Abstract
Purpose
To evaluate a chemical shift-based fat quantification technique in the rotator cuff muscles in comparison with the semi-quantitative Goutallier fat infiltration classification (GC) and to assess their relationship with clinical parameters.
Materials and Methods
The shoulders of 57 patients were imaged using a 3T MR scanner. The rotator cuff muscles were assessed for fat infiltration using GC by two radiologists and an orthopedic surgeon. Sequences included oblique-sagittal T1-, T2- and proton density-weighted fast spin echo, and six-echo gradient echo. The iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) was used to measure fat fraction. Pain and range of motion of the shoulder were recorded.
Results
Fat fraction values were significantly correlated with GC grades (p< 0.0001, kappa>0.9) showing consistent increase with GC grades (grade=0, 0%–5.59%; grade=1, 1.1%–9.70%; grade=2, 6.44%–14.86%; grade=3, 15.25%–17.77%; grade=4, 19.85%–29.63%). A significant correlation between fat infiltration of the subscapularis muscle quantified with IDEAL versus a) deficit in internal rotation (Spearman Rank Correlation Coefficient=0.39, 95% CI 0.13–0.60, p<0.01) and b) pain (Spearman Rank Correlation coefficient=0.313, 95% CI 0.049–0.536, p=0.02) was found but was not seen between the clinical parameters and GC grades. Additionally, only quantitative fat infiltration measures of the supraspinatus muscle were significantly correlated with a deficit in abduction (Spearman Rank Correlation Coefficient=0.45, 95% CI 0.20–0.60, p<0.01).
Conclusion
We concluded that an accurate and highly reproducible fat quantification in the rotator cuff muscles using water-fat MRI techniques is possible and significantly correlates with shoulder pain and range of motion.
Keywords: Shoulder, Fat Quantification, Rotator Cuff, Water-fat Imaging, Magnetic Resonance Imaging (MRI)
INTRODUCTION
The relationship between fatty infiltration of the rotator cuff (RC) muscles and a rotator cuff tear has been previously documented (1–4). The natural history of fatty infiltration has been described as an irreversible process that is often progressive. It has been recommended that surgical repair of the rotator cuff tear should be performed prior to advanced fatty infiltration. The amount of fatty infiltration of the rotator cuff muscle is one of the main factors in the clinical decision making in the management of patients with RC tear (5,6). High grades of fatty infiltration are associated with higher rates of repair failure and more limitations in the achievable outcome in the post-operative period (6). Thus, an accurate determination of fatty infiltration in the rotator cuff muscles is of high clinical significance.
Both computed tomography (CT) and magnetic resonance imaging (MRI) have shown capability to detect fatty infiltration within the RC muscles. For the assessment of fatty infiltration, CT and MRI-based classifications have been established by Goutallier et al. (7) and by Fuchs et al. (8,9) respectively. These classifications have become widely used in the orthopaedic literature and have been accepted as a standard five-grade scoring system for muscle fatty infiltration of the RC. The limited soft tissue contrast and high exposure of ionizing radiation are substantial drawbacks of CT, which have led to adoption of MRI as the preferred modality for the assessment of fatty infiltration. Although the Goutallier grading has been widely used, multiple previous studies have shown that this system is highly observer-dependent (10,11). Given the significant implications of the grade of fatty infiltration on treatment recommendations, there is a need for a reliable and reproducible method for quantitative fat content measurements.
Imaging-based fat quantification has been performed in different skeletal muscles using multiple different techniques, including fat selective imaging (12) and T2-based water-fat separation (13). Previous fat quantification approaches applied in the rotator cuff muscles included single-voxel MR spectroscopy (14), spectroscopic gradient echo imaging (15) and two-point Dixon imaging (16). The development of new water-fat imaging techniques enabling the generation of accurate quantitative fat-fraction maps (17), makes MRI even more appealing for the assessment of RC in comparison to semi-quantitative Goutallier scores, which are based on the subjective evaluation of the readers (18). In this study, a chemical shift-based water-fat separation technique, accounting for different confounding factors, based on the iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL (19,20)) was used to quantify fatty infiltration in the RC muscles. The purpose of this study was threefold: (i) to show the feasibility of applying water-fat imaging for fat quantification of the rotator cuff muscles and assess its inter-observer reproducibility, (ii) to compare the previously used semi-quantitative fat infiltration Goutallier’s classification (GC) of RC muscles with a MRI-based fat quantification technique (IDEAL), and (iii) to compare GC grades and IDEAL-based fat fraction values in predicting functional measurements of shoulder range of motion and pain.
MATERIALS AND METHODS
Patients and clinical evaluation
The shoulders of 57 patients (31 men, 26 women; mean 52.44 years; range 17–76 years) with symptoms of rotator cuff tears were imaged using MRI. Previous shoulder surgery and MRI contraindication were criteria for exclusion. The study was approved by the Institutional Review Board and conducted in accordance with the Committee for Human Research. All subjects gave written informed consent prior to participation in the study.
