classification based on the position of the maxilla, mandible, dento-alveolar units and vertical relationships - contribute to the development of the Class III deformity 24. They identified 7 clusters in their Asian sample of 106 untreated Class III subjects with a mean age of 21-years-old (range 16-32). Their clusters illustrated that in addition to the facial bones and dentition, the cranial base, cranial vault and the cervical spine were also involved in different but specific architectural patterns.
Abu Alhaija and Richardson (2003) studied 115 class III children of ages 11.6-12.7 with cluster analysis and discriminant function analyses to differentiate between favorable and unfavorable growers. This study found three main clinical clusters according to long, short and intermediate facial heights and determined that the power of discriminant function analyses to discriminate between favorable and unfavorable growers increases from 80% to 100% in some cases when cluster analysis is applied prior to discriminant function analysis 26.
The latest article and the one most directly relevant to this work was carried out by Bui et al. in 2006 and characterized Class III malocclusion phenotypes using cluster analysis and PCA of 67 cephalometric variables derived from 309 Class III individuals 27. Their sample included a wide age range (average age of 19.1; range 5.9 – 56.3) and was racially/ethnically diverse, consisting of 73% Caucasians, 17% African Americans, 5% Asians, 3% Hispanics and 2% individuals of other race/ethnicity. Subjects with previous orthodontic treatment, congenital abnormalities, trauma or incomplete or low quality cephalograms were excluded. Five clusters were identified representing distinct subgroups of Class III malocclusion. In addition to the spectrum of phenotypic variation evidenced by the clusters, the investigators found that the first five principal components derived from the data explained 67% of variation within the sample. Based upon these combined findings, the authors suggested that different genes may be involved in controlling dimensions versus structures and questioned current treatment modalities that target the growth of the maxillary or mandibular skeletal structures. Although these data are informative, the sample included subjects who were still growing, and would not have had fully expressed phenotypes. In addition, the very small numbers of ethnicities represented may not be large enough to be statistically meaningful increasing phenotypic heterogeneity and limiting generalizability. Still, that study clearly demonstrated that Class III malocclusion exists in morphologically diverse patterns that can be classified into phenotypes using multivariable methods such as cluster analysis and PCA.
While previous studies have contributed to our understanding of the craniofacial components of the Class III malocclusion, limitations exist in sample sizes, sample selection criteria such as including growing individuals and not excluding other genetic or environmental traits such as missing or impacted teeth, heterogeneity due to race/ethnicity, and lack of or limited standardization of data with respect to key variables such as age and sex before applying the data reduction methods. Therefore, there is uncertainty regarding the extent to which the results from previous work – particularly from the most recent and methodologically advanced Bui et al. study – are generalizable to other samples and populations and whether one can identify additional phenotypic variation in other samples.
In this study, we aimed at extracting phenotypes that could best capture the phenotypic variation present in a large adult Caucasian Class III sample by using multivariate reduction methods. Using similar methods to those in Bui et al., one goal was to evaluate if their phenotypes replicate in an ethnically homogeneous sample limited to post-pubertal individuals. In light of the uncertainty about generalizability of previous findings, replication studies are essential to evaluating the validity of this approach for phenotypic characterization. Another goal was to see if we could explain meaningful additional variation in this sample. Such improvement in phenotypic variation can be important both clinically and for increasing the power of genetic studies. We apply rigorous sample inclusion criteria and carefully account for age and gender effects to increase the precision of the estimation. Our work builds on previous studies and provides a comprehensive set of Class III phenotypes that can be readily applied for phenotypic characterization of Class III individuals in other samples, which would facilitate large future collaborations of genetic studies.
MATERIALS AND METHODS
The study protocol was reviewed and approved by the Institutional Review Board at the University of Iowa. The study sample included adult Class III patients who were seeking treatment at the University of Iowa Orthodontic Graduate Clinic, University of Iowa Hospital Dentistry Clinic or surrounding area Private Practice Clinics. The sample consisted of 292 Caucasian post-pubertal subjects (126 males ≥18, 166 females ≥ 16; age range 16-57 years) who would have completed 98% of their growth at the time of initial records and met our eligibility criteria (Table I), which selected for moderate (ANB or Witts from 0 to-3) to severe (ANB or Witts < 3) Class III malocclusion with a skeletal component. The pool of available subjects included 311 individuals; eighteen of non-Caucasian race were excluded due to lack of power and one additional subject was found to be ineligible based on inclusion criteria.
Table 1.
Eligibility Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Adult (female ≥ 16 years, male ≥ 18 years) | |
|
| |
| At least 2 of the following clinical criteria required: | History of severe facial trauma |
|
| |
| ANB ≤ 0 | Previous orthodontic treatment |
|
| |
| Overjet ≤ 0 at least edge-to-edge or anterior crossbite | Presence of facial syndromes |
|
| |
| Wits (female ≤ 0, male ≤ -1) | Missing or poor quality records |
|
| |
| Angle CIII molar or canine relationship on at least one side | Missing or impacted teeth other than 3rd molars |
|
| |
| Concave profile | Retained primary teeth |
Cephalometric Procedure
2D pre-treatment lateral cephalometric films of 292 Class III adults were digitized using Dolphin Imaging, version 11.0 (Dolphin Imaging Systems, Chatsworth, Calif). Sixty-three cephalometric measurements were taken representing distance (mm), degree, percentage and difference measures between cephalometric landmarks, which were derived from commonly used lateral cephalometric analyses 27, 31, 32 (Table II). Data were obtained from two different sources (film and digital radiographs). All films taken on conventional/analog cephalometric units from either the College of Dentistry Graduate Orthodontic Clinic or the Hospital Dentistry Clinic were scanned into Dolphin with a 100mm ruler and corrected for magnification by 12% and 13%, respectively. Distance measures for film radiographs were scaled (multiplied by 0.8929 for 12% magnified cephalometric radiographs from the College of Dentistry Graduate Clinic and 0.8850 for 13% magnified cephalometric radiographs from Hospital Dentistry Clinic) to match the digital radiographs which were not corrected for magnification 33. In order to reduce landmark identification errors, all scanned analog films were traced twice (by K.V.) and the average value for each variable was used in data analysis 34.
Table 2.
63 Cephalometric Variables
| Cranial Base | Intermaxillary | Dental |
|---|---|---|
| Saddle/Sella Angle (SN-Ar) (°) | ANB (°) | U1 - SN (°) |
| Ant Cranial Base (SN) (mm) | Facial Plane to AB (AB-NPg) (°) | U1 - NA (°) |
| Post Cranial Base (S-Ar) (mm) | Facial Plane to SN (SN-NPg) (°) | U1 - NA (mm) |
| Midface Length (Co-A) (mm) | U1 - FH (°) | |
| Maxilla | P-A Face Ht (S-Go/N-Me) (%) | IMPA (L1-MP) (°) |
| SNA (°) | Y-Axis (N-S-Gn) (°) | L1 - NB (°) |
| Convexity (NA-APg) (°) | Mx/Md Diff (Co-Gn - Co-ANS) (mm) | L1 - NB (mm) |
| N-A ∥ HP (mm) | Wits Appraisal (AO-BO) (mm) | L1 Protrusion (L1-APg) (°) |
| A to N Perp (FH) (mm) | Ant Face Ht (N-Me) (mm) | L1 Protrusion (L1-APg) (mm) |
| Mx Unit Length (Co-ANS) (mm) | Upper Face Ht (N-ANS) (mm) | FMIA (L1-FH) (°) |
| Lower Face Ht (ANS-Me) (mm) | Interincisal Angle (U1-L1) (°) | |
| Mandible | Nasal Ht (N-ANS/N-Me) (%) | UADH (U1-PP) (mm) |
| SNB (°) | PFH:AFH (Co-Go/N-Me) (%) | LADH (L1-MP) (mm) |
| Facial Angle (FH-NPg) (°) | FMA (FH-MP) (°) | UPDH (U6-PP) (mm) |
| Gonial/Jaw Angle (Ar-Go-Me) (°) | SN - GoGn (°) | LPDH (L6 - MP) (mm) |
| Chin Angle (Id-Pg-MP) (°) | Occ Plane to SN (°) | Overjet (mm) |
| Ramus Height (Ar-Go) (mm) | Occ Plane to FH (°) | Overbite (mm) |
| Length of Mn Base (Go-Pg) (mm) | FH - SN (°) | |
| Facial Taper (N-Gn-Go) (°) | Soft Tissue | |
| Articular Angle (S-Ar-Go) (°) | Upper Lip to E-Plane (mm) | |
| N-B ∥ HP (mm) | Lower Lip to E-Plane (mm) | |
| N-Pg ∥ HP (mm) | U Lip to ST N Perp (FH) (mm) | |
| B to N Perp (FH) (mm) | L Lip to ST N Perp (FH) (mm) | |
| Pg to N Perp (FH) (mm) | ST Pg to ST N Perp (FH) (mm) | |
| Mn Unit Length (Co-Gn) (mm) | ||
| Pg - NB (mm) | ||
| Post Facial Ht (mm) (Co-Go) |
Method Error
Reliability in landmark location and resulting calculation of craniofacial measurements was determined by means of inter-rater and intra-rater methods using the intraclass correlation (ICC) 35 and difference testing. A sample of 15 random cephalometric radiographs were traced by two different raters (K.V. and L.M.) in order to assess inter-rater reliability and traced two times at least three weeks apart by the same rater (K.V.) to assess intra-rater reliability. In addition, the possibility of systematic differences between raters or between first and second ratings was assesses using the Wilcoxon Rank Sum procedure. All analyses were performed using SAS for Windows (v9.2, SAS Institute Inc, Cary, NC, USA), and a type I error of 0.05 was assumed.
Statistical Analysis
PCA and cluster analysis were used to capture the most significant components of variation and identify the most homogeneous groups of individuals representing distinct Class III phenotypes to reduce genetic heterogeneity. Data were standardized using a linear model to assess possible effects of age and gender and to consider the possibility of age-by-gender interactions. A separate model was fit for each of the 63 cephalometric measures using standard multiple regression methods. In all, four different configurations of covariate adjustment were used among the 63 models: all included an adjustment for gender, some also required an age adjustment, and others an additional consideration of gender by age interaction, i.e., different age adjustment for each gender. Model diagnostic procedures were performed on all standardization models and assumptions were validated. The studentized (normalized) residuals were extracted from these models and used as the standardized data for the PCA. Standardized PCA scores were the basis for the formation of clusters defining different phenotypes within the Class III malocclusion. Criterion-based model selection methods were used to determine the cluster configuration that illustrated the most distinct clusters graphically. Cluster analysis was performed via a partitional cluster analysis of extracted principal components using SAS 9.3 statistical software with methods based on the leader 36 and the k-means 37algorithms using the method of Anderberg 38called nearest centroid sorting.