Clinical exam notes from all patients were reviewed. The clinic visit closest and prior to the imaging study date was chosen for review. All range of motion and strength testing was performed by one of three senior orthopedic surgeons with fellowship training in Sports Medicine and Shoulder Surgery. Four patients were not tested due to a recent dislocation. Each patient was asked to report a pain level with a numeric value from 0 to 10, with 10 being the worst pain according to the Visual Analog Scale (VAS) (21). Range of motion was measured for forward flexion, abduction, external rotation and internal rotation. External rotation was performed with the elbows flexed to 90 degrees and held at the side of the patient. Internal rotation was recorded as the most proximal vertebral level that the patient could reach. Strength was measured and recorded on a standard 0–5 scale (22,23) with full strength to resistance rated as 5, strength to some resistance as 4, anti-gravity strength as 3, full range of motion in a plane without gravity as 2, muscle contraction without range of motion as 1, and no strength as 0. The supraspinatus was tested as forward flexion in the scapular plane. Infraspinatus was measured as external rotation with the elbows flexed to 90 degrees and held at the side of the patient. Subscapularis was tested with the lift-off test.
MR imaging
Shoulder MRI examinations were performed on a 3.0-T scanner (MR750; GE Healthcare, Milwaukee, Wis) using an 8-channel shoulder surface coil (Clinical MR Solutions, Brookfield, Wis). In addition to the standard clinical MRI protocol, which included T2-weighted fat-suppressed fast spin-echo (FSE) sequences, T1-weighted FSE sequences and proton density (PD) FSE sequences, a 3D fast gradient echo pulse sequence with 6 echoes was used for chemical shift-based water fat separation. From the clinical protocol only the sagittal PD weighted sequence was used to semi-quantitatively score fat infiltration using GC. IDEAL was used to quantitatively measure the amount of fat in the muscle bulk. The clinical sagittal T2-weighted sequence had a marginal role and was only used to double-check the boundaries of the muscles for the segmentation. The total acquisition time for the clinical protocol was about 25 minutes, excluding the 3 minutes used to run the IDEAL sequence. The IDEAL algorithm (19,20,24) was employed with T2* correction and a multi-peak model for the fat spectrum (25). A hybrid (complex/magnitude)-based approach was used to correct for eddy current effects (26). A small flip angle was used to reduce the T1-bias effect (17,27). A six-echo acquisition was employed as reconstructions of six-echo data have been previously frequently used in single T2*-corrected water-fat separation and have been shown to be not affected by the susceptibility induced fat resonance shift effect in skeletal muscle (28). The IDEAL reconstruction was performed online and produced water-only, fat-only, in-phase (water and fat), and out-of-phase (water minus fat) images and fat fraction maps. All sequences were acquired with a sagittal-oblique orientation, slice thickness of 4 mm, and a field of view (FOV) of 12 cm for a final in-plane spatial resolution of 0.468 × 0.468 mm (Table 1). The fat fraction map defined as the ratio of the fat proton density weighted signal to the sum of the water and fat proton density weighted signals reflected the percentage amount of fat in each voxel.
Table 1.
Parameters of oblique sagittal proton density 2D FSE and oblique sagittal 3D SPGR MRI sequences
| Imaging Sequence | Relevant Acquisition Parameters | Resolution (mm3) | Purpose |
|---|---|---|---|
| Oblique sagittal proton density 2D FSE | TR/TE = 2200/25 ms, rBW = 62.5 kHz, ETL = 10, NEX = 4 FOV = 12 cm AT= 3 min |
0.23×0.23×4 | Goutallier classification |
| Oblique sagittal 3D SPGR | 6 points, ETL = 2, TE/ΔTE = 2.1/1.0 ms TR = 10.8 ms, flip angle = 3° rBW = 62.5 kHz, NEX = 1 FOV=12 AT= 3 min |
0.46×0.46×4 | Fat quantification |
TR= relaxation time; TE= echo time; FOV= Field of View; ETL= echo train length; rBW= receiver bandwidth; NEX= number of excitations; AT= acquisition time
Image analysis
Semi-quantitative grading of the fatty infiltration in the RC muscles
Following the protocol described by Fuchs et al. (9,29) for MRI, we identified the most lateral sagittal-oblique slice where the acromion, coracoid and scapular body were all visible, together with the next two consecutive lateral slices and with the next consecutive medial slice. From these slices, the presence of fatty infiltration was graded according to a 5-point semi-quantitative scale described by Goutallier et al. (18,30) and shown in Figure 1: (grade 0) normal, (grade 1) some fatty streaks, (grade 2) less than 50% fatty muscle, (grade 3) as much fat as muscle and (grade 4) more fat than muscle. After a calibration session during which two radiologists (L.N., T.M.L.) and an orthopedic surgeon (D.L.) reviewed 20 studies, all MRI studies were interpreted by a board-certified radiologist and an orthopedic surgeon, independently, using the modified GC as proposed by Fuchs et al. (9). The two readers reviewed these twenty studies on two separate occasions separated by three weeks. A second radiologist (T.M.L., 25 years of experience) was consulted in case of disagreement; all images were reviewed on a picture archiving communication system workstation (Agfa, Ridgefield Park, NJ). Inter-reader agreement was assessed between two readers. Intra-reader agreement was assessed for each individual reader.
Figure 1.