To visualize the cluster analysis results, a canonical discriminant analysis was performed and scored canonical variables were computed. The scored canonical variables were used to plot pairs or triads of canonical variables in order to aid visual interpretation of cluster differences. R statistical program along with the rgl package were used to produce three-dimensional graphs of the data.
The k-means clustering algorithm is sensitive to extreme values as a consequence of the least squares condition; however no subjects in this dataset appeared to represent extreme observations. The clustering algorithm was performed separately for a range of number of clusters, from 3 to 7 clusters. The criterion based methods of pseudo F statistic 39, approximate expected over-all R2, and cubic clustering criterion (valid because of the uncorrelated nature of principal components) 40 as well as data visualization techniques of scored canonical variables were used to determine the appropriate number of clusters 41. Of the range of clusters considered, the five cluster model best optimized the criterion and presented the most distinct clusters graphically. Cluster validation was performed by locating subjects closest to the final cluster means and examining the subject’s cephalometric data and profile to ensure that clusters represented distinct clinical phenotypes. All analyses used SAS 9.3 with a 0.05 level of significance.
RESULTS
Reliability testing of landmark location and derived craniofacial measurements showed inter-rater reliability values for intraclass correlation ranging from ICC=0.8594 to ICC=0.9987, with only 4 variables in which ICC< 90%. Intra-rater reliability ranged from ICC=0.9021 to ICC=0.9999, with only 2 variables in which ICC< 94%. In general, inter- and intra-rater reliability is deemed acceptable with values above 85% 35. Thus, excellent agreement between the two measures was achieved for all 63 variables. Results from difference testing showed 16 significant differences between the two sets of measures for inter-rater reliability using the Wilcoxon signed rank test. The median difference was greater than 0.5 (mm) for only 7 of the 16 variables. When intra-rater reliability was assessed, 5 significant differences were found to exist between the two sets of measures. The median difference for CoAmm was 0.5 (mm); the median difference for SNA° was 0.1°. After examining variables with significant differences, outliers were identified and techniques utilized to improve reliability to acceptable values. In general, discrepancies in cephalometric measurements within 0.5-1mm are acceptable in the literature due to the inherent difficulty in landmark location.
The results of the PCA revealed that six principal components accounted for 81.2% of the total variance in the data (Figure 1). The first six principal components (PCs) were selected because they explained the most variation in the data set and were specific in their anatomic explanation. As shown in Figure 1, PCs beyond the 6th component were deemed not informative as the additional variation explained decreased significantly. About half of the variation in this sample was explained by the AP position of the mandible in relation to the cranial base, the size of the maxillo-mandibular horizontal discrepancy and the lower incisor position and its effect on lower lip protrusion. Table III contains the variance explained by each of the 6 components and the set of cephalometric variables that contributed the most to each PC. Figure 2 displays cephalometric profiles of individuals with extreme PC score values (i.e. most negative and most positive scores) for each of the six PCs together with the highest loading cephalometric variables within each PC.
Figure 1.
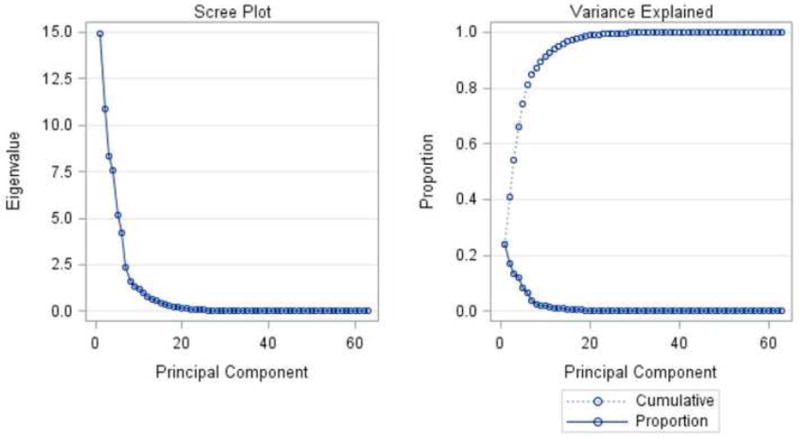
Principal Component Analyses. Six principal components accounted for 81.2% of the variation.
Table 3.
Principal Component Analysis.
| Principal Component | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Variance Explained | 0.2374 | 0.1729 | 0.1325 | 0.1199 | 0.0825 | 0.0665 |
| Cumulative Variance | 0.4103 | 0.5428 | 0.6627 | 0.7452 | 0.8117 | |
| *Variables | Facial Plane to SN (SN-NPg) (°) | Mn Unit Length (Co-Gn) (mm) | L1 Protrusion (L1-APg) (mm) | IMPA (L1-MP) (°) | U1 - NA (°) | FH - SN (°) |
| N-Pg ∥ HP (mm) | Post Facial Ht (mm) (Co-Go) | L1 - NB (mm) | Mx/Md Diff (Co-Gn - Co-ANS) (mm) | U1 - NA (mm) | Saddle/Sella Angle (SN-Ar) (°) | |
| Y-Axis (N-S-Gn) (°) | Midface Length (Co-A) (mm) | Lower Lip to ST N Perp (FH) (mm) | Chin Angle (Id-Pg-MP) (°) | A to N Perp (FH) (mm) | Occ Plane to FH (°) | |
| N-B ∥ HP (mm) | Mx Unit Length (Co-ANS) (mm) | L1 - NB (°) | Wits Appraisal (AO-BO) (mm) | SNA (°) | Upper Lip to ST N Perp (FH) (mm) | |
| SNB (°) | Ramus Height (Ar-Go) (mm) | Pg - NB (mm) | Facial Taper (N-Gn-Go) (°) | N-A ∥ HP (mm) | Lower Lip to ST N Perp (FH) (mm) |
Variables making the greatest contribution to the respective principal component.
Figure 2.
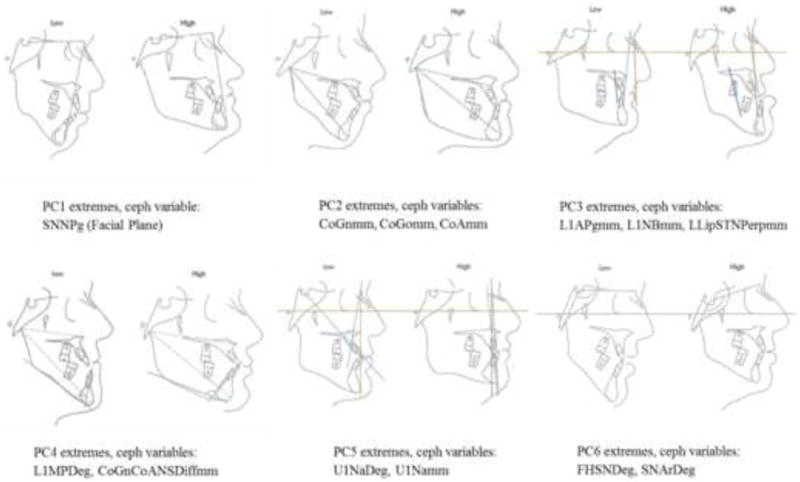
Ceph profiles of individuals with PC scores that fall on opposite ends (i.e. the most positive and most negative scores) on each of the six principal components together with the highest loading ceph variables within each component. PC 1 refers to the antero-posterior position of the mandible in relationship to the cranial base and explains 23.7% of the variation. PC 2 refers to the maxillo-mandibular horizontal and vertical size discrepancies and explains 17.3% of the variation. PC 3 refers to the position and inclination of the lower incisor and its effect on lower lip protrusion and explains 13.3% of the variation. PC 4 refers to lower incisor angulation, facial taper and variation in maxillo-mandibular discrepancies and explains 12.0% of the variation. PC 5 refers to variation in the upper incisor and the maxillary horizontal position and explains 8.3% of the variation. PC 6 refers to variation in the cranial base and explains 6.7% of the variation.
The cluster analysis resulted in the identification of five phenotypes within Class III individuals (Figure 3). The preliminary cluster analysis explored configurations of three to seven clusters of Class III phenotypes based upon the cephalometric measurements. During this process, the iterative reassignment of cluster centroids progressed until no observations changed clusters and convergence was achieved by the cluster algorithm in all configurations.
Figure 3.
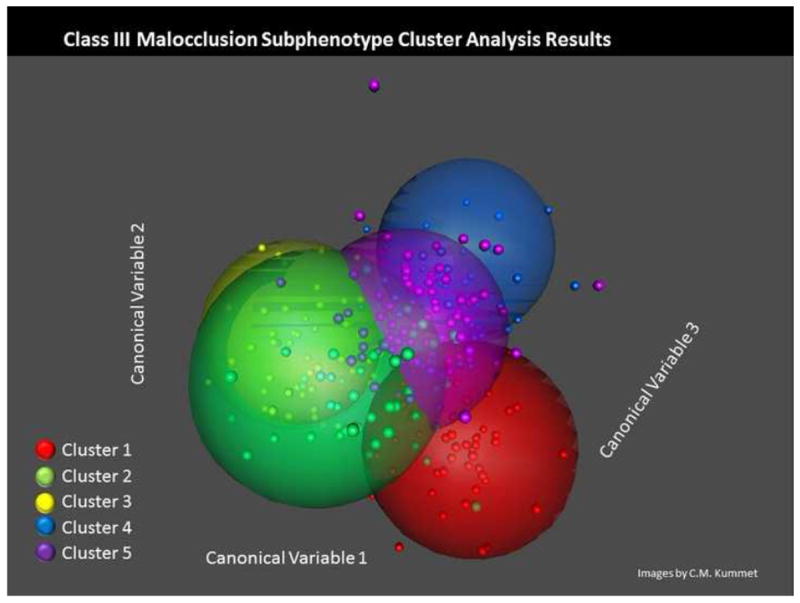
3-D Plot showing 5 spatially distinct clusters of CIII malocclusion subjects.