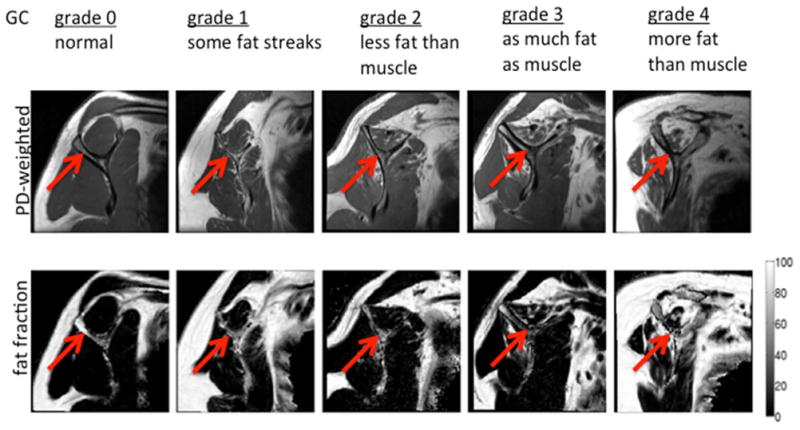
Representative proton density-weighted images and fat fraction maps for different degrees of fat infiltration of the supraspinatus muscle using the GC scale. The images were obtained in the sagittal-oblique plane. Gray scale bar corresponds to fat fraction values (in %).
Quantitative grading of the fatty infiltration in the RC muscles
The segmentation of the RC muscles was performed by one radiologist (L.N.) and one orthopedic surgeon (D.L.) using proprietary software written in MATLAB (The Mathworks, Inc., Natick, MA). Inter-operator reproducibility of mean fat fraction values between both operators was assessed. To standardize segmentation the sagittal-oblique intermediate-weighted FSE sections were identified, where the scapular body, coracoid process, and acromion were all depicted and formed the “ScapularY”. This section and the adjacent medial section as well as the next two consecutive lateral sections were used for muscle segmentation. The regions of interest (ROIs) for the 4 RC muscles (Supraspinatus, Infraspinatus, Subscapularis and Teres Minor) were defined so that the boundaries of the ROIs were within 1–2 mm from the muscle boundaries and the muscle fascia, as visually inspected on the T2-weighted images. This was done to assure that fat regions in the periphery of the muscle compartments were excluded from the ROIs. The only exception was the subscapularis muscle: its caudal part was not included in the segmentation in order to avoid the inclusion of low-signal regions. The caudal boundary of this muscle was identified as a horizontal line passing through the “L” point (Figure 2). The “L” point was determined as the most anterior point of the scapula surface, 10 mm distant from the tip of the scapular. Mean intramuscular fat fraction values were computed in all ROIs based on the IDEAL-fat fraction maps. Total time to process an IDEAL sequence from its acquisition to obtain quantitative score was about 15 minutes per study, including 6–8 minutes for the segmentation.
Figure 2.
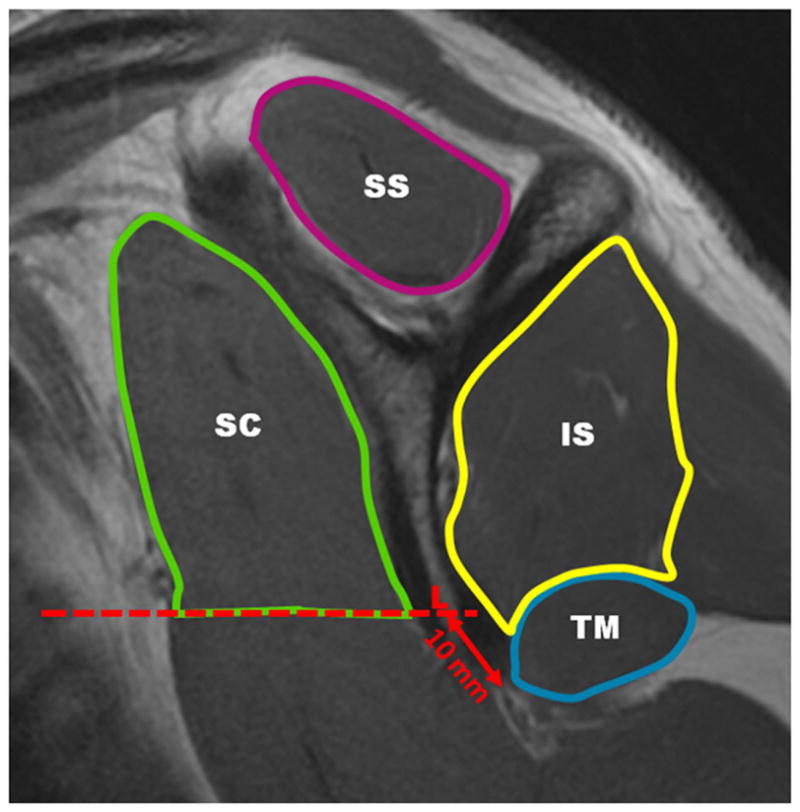
Muscle regions on an proton density-weighted image, sagittal-oblique plane.
IS= infraspinatus; SC= subscapularis (caudal boundary drawn using horizontal line passing through L point); SS= supraspinatus; TM= teres minor
Statistical analysis
Statistical analysis was performed using JMP software Version 7 (SAS Institute, Cary, NC) and SPSS Version 14 (IBM, Chicago, IL). Fisher’s exact test was used to compare GC grades and IDEAL fat fraction values. Spearman rank correlation was used to compare GC grades and IDEAL fat fraction values to clinical variables. Inter-reader agreement was assessed using Cohen’s kappa coefficient, while the absolute precision error was used to assess the reproducibility of mean fat fraction quantification based on the IDEAL fat fraction maps. The level of significance for all calculations was defined at p<0.05.
RESULTS
Figure 2 demonstrates a sagittal proton density-weighted FSE image of the shoulder with the segmented ROIs of the rotator cuff muscles. Segmentation of all four RC muscles took between six and eight minutes, while GC assessment took approximately two minutes for each MRI study and all four muscles.
Comparison of GC grades and IDEAL fat fraction values
Significant differences of the mean fat fraction values between the individual Goutallier grades were found (p<0.001). The mean fat fraction and standard deviation values calculated for the RC muscles, as well as the corresponding clinical grade based on GC, are presented in Figure 3. The Goutallier grade was greater than 2 for 4 patients in the supraspinatus, 8 patients in the infraspinatus, 5 patients in the subscapularis, and 3 patients in the teres minor. The mean fat fraction values consistently increased with Goutallier grade: grade 0 ranged from 0% to 5.59%, grade 1 from 1.1% to 9.70%, grade 2 from 6.44% to 14.86%, grade 3 from 15.25% to 17.77%, and grade 4 from 19.85% to 29.63%. A significant correlation between the semi-quantitative clinical grading and IDEAL fat fraction values was found in all muscles. The mean fat fraction for grade 2 was significantly different from the mean fat fraction for grade 3 in all muscle compartments of the shoulder (p<0.01). A statistically significant difference was also found when comparing Goutallier grade less than 3 with grade 3 and higher (p<0.01). Both IDEAL and GC could significantly distinguish two groups of patients with fatty infiltration classified as GC≤2 and GC >2 (Fisher’s exact test and simple kappa tests both > 90%).
Figure 3.
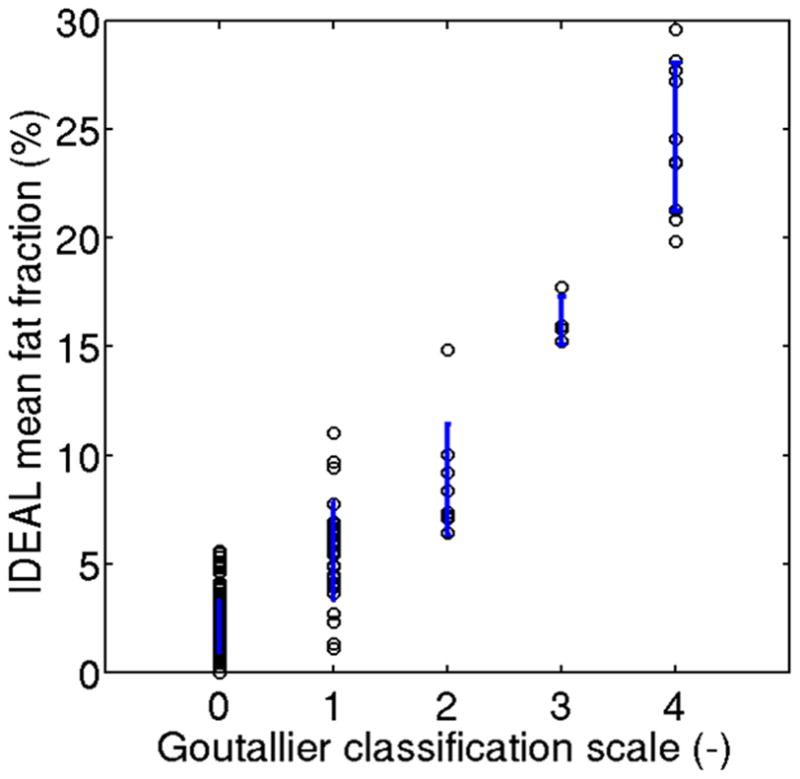
Comparison of computerized fat fraction metrics with Goutallier grade in all four muscle compartments. The vertical lines represent the standard deviation values for the computerized fat fraction metrics at every Goutallier grade.
The mean IDEAL fat fraction values were significantly lower than the corresponding mean fat fractions commonly associated with each Goutallier grade (Figure 3). For example, eighteen muscles were graded as Goutallier grade 3 or 4 (which would be associated with a fatty infiltration above 50%), and none of them had an IDEAL fat content greater than 50%.
Relationship of GC grades and IDEAL fat fraction values with clinical variables
Table 2 summarizes the results of the comparison of GC grades and IDEAL fat fraction values with clinical variables. The observed correlations suggest that correlations with pain and clinical measurements for IDEAL are higher than those for the Goutallier classification: Spearman rank Correlation coefficients and p values showed a trend towards a stronger relationship between clinical scores and IDEAL fat fraction values yielding higher Spearman Rank Correlation coefficients.
Table 2.
Spearman rank correlation coefficients of fat infiltration metrics (computerized IDEAL-based quantitative fat fraction and Goutallier grade) compared with clinical data.
| Muscles | Spearman Rank Correlation quantitative | Spearman Rank Correlation Goutallier | Spearman p-values quantitative | Spearman p-values Goutallier |
|---|---|---|---|---|
| IS Variables | ||||
|
| ||||
| Pain | 0.119 | 0.087 | 0.3910 | 0.5336 |
| ER | 0.368 | 0.370 | 0.0067* | 0.0064* |
| SC Variables | ||||
|
| ||||
| Pain | 0.313 | 0.193 | 0.0214* | 0.1625 |
| IR | 0.39 | 0.30 | 0.0051* | 0.0357* |
| SS Variables | ||||
|
| ||||
| Pain | 0.128 | 0.012 | 0.3557 | 0.9318 |
| FF | 0.206 | 0.032 | 0.1382 | 0.8215 |
| Ab | 0.448 | 0.223 | 0.0011* | 0.1201 |
IR= intra-rotation; ER= extra-rotation; Ab= Abduction; FF= forward flexion; IS= infraspinatus; SC= subscapularis; SS= supraspinatus
statistically significant p-value
The only statistically significant correlation of Visual Analog Scale pain assessment with fatty infiltration was observed in the subscapularis muscle, and only for the IDEAL fat fraction values (Spearman Rank correlation coefficient=0.313; p= 0.02).