The model with three clusters was too simplistic clinically while the seven cluster model contained redundant information. Although the cluster validation graph showed the ideal statistical criteria at four clusters, an important Class III phenotype – the vertical subtype – was not represented; thus, a five cluster model was selected because it yielded the most spatially distinct and clinically meaningful phenotypes that were statistically acceptable (Table IV). Cluster 5 (severely retrusive maxilla, normal mandible) was the central cluster and contained the most observations (n=86); however, cluster 4 (normal maxilla, severely protrusive mandible) had the largest standard deviation (spread of observations). Cluster 4 also had the fewest observations (n=44). Cluster centroids representing the average phenotype within each cluster are illustrated in Figure 4. Clusters 1 and 2 depict borderline Class III phenotypes with a combination of mild maxillary retrognathism and mandibular prognathism, yet with either a flat or normal mandibular plane, respectively. Cluster 3 corresponds with the vertical Class III phenotype with a large anterior facial height, while Cluster 4 and Cluster 5 represent the severely mandibular prognathic and severely maxillary retrognathic phenotypes, respectively. Complete descriptions of clusters phenotypes are given in Table V.
Table 4.
Cluster Summary
| Cluster Summary-5 Clusters
| ||||
|---|---|---|---|---|
| Frequency (% total) | *Root mean Squares (St.Dev) | Nearest Cluster | Distance Between Centroids | |
|
| ||||
| 1 | 56 (19.2%) | 0.80 | 5 | 2.19 |
| 2 | 56 (19.2%) | 0.84 | 5 | 2.23 |
| 3 | 50 (17.1) | 0.85 | 5 | 2.06 |
| 4 | 44 (15.1%) | 0.89 | 5 | 2.18 |
| 5 | 86 (29.5%) | 0.73 | 3 | 2.06 |
N=292 Cuacasian. Data age, gender adjusted and normalized PCA.
Indicates the average distance between observations in the cluster.
Figure 4.
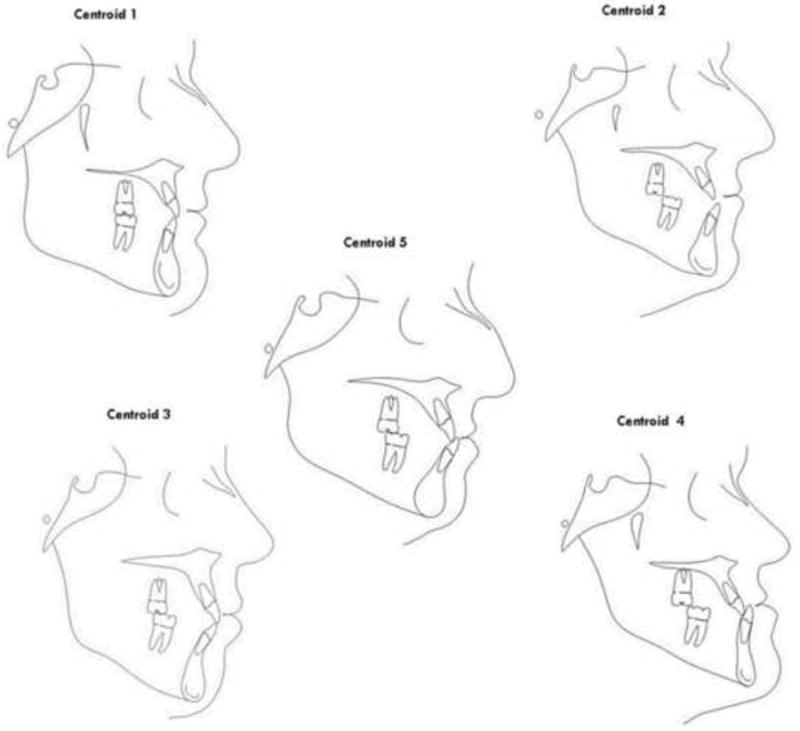
Cluster centroids. Clusters 1 and 2 represent borderline CIII phenotypes with a combination of mild maxillary retrognathism and mandibular prognathism, yet with either a flat or a normal mandibular plane, respectively. Cluster 3 corresponds with the vertical CIII phenotype with a large mandible expressed vertically. Cluster 4 and Cluster 5 represent the severely mandibular prognathic and severely maxillary retrognathic phenotypes, respectively.
Table 5.
Description of Clusters
| Atribute | Cluster 1 (N=56) | Cluster 2 (N=56) | Cluster 3 (N=50) | Cluster 4 (N=44) | Cluster 5 (N=86) |
|---|---|---|---|---|---|
|
| |||||
| Cranial Base | Acute Short Ant CB | Acute Short Ant & Post CB | Normal Angle Long Ant & Post CB | Acute Short Ant. & Post CB | Normal Angle Slighlty Short Ant & Post CB |
|
| |||||
| Maxilla | Slightly Retrusive | Moderately Retrusive | Normal | Normal | Severly Retrusive |
|
| |||||
| Mandible | Slightly Protrusive | Slighlty Protrusive | Protrusive, expressed vertically | Severly Protrusive | Normal |
|
| |||||
| Vertical | Slighlty Flat MP ↑ Ant. Facial height Normal Ramus | Normal MP ↓ Ant. Facial Height Short Ramus | Steep MP ↑ Ant Facial Height Long Ramus | Normal MP Slightly Short Ramus | Normal MP ↑ Lower Ant. Face Height Short Ramus |
|
| |||||
| U1 | Normal | Protrusive | Normal | Protrusive | Normal |
|
| |||||
| L1 | Retrusive | Normal | Protrusive | Retrusive | Slightly Protrusive |
|
| |||||
| Lips | Retrusive | Retrusive | Protrusive Lower Lip | Retrusive Upper Lip | Retrusive Upper |
| Protusive Lower lip | Lip Normal Lower Lip | ||||
MP, Mandibular plane. CB, Cranial base. U1 Upper incisor. L1 Lower incisor.