Regarding the supraspinatus muscle, abduction strength was significantly correlated with high IDEAL fat fraction values (Spearman Rank Correlation coeffient=0.45; p< 0.01) but not with Goutallier scores (p=0.12). However, forward flexion strength was not statistically significantly correlated with either Goutallier score (p=0.82) or IDEAL fat fraction values (p=0.14). In the subscapularis muscle semi-quantitative internal rotation muscle strength grading was significantly correlated with both Goutallier score (Spearman Rank Correlation Coefficient=0.30, p=0.04) and IDEAL fat fraction values (Spearman Rank Coefficient: 0.39; p<0.01). In the infraspinatus muscle external rotation strength was statistically significantly correlated with both Goutallier score (SRC=0.37, p<0.01) and IDEAL fat fraction values (SR: 0.37; p<0.01).
B0 Fieldmaps and T2* Relaxation
Figure 4 demonstrates reconstructed IDEAL images of a subject with GC of 3 at the subscapularis. Shown are the water only images, the fat only images, the B0 fieldmaps and the R2* relaxation maps. The mean off-resonances in the muscle regions were −19.5 ± − 5.5 Hz. The R2* maps revealed a mean of 44 ±14 [1/s]. This corresponds to a T2* mean value of 22ms.
Figure 4.
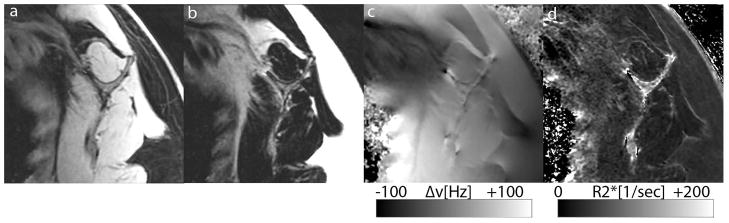
Examples of IDEAL reconstructed water only (Figure 4a) and fat only (Figure 4b) images are shown. Furthermore the B0 fieldmaps are presented demonstrating the off-resonance distribution in the regions of interest (Figure 4c). Finally, Figure 4d depicts the R2* relaxation map of the same subject.
Reproducibility Analyses
As shown in Table 3, comparing the readings of D.L. and L.N., a Cohen’s kappa value for inter-observer agreement on presence/absence of fatty infiltration of 0.84 was calculated. The Cohen’s kappa values for intra-observer agreement were 0.90 for D.L. and 0.92 for L.N. These values correspond with good reader agreement.
Table 3.
Cohen’s Kappa values for the clinical GC reading for both inter- and intra reproducibility are presented separately for each muscle and for the four muscle compartments together.
| SS | IS | SC | TM | All muscles | |
|---|---|---|---|---|---|
| Intra-reproducibility LN | 0.96 | 0.84 | 0.92 | 0.96 | 0.92 |
| Intra-reproducibility DL | 0.85 | 0.87 | 0.82 | 0.96 | 0.90 |
| Inter-reproducibility | 0.81 | 0.84 | 0.81 | 0.91 | 0.84 |
SS= Supraspinatus; IS= infraspinatus; SC= subscapularis; TM= teres minor
The absolute precision errors for inter-user reproducibility of intramuscular fat quantification based on ROIs generated by manual segmentation of the RC muscles ranged between fat fractions of 0.6% and 1.0% with 0.60%, 0.65%, 0.87% and 1.0% for supraspinatus, infraspinatus, subscapularis, and teres minor, respectively considering all muscles separately.
DISCUSSION
In this study, semi-quantitative fat infiltration based on GC was compared with an MR-based fat quantification approach using the IDEAL water-fat separation technique. To this end, muscle regions of interests were defined using anatomical landmarks. Intra- and inter-reader reproducibility of GC, inter-user reproducibility of IDEAL fat quantification, and correlation of both techniques with clinical parameters were investigated. A high correlation between GC and the IDEAL based fat quantification was found. Furthermore, good levels of intra- and inter-reader agreement for GC, good inter-user reproducibility for the IDEAL-based technique, and correlation of both techniques with clinical parameters were found. However, IDEAL fat quantification showed higher and more consistent correlation coefficients with clinical parameters compared to those found for GC grades.
The accuracy of IDEAL fat quantification has previously been validated by Hines et al. (31) in the liver of obese mice. The authors found high correlation between IDEAL imaging and lipid extraction, and quantitative histologic analysis. The authors concluded, that IDEAL can be used to accurately quantify fat in vivo and that correction for T2* decay and accurate spectral modeling are essential for accurate fat quantification in the liver. In another recent study, Hu et al. (32) investigated the accuracy of IDEAL-based fat mass measurement using chemical analysis as the reference standard and found a high correlation between MR fat mass quantification and chemical analysis in samples from different organs. The present study attempted to quantify the amount of fatty infiltration in the RC muscles of patients with a novel water-fat MRI technique. Different approaches such as CT, MRI, and proton MR spectroscopy have been used in the past for fat quantification within the muscle (14). Proton MR spectroscopy resulted in a reliable quantification of fat compared to biochemical measures (33) but does not allow spatially resolved measures. Chemical shift-based water-fat separation pulse sequences accounting for different confounding factors have shown to be accurate, reproducible, and fast (34). They also offer the unique advantage of spatially distributed information of the fat content. The fat content is determined as a ratio of signals and therefore independent of the receive coil profile effects, which are prominent while imaging the shoulder using surface coils (9). For all the above reasons, a chemical shift-based water-fat separation technique was selected amongst the other techniques for this study.
Fatty infiltration and atrophy in the RC muscles have been shown to be negative prognostic factors for successful outcomes following rotator cuff repairs (35–40). Radiographic grading systems as proposed by Goutallier et al (37) and Fuchs et al (35) assess fatty degeneration in a semi-quantitative and subjective manner with poor reproducibility as previously reported (41). Nevertheless, outcomes of RC repair are dependent on the Goutallier grade of the RC muscles. Using an optimized IDEAL computerized quantification approach, fatty infiltration could be determined in each RC muscle in an objective and reproducible way. The IDEAL-based fat content range that corresponded to the clinical Goutallier grade was determined. Surprisingly, the percentage of fat within the RC muscles was very different compared to the original GCs. Grades 3 and 4, according to GC, should correspond to more than 50% fat content in the muscle. However, the range of IDEAL fat content within the muscle was 15.25% to 17.77% for grade 3, and 19.85% to 29.63% for grade 4. One possible explanation for the overestimation of the fat content using GC is the heterogeneity of the tissues within the muscle (vessels, connective tissue, fat) which cannot be appreciated using a FSE intermediate-weighted sequence and may result in a signal intensity similar to fat. Furthermore small differences of the fat fraction within a single voxel are difficult to detect using an intermediate-weighted sequence and may cause misinterpretation of the findings. For instance, GC grading of Proton density weighted images cannot differentiate a voxel with intermediate fat fraction from a voxel with high fat fraction. Therefore, using GC, the reader would usually assign individual voxels either to fat or to muscle depending on its signal intensity, whereas IDEAL quantification is a continuous variable that provides an exact number for each voxel between 0 (no fat) and 100 (only fat). This approach allows a wider range of values to characterize and quantify more precisely the fat infiltration with high spatial resolution within the muscle. Further studies based on clinical outcome may redraw the limits for different degrees of fat infiltration.
Goutallier grade 2 is considered a transition point from normal to pathologic fatty infiltration. A GC higher than two is commonly considered a negative prognostic factor and a relative contraindication for surgical repair of a rotator cuff tear (5,42). In our work we used a regression model to analyze the difference in mean fat fraction between Goutallier grade 2 and grade 3. The mean fat fraction in grade 2 was significantly different from grade 3 in all muscle compartments of the shoulder and both IDEAL and GC could distinguish these two groups.
To the best of our knowledge our study is the first that used quantitative chemical shift based water-fat imaging to combine quantitative MR imaging strategies and clinical data. It is also the first study that applies advanced IDEAL fat quantification MRI to the shoulder. Compared to GC, IDEAL showed a trend towards a stronger relationship with clinical data. Previous studies have not found any correlation between RC tear and symptoms and have demonstrated that the physical examination had low sensitivity and specificity for detecting tears (43). However, none of these publications correlated the fatty infiltration with the symptoms. The limited range of motion or strength may depend on the substitution of the fat within the muscle, which, in turn may result in less elasticity of the muscle limiting the motion of the articulation itself. It is important to develop an objective measure of fatty infiltration to aid clinical decision-making. While it has been shown that a high grade of fatty infiltration is a negative prognostic factor for surgical repair, the current GC grading system is a qualitative assessment with large variability and poor reliability: Slabaugh et al. (41) reported an inter-reproducibility for Goutallier score below 60% and Oh et al. (44) reported a very wide range of reproducibility with a Cohen’s kappa value ranging from 26% to 81%. The quantitative scoring system we are proposing has values of absolute precision errors for inter-user reproducibility ranging between fat fractions of 0.6% and 1.0%. Given the strong importance of the fat infiltration on the decision making process for or against intervention and the prognosis of rotator cuff tears, a test with high reproducibility should be considered. Our methods described here provide an objective measure of fatty infiltration, which allows following muscle deterioration longitudinally and also possible recovery following successful surgical repair. We are currently following patients longitudinally after non-operative and operative repair of rotator cuff injuries. A quantitative approach will allow us to better stratify injuries and predict success of treatment.
The additional scan time needed for performing the 3D 6-echo gradient-echo sequence with the present resolution and without parallel imaging is of the order of 3 minutes. The resolution of the quantitative sequence could be potentially further reduced or parallel imaging could be used to reach scan times between 1 and 2 minutes, to increase time efficiency for wide spread use in clinical setting. This study is not without limitations. First, the number of subjects with high-grade fatty infiltration (Goutallier classification >2) was small. This bias towards patients with less fatty infiltration is likely responsible for the wider distribution of fatty infiltration values with higher GC scores. A second limitation was the lack of a histological proof regarding fatty infiltration in the muscle of the shoulder. Previous animal studies have demonstrated a strong correlation. Hence, there is little to suggest that the same would not be true with regards to the shoulder musculature. Moreover, examining at a single time-point may limit the conclusions we can draw from the clinical data. Furthermore, the calibration session before the independent reading of the study for fat infiltration may have inflated the inter-reproducibility of Goutallier Classification and might have rendered the difference between the subjective Goutallier Classification and the objective IDEAL fat quantification less appreciable.