DISCUSSION
An important step towards the identification of genes implicated in class III malocclusion is the comprehensive characterization of the phenotypic expression of this condition. Conventional pre-treatment orthodontic records constitute an invaluable resource for the characterization of craniofacial variation since they provide skeletal, soft tissue and 3D dento-alveolar data that can be analyzed to construct comprehensive craniofacial phenotypes. Integration of genetic and environmental data with carefully characterized craniofacial phenotypes will eventually lead to identification of the etiologic genetic and environmental factors that predispose to disproportionate craniofacial growth and Class III malocclusion.
In our study, six PCs of various multivariate traits as well as five clusters within the sample of Class III individuals were identified confirming several results from previous studies. We replicated the clusters and most of the principal components in Bui et al., (2006) and were able to explain 81% of the total variation based on the first 6 components, which adds 14% of explained variation above the 67% reported by Bui et al. based on 5 components- 7% variation is due to the sixth principal component. While this additional explained variation is relatively modest, it may be meaningful both clinically and in research studies by capturing some of the “higher hanging fruit” and enhancing the power of genetic studies.
In both studies, PC 1 represented sagittal parameters such as the facial plane to SN and the facial angle. PCs 2 and 3 consisted mostly of vertical and antero-posterior measures as well as lower incisor and lower lip position. Together, approximately half of the variation in both studies was explained by the heavily weighted variables in these three components. Interestingly, the maxilla and upper incisor position were not captured in the Bui PCA to the same extent as ours (PC5, explaining 8%). Perhaps the inclusion of only Caucasian samples and exclusion of individuals with missing or impacted teeth in our study could account for these differences. However, our results overall independently replicated the main findings of the Bui et al., study.
Similarly, the ability to capture an additional 14% of variation in our study may also be in part due to differences in sample eligibility and some analytical specifics between the two studies. As mentioned above, the sample in Bui et al was racially diverse compared to our solely Caucasian group. Also, we only included post-pubertal individuals with nearly completed growth at the time of initial records in our sample, ensuring nearly full expression of the malocclusion phenotype. In contrast, their sample age ranged from about 6 to 56 years, which could affect interpretation of the results since skeletal components of class III malocclusion worsen with age. In addition, subjects with missing or impacted teeth were excluded from our study to further reduce confounding variables such as early tooth loss which can result in Class III malocclusion irrespective of the patient’s genotype. It is also possible that the additional explanatory power is driven by other uncharacterized differences between the two samples Yet, despite differences in sample composition between the two studies, it was very gratifying to find that the PCs derived in the two studies were similar in terms of the most informative cephalometric variables emphasizing the validity of these phenotypic methods and providing support to the use of these phenotypes and sub classifications in future genetic studies.
The six PCs were used as the basis for the formation of clusters defining phenotypes of Class III malocclusion in our study. Instead of using standard PCA scores, Bui et al. used normalized cephalometric values to form their clusters. Other studies have employed different methods such as the centroid method used by Mackay or the Delaire analysis used by Hong and Yi to evaluate craniofacial morphology, which may account for the slightly different results between studies.
Determination of the number of clusters is subjective and can result in variability between studies. Models using three to seven clusters were tested in our sample to determine the model that best optimized the criterion and presented the most spatially distinct clusters graphically. Additional cluster validation was performed by locating subjects closest to the cluster means and examining the subjects’ cephalometric data to ensure that each cluster represented clinically meaningful Class III phenotypes. We selected five clusters, which is in accordance with previous studies. Bui and Mackay identified five cluster groups, while Abu Alhaija and Richardson identified three clusters and Hong and Yi identified seven. Our description of the cluster centroids is more complex than Bui’s and Abu Alhaija’s, which only included variation in three components: maxillary position, mandibular position and vertical dimensions. While is tempting to oversimplify the facial morphology in this way, it prevents using multivariate data reduction procedures such as PCA and CA to their full potential. Inclusion of additional morphological features that contribute to the Class III malocclusion phenotype such as cranial base dimensions, incisor angulation and lip posture, as also suggested by Hong and Li, may permit a more comprehensive characterization.
Similar to Bui et al., our results also support a contributory role for other cephalometric variables in evaluating the morphological characteristics of Class III subjects as opposed to the more commonly used cephalometric variables ANB, Overjet and Wits. While direct comparison of our results to previous studies is restricted due to the different sample sizes, age ranges, ethnicity and malocclusion severity 24-26, 42, the similarity between results is encouraging as it indicates an independent replication of the underlying skeletal structure in the phenotypes of subjects with Class III malocclusion. Therefore, we believe that our phenotypic classification can be applied to other class III subjects with less restriction which can facilitate multicenter collaborations for genetic studies in the future.
Ongoing studies at the College of Dentistry of the University of Iowa are using these data to target individuals for collection of DNA and environmental data; however, current genetic and environmental studies will necessitate much larger samples and therefore multicenter collaborative projects will constitute the ideal scenario for the identification of malocclusion etiology. Moreover, similar studies in the future utilizing three-dimensional hard and soft tissue images will expand the scale and scope of phenotypic approaches in the craniofacial complex that could facilitate gene discovery. Understanding the genetic etiology of unbalanced craniofacial growth will have a large impact on orthodontic patient care worldwide via novel and improved therapy and prevention approaches. In the future, gene therapy will be capable of reestablishing harmony in the growing face, ultimately translating into improved quality of life for individuals affected with these conditions.