In conclusion our work demonstrated that IDEAL-based fat quantification can be used as a reproducible and objective assessment tool for the fatty infiltration within the muscles of the RC, providing a continuous variable for characterizing fat content. This improved method of evaluating fatty infiltration will allow for further studies on the effects of treatment on altering the progression of this pathologic change. The IDEAL-based fat quantification methodology as outlined here has the potential to become an important clinical resource in evaluating the muscles of the rotator cuff and objectively monitoring fatty infiltration changes over time. Furthermore, IDEAL-based fat quantification correlated with strength and range of motion of the shoulder muscles and may be superior to standard GC measurements. Further data collection to compare the performance of IDEAL-based fat quantitation to GC grading as a predictor of surgical outcome is needed to assess the full potential value of the technique.
Acknowledgments
Grant Support
NIH Grant numbers: R01AR057336, 1P30AR058899, U01 AR059507, and P50 AR060752
The authors would like to acknowledge Ann Shimakawa (Global Science Laboratory, GE Healthcare) for help with the pulse sequence and Warapat Virayavanich for the statistical support (Department of Radiology, Ramathibodi Hospital, Mahidol University).
References
- 1.Goutallier D, Godefroy D, Postel JM, Radier C, Bernageau J. Comments on: muscle fatty infiltration in rotator cuff tears: descriptive analysis of 1,688 cases by B. Melis, C. Nemoz and G. Walch, published in 10.1016/j.otsr. 2009.05. 001. Orthop Traumatol Surg Res. 2010;96(8):918–919. doi: 10.1016/j.otsr.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 3.Rubino LJ, Stills HF, Jr, Sprott DC, Crosby LA. Fatty infiltration of the torn rotator cuff worsens over time in a rabbit model. Arthroscopy. 2007;23(7):717–722. doi: 10.1016/j.arthro.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12(6):550–554. doi: 10.1016/s1058-2746(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 5.Schaefer O, Winterer J, Lohrmann C, Laubenberger J, Reichelt A, Langer M. Magnetic resonance imaging for supraspinatus muscle atrophy after cuff repair. Clin Orthop Relat Res. 2002;403:93–99. doi: 10.1097/00003086-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 Suppl):1S–7S. doi: 10.1016/j.jse.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 8.Fuchs T, Immich H, Nedden R. Quantitative relations between sero-chemical findings, fatty degeneration of the liver, alcohol drinking and body weight in a diabetic and non-diabetic collective with fatty degeneration of the liver. Med Welt. 1971;10:392–399. [PubMed] [Google Scholar]
- 9.Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 10.Shahabpour M, Kichouh M, Laridon E, Gielen JL, De Mey J. The effectiveness of diagnostic imaging methods for the assessment of soft tissue and articular disorders of the shoulder and elbow. Eur J Radiol. 2008;65(2):194–200. doi: 10.1016/j.ejrad.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Alizai H, Nardo L, Karampinos DC, et al. Comparison of clinical semi-quantitative assessment of muscle fat infiltration with quantitative assessment using chemical shift-based water/fat separation in MR studies of the calf of post-menopausal women. Eur Radiol. 2012;22(7):1592–1600. doi: 10.1007/s00330-012-2404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schick F, Machann J, Brechtel K, et al. MRI of muscular fat. Magn Reson Med. 2002;47(4):720–727. doi: 10.1002/mrm.10107. [DOI] [PubMed] [Google Scholar]
- 13.Kan HE, Scheenen TW, Wohlgemuth M, et al. Quantitative MR imaging of individual muscle involvement in facioscapulohumeral muscular dystrophy. Neuromuscular disorders: NMD. 2009;19(5):357–362. doi: 10.1016/j.nmd.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Pfirrmann CW, Schmid MR, Zanetti M, Jost B, Gerber C, Hodler J. Assessment of fat content in supraspinatus muscle with proton MR spectroscopy in asymptomatic volunteers and patients with supraspinatus tendon lesions. Radiology. 2004;232(3):709–715. doi: 10.1148/radiol.2323030442. [DOI] [PubMed] [Google Scholar]
- 15.Kenn W, Bohm D, Gohlke F, Hummer C, Kostler H, Hahn D. 2D SPLASH: a new method to determine the fatty infiltration of the rotator cuff muscles. European radiology. 2004;14(12):2331–2336. doi: 10.1007/s00330-004-2410-5. [DOI] [PubMed] [Google Scholar]
- 16.Gokalp G, Yildirim N, Yazici Z, Ercan I. Using chemical-shift MR imaging to quantify fatty degeneration within supraspinatus muscle due to supraspinatus tendon injuries. Skeletal Radiol. 2010;39(12):1211–1217. doi: 10.1007/s00256-010-0927-z. [DOI] [PubMed] [Google Scholar]
- 17.Karampinos DC, Yu H, Shimakawa A, Link TM, Majumdar S. T(1)-corrected fat quantification using chemical shift-based water/fat separation: application to skeletal muscle. Magn Reson Med. 2011;66(5):1312–1326. doi: 10.1002/mrm.22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippe J, Spang JT, Leger RR, Arciero RA, Mazzocca AD, Shea KP. Inter-rater agreement of the Goutallier, Patte, and Warner classification scores using preoperative magnetic resonance imaging in patients with rotator cuff tears. Arthroscopy. 2012;28(2):154–159. doi: 10.1016/j.arthro.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Reeder SB, Pineda AR, Wen Z, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005;54(3):636–644. doi: 10.1002/mrm.20624. [DOI] [PubMed] [Google Scholar]