CONCLUSIONS
In this study we characterized Class III malocclusion phenotypes via data reduction methods including principal component analysis and cluster analysis applied to cephalometric data of a large adult Class III sample. The PCA reduced 63 cephalometric variables into six principal components, which captured 81% of variation within our sample and the cluster analysis identified five distinct phenotypic subgroups. Our study replicates the main findings in previous studies which supports the validity of these phenotypic measures for future research of genetic and environmental etiology.
Acknowledgments
We thank Drs. Robert N. Staley, James S. Wefel and George Wehby for their helpful discussions during the preparation of this manuscript. We also thank Chika Takeuchi, Mary E. Hoppens and Patricia Hancock for their assistance with orthodontic record review. We also thank the private practices of Drs. Clayton Parks, Jason Schmit, Paul and John Hermanson, Tom Stark, David Gehring, Carney Loucks and Jennifer Buren for their contribution with orthodontic records.
Funding: AAOF OFDFA_2008-2011 and also supported by the National Center for Advancing Translational Sciences, and the National Institutes of Health (NIH), through Grants 2 UL1 TR000442-06 and T32-DEO14678-09. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Litton SF, Ackermann LV, Isaacson RJ, Shapiro BL. A genetic study of Class 3 malocclusion. Am J Orthod. 1970;58:565–577. doi: 10.1016/0002-9416(70)90145-4. [DOI] [PubMed] [Google Scholar]
- 2.Markovic M. Results of a genetic study of triplets with class III malocclusion. Zahn Mund Kieferheilkd Zentralbl. 1983;71:184–190. [PubMed] [Google Scholar]
- 3.Mossey PA. The heritability of malocclusion: Part 1--Genetics, principles and terminology. Br J Orthod. 1999;26:103–113. doi: 10.1093/ortho/26.2.103. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi T, Park SB, Narita A, Maki K, Inoue I. Genome-wide linkage analysis of mandibular prognathism in Korean and Japanese patients. J Dent Res. 2005;84:255–259. doi: 10.1177/154405910508400309. [DOI] [PubMed] [Google Scholar]
- 5.Frazier-Bowers S, Rincon-Rodriguez R, Zhou J, Alexander K, Lange E. Evidence of linkage in a Hispanic cohort with a Class III dentofacial phenotype. J Dent Res. 2009;88:56–60. doi: 10.1177/0022034508327817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Zhang F, Li X, Chen F. Genome scan for locus involved in mandibular prognathism in pedigrees from China. PLoS One. 2010;5:e12678. doi: 10.1371/journal.pone.0012678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Li X, Zhang F, Chen F. The identification of a novel locus for mandibular prognathism in the Han Chinese population. J Dent Res. 2011;90:53–57. doi: 10.1177/0022034510382546. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Lu Y, Gao XH, et al. The growth hormone receptor gene is associated with mandibular height in a Chinese population. J Dent Res. 2005;84:1052–1056. doi: 10.1177/154405910508401116. [DOI] [PubMed] [Google Scholar]
- 9.Xue F, Wong R, Rabie AB. Identification of SNP markers on 1p36 and association analysis of EPB41 with mandibular prognathism in a Chinese population. Arch Oral Biol. 2010;55:867–872. doi: 10.1016/j.archoralbio.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Tassopoulou-Fishell M, Deeley K, Harvey EM, Sciote J, Vieira AR. Genetic variation in myosin 1H contributes to mandibular prognathism. Am J Orthod Dentofacial Orthop. 2012;141:51–59. doi: 10.1016/j.ajodo.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilcox MA, Wyszynski DF, Panhuysen CI, et al. Empirically derived phenotypic subgroups - qualitative and quantitative trait analyses. BMC Genet. 2003;4(Suppl 1):S15. doi: 10.1186/1471-2156-4-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanborn RT. Differences between the facial skeletal patterns of Class III maloccusion and normal occlusion. Angle Orthod. 1955;25 [Google Scholar]
- 13.Dietrich UC. Morphological variability of skeletal Class 3 relationships as revealed by cephalometric analysis. Rep Congr Eur Orthod Soc. 1970:131–143. [PubMed] [Google Scholar]
- 14.Jacobson A, Evans WG, Preston CB, Sadowsky PL. Mandibular prognathism. Am J Orthod. 1974;66:140–171. doi: 10.1016/0002-9416(74)90233-4. [DOI] [PubMed] [Google Scholar]
- 15.Ellis E, 3rd, A MJ., Jr Components of adult Class III malocclusion. J Oral Maxillofac Surg. 1984;42:295–305. doi: 10.1016/0278-2391(84)90109-5. [DOI] [PubMed] [Google Scholar]
- 16.Miyajima K, A MJ, Jr, Sana M, Murata S. An estimation of craniofacial growth in the untreated Class III female with anterior crossbite. Am J Orthod Dentofacial Orthop. 1997;112:425–34. doi: 10.1016/s0889-5406(97)70051-9. [DOI] [PubMed] [Google Scholar]
- 17.Singh GD, A MJ, Jr, Lozanoff S. Thin-plate spline analysis of the cranial base in subjects with Class III malocclusion. Eur J Orthod. 1997;19:341–53. doi: 10.1093/ejo/19.4.341. [DOI] [PubMed] [Google Scholar]
- 18.Singh GD, A MJ, Jr, Lozanoff S. Spline analysis of the mandible in human subjects with class III malocclusion. Arch Oral Biol. 1997;42:345–53. doi: 10.1016/s0003-9969(97)00032-0. [DOI] [PubMed] [Google Scholar]
- 19.Singh GD, A MJ, Jr, Lozanoff S. Localisation of deformations of the midfacial complex in subjects with class III malocclusions employing thin-plate spline analysis. J Anat. 1997;191(Pt 4):595–602. doi: 10.1046/j.1469-7580.1997.19140595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh GD, McNamara JA, Jr, Lozanoff S. Procrustes, Euclidean and cephalometric analyses of the morphology of the mandible in human Class III malocclusions. Arch Oral Biol. 1998;43:535–543. doi: 10.1016/s0003-9969(98)00036-3. [DOI] [PubMed] [Google Scholar]
- 21.Chang HP, Hsieh SH, Tseng YC, Chou TM. Cranial-base morphology in children with class III malocclusion. Kaohsiung J Med Sci. 2005;21:159–165. doi: 10.1016/S1607-551X(09)70295-5. [DOI] [PubMed] [Google Scholar]
- 22.Proff P, Will F, Bokan I, Fanghanel J, Gedrange T. Cranial base features in skeletal Class III patients. Angle Orthod. 2008;78:433–9. doi: 10.2319/013007-48.1. [DOI] [PubMed] [Google Scholar]
- 23.Mouakeh M. Cephalometric evaluation of craniofacial pattern of Syrian children with Class III malocclusion. Am J Orthod Dentofacial Orthop. 2001;119:640–649. doi: 10.1067/mod.2001.112671. [DOI] [PubMed] [Google Scholar]
- 24.Hong SX, Yi CK. A classification and characterization of skeletal class III malocclusion on etio-pathogenic basis. Int J Oral Maxillofac Surg. 2001;30:264–271. doi: 10.1054/ijom.2001.0088. [DOI] [PubMed] [Google Scholar]
- 25.Mackay F, Jones JA, Thompson R, Simpson W. Craniofacial form in class III cases. Br J Orthod. 1992;19:15–20. doi: 10.1179/bjo.19.1.15. [DOI] [PubMed] [Google Scholar]
- 26.Abu Alhaija ES, Richardson A. Growth prediction in Class III patients using cluster and discriminant function analysis. Eur J Orthod. 2003;25:599–608. doi: 10.1093/ejo/25.6.599. [DOI] [PubMed] [Google Scholar]
- 27.Bui C, King T, Proffit W, Frazier-Bowers S. Phenotypic characterization of Class III patients. Angle Orthod. 2006;76:564–569. doi: 10.1043/0003-3219(2006)076[0564:PCOCIP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Lindeman RH, Merenda PF, Gold RZ. Introduction to Bivariate and Multivariate Analysis. 1. Glenview, Ill: Scott, Foresman; 1980. [Google Scholar]
- 29.Liebgott B. Factors of human skeletal craniofacial morphology. Angle Orthod. 1977;47:222–30. doi: 10.1043/0003-3219(1977)047<0222:FOHSCM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Landauer CA. A factor analysis of the facial skeleton. Hum Biol. 1962;34:239–53. [PubMed] [Google Scholar]
- 31.Bishara SE. Longitudinal cephalometric standards from 5 years of age to adulthood. Am J Orthod. 1981;79:35–44. doi: 10.1016/0002-9416(81)90099-3. [DOI] [PubMed] [Google Scholar]
- 32.McNamara JA., Jr A method of cephalometric evaluation. Am J Orthod. 1984;86:449–469. doi: 10.1016/s0002-9416(84)90352-x. [DOI] [PubMed] [Google Scholar]
- 33.Cohen JM. Comparing digital and conventional cephalometric radiographs. Am J Orthod Dentofacial Orthop. 2005;128:157–160. doi: 10.1016/j.ajodo.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Baumrind S, Frantz RC. The reliability of head film measurements. 1 Landmark identification Am J Orthod. 1971;60:111–27. doi: 10.1016/0002-9416(71)90028-5. [DOI] [PubMed] [Google Scholar]
- 35.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 36.Hartigan JA. Clustering Algorithms. New York: Wiley; 1975. [Google Scholar]
- 37.Macqueen JB. Some Methods for classification and analysis of multivariate observations. Vol. 1. University of California Press; 1967. pp. 281–297. [Google Scholar]
- 38.Anderberg M. Cluster Analysis for Applications. New York, NY: Academic Press, Inc.; 1973. [Google Scholar]
- 39.Caliński T, Harabasz J. A dendrite method for cluster analysis. Communications in Statistics. 1974;3:1–27. [Google Scholar]
- 40.Sarle WS. The Cubic Clustering Criterion. Cary, NC, USA: SAS Institute; 1983. p. A-108. [Google Scholar]
- 41.Cooper MC, Milligan GW. In: Data, expert knowledge and decisions. Gaul W, Schader M, editors. London, UK, UK: Springer-Verlag; 1988. pp. 319–328. [Google Scholar]
- 42.Lu YC, Tanne K, Hirano Y, Sakuda M. Craniofacial morphology of adolescent mandibular prognathism. Angle Orthod. 1993;63:277–282. doi: 10.1043/0003-3219(1993)063<0277:CMOAMP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]