- 20.Reeder SB, Wen Z, Yu H, et al. Multicoil Dixon chemical species separation with an iterative least-squares estimation method. Magn Reson Med. 2004;51(1):35–45. doi: 10.1002/mrm.10675. [DOI] [PubMed] [Google Scholar]
- 21.Rolfsson H. Pitfalls in pain measurement. Visual analog scale as pain assessment method questioned. Lakartidningen. 2009;106(9):591–593. [PubMed] [Google Scholar]
- 22.Zaslav KR. Internal rotation resistance strength test: a new diagnostic test to differentiate intra-articular pathology from outlet (Neer) impingement syndrome in the shoulder. J Shoulder Elbow Surg. 2001;10(1):23–27. doi: 10.1067/mse.2001.111960. [DOI] [PubMed] [Google Scholar]
- 23.Bostrom C. Shoulder rotational strength, movement, pain and joint tenderness as indicators of upper-extremity activity limitation in moderate rheumatoid arthritis. Scand J Rehabil Med. 2000;32(3):134–139. doi: 10.1080/003655000750045488. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Reeder SB, Shimakawa A, Brittain JH, Pelc NJ. Field map estimation with a region growing scheme for iterative 3-point water-fat decomposition. Magn Reson Med. 2005;54(4):1032–1039. doi: 10.1002/mrm.20654. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60(5):1122–1134. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu H, Shimakawa A, Hines CD, et al. Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magn Reson Med. 2011;66(1):199–206. doi: 10.1002/mrm.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58(2):354–364. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 28.Karampinos DC, Yu H, Shimakawa A, Link TM, Majumdar S. Chemical shift-based water/fat separation in the presence of susceptibility-induced fat resonance shift. Magn Reson Med. 2012;68(5):1495–1505. doi: 10.1002/mrm.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung S, Dillon E, Tham SC, et al. The presence of fatty infiltration in the infraspinatus: its relation with the condition of the supraspinatus tendon. Arthroscopy. 2011;27(4):463–470. doi: 10.1016/j.arthro.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Thompson SM, Reilly P, Emery RJ, Bull AM. A comparison of the degree of retraction of full-thickness supraspinatus tears with the Goutallier grading system. J Shoulder Elbow Surg. 2012;21(6):749–753. doi: 10.1016/j.jse.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Hines CD, Yu H, Shimakawa A, et al. Quantification of hepatic steatosis with 3-T MR imaging: validation in ob/ob mice. Radiology. 2010;254(1):119–128. doi: 10.1148/radiol.09090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu HH, Nagy TR, Goran MI, Nayak KS. Quantification of absolute fat mass by magnetic resonance imaging: a validation study against chemical analysis. International Journal of Body Composition Research. 2011;9(3):111–122. [PMC free article] [PubMed] [Google Scholar]
- 33.Mengiardi B, Schmid MR, Boos N, et al. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: quantification with MR spectroscopy. Radiology. 2006;240(3):786–792. doi: 10.1148/radiol.2403050820. [DOI] [PubMed] [Google Scholar]
- 34.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36(5):1011–1014. doi: 10.1002/jmri.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 36.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. The American journal of sports medicine. 2007;35(5):719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 37.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clinical orthopaedics and related research. 1994;304:78–83. [PubMed] [Google Scholar]
- 38.Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clinical orthopaedics and related research. 2003;415:111–120. doi: 10.1097/01.blo.0000092974.12414.22. [DOI] [PubMed] [Google Scholar]
- 39.Rubino LJ, Stills HF, Jr, Sprott DC, Crosby LA. Fatty infiltration of the torn rotator cuff worsens over time in a rabbit model. Arthroscopy. 2007;23(7):717–722. doi: 10.1016/j.arthro.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 40.Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 Suppl):1S–7S. doi: 10.1016/j.jse.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Slabaugh MA, Friel NA, Karas V, Romeo AA, Verma NN, Cole BJ. Interobserver and intraobserver reliability of the Goutallier classification using magnetic resonance imaging: proposal of a simplified classification system to increase reliability. Am J Sports Med. 2012;40(8):1728–1734. doi: 10.1177/0363546512452714. [DOI] [PubMed] [Google Scholar]
- 42.Jost B, Pfirrmann CW, Gerber C, Switzerland Z. Clinical outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am. 2000;82(3):304–314. doi: 10.2106/00004623-200003000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Kim HA, Kim SH, Seo YI. Ultrasonographic findings of painful shoulders and correlation between physical examination and ultrasonographic rotator cuff tear. Mod Rheumatol. 2007;17(3):213–219. doi: 10.1007/s10165-007-0577-8. [DOI] [PubMed] [Google Scholar]
- 44.Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clinical orthopaedics and related research. 2010;468(6):1558–1564. doi: 10.1007/s11999-009-0818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


