Abstract
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer and remains the deadliest form of cancer in the US and worldwide. New therapies are highly sought after to improve outcome. The effect of sodium-R-alpha lipoate on camptothecin- and paclitaxel-induced cytotoxicity was evaluated on A549 NSCLC and BEAS-2B ‘normal’ lung epithelial cells. Combination indices (CI) and dose reduction indices (DRI) were investigated by studying the cytotoxicity of sodium-R-alpha lipoate (0–16 mM), camptothecin (0–25 nM) and paclitaxel (0–0.06 nM) alone and in combination. 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium-bromide (MTT) was used to assess cytotoxicity. The combinational cytotoxic effects of sodium-R-alpha lipoate with camptothecin or paclitaxel were analyzed using a simulation of dose effects (CompuSyn®3.01). The effects of sodium-R-alpha lipoate on camptothecin- and paclitaxel-induced cytotoxicity varied based on concentrations and treatment times. It was found that sodium-R-alpha lipoate wasn’t cytotoxic towards BEAS-2B cells at any of the concentrations tested. For A549 cells, CIs [(additive (CI=1); synergistic (CI<1); antagonistic (CI>1)] were lower and DRIs were higher for the camptothecin/sodium-R-alpha-lipoate combination (CI=~0.17–1.5; DRI=~2.2–22.6) than the paclitaxel/sodium-R-alpha-lipoate combination (CI=~0.8–9.9; DRI=~0.10–5.8) suggesting that the camptothecin regimen was synergistic and that the addition of sodium-R-alpha lipoate was important for reducing the camptothecin dose and potential for adverse effects.
INTRODUCTION
Lung cancer is the most common cause of cancer-related deaths among both men and women in the United States and worldwide (1). Lung cancer is also among the three most common cancers in both men and women in the United States. NSCLC is by far the most prevalent type of lung cancer, accounting for nearly 85% of all lung cancer cases (2). With a 5-year survival rate of ~15% in the United States and ~8% in Europe and the developing world, NSCLC remains a highly lethal cancer despite current therapeutic options (2). Thus, there remains a critical need for more effective therapies. Early-stage NSCLC presents with vague and variable symptoms. For this reason, the majority of NSCLC cases are diagnosed at an advanced stage, during which surgical therapy can no longer offer a curative outcome and chemotherapy becomes a mainstay of therapy. Thus, improvement of current chemotherapeutic regimens is highly sought after. One such approach, combination therapy, has the goal of enhancing or replacing the desired cytotoxic effect of a chemotherapeutic agent with another agent, allowing for an overall reduction in the chemotherapeutic drug and/or delivery system load. Theoretically, the use of benign agents that are selective for malignant cells (i.e., benign towards normal tissues) may not only enhance or replace the effects of chemotherapy and lower drug/delivery system load, but may also impart the additional benefit of greatly reducing the potential for toxic side effects. In addition, agents with antioxidant and/or anti-inflammatory activities may exert a chemoprotective effect on normal tissues against the toxic insults of chemotherapy (3).
We hypothesized that one agent that fulfills these criteria is sodium-R-alpha lipoate, the sodium salt of the R-enantiomer of alpha lipoic acid (ALA). ALA is an organosulfur compound that serves as an essential metabolic cofactor for several enzyme complexes, including pyruvate dehydrogenase complex, 2-oxoglutarate dehydrogenase complex, branched chain oxoacid complex, and acetoin dehydrogenase complex (4, 5). ALA, whose R-enantiomer (R-alpha lipoic acid) is endogenously synthesized and obtained through the diet, has been shown to have several beneficial effects in addition to its essential metabolic function, including antioxidant, metal-chelating, anti-inflammatory, neuroprotective, wound-healing, anti-aging, and hypoglycemic activities (4, 5). For this reason, ALA has been used clinically in a number of conditions, including diabetic neuropathy (6), liver disease (7), and human immunodeficiency virus/acquired immunodeficiency syndrome (8). Recently, ALA has gained considerable attention due to in vitro (9–12) and in vivo (13, 14) anticancer effects. Although the exact mechanisms responsible for these effects have yet to be completely elucidated, it is most likely related to ALA’s metabolic effects (9, 13). Wenzel et al. showed that ALA induces apoptosis in the human colon cancer cell line HT-29 by increasing mitochondrial respiration and causing a resultant increase in superoxide production. In the same study, it was shown that ALA was selective for cancer cells, as it did not induce apoptosis in the ‘normal’ counterpart cell line tested (9). In addition, certain types of cancer present with a metabolic anomaly, hypothesized to promote cancer growth, which results in a switch from a primary reliance on oxidative phosphorylation for energy production to aerobic glycolysis, termed the ‘Warbug Effect’ (15). The observations that the enzyme pyuruvate dehydrogenase kinase (PDK) is involved in the expression of this metabolic switch (16) and that ALA has been shown to inhibit PDK (17), and thus potentially reverse the ‘Warburg Effect’, add further credence to the hypothesis that a metabolic effect is responsible for the cytotoxic effects of ALA. Interestingly, dichloroaceate, an analogue of acetic acid and an inhibitor of PDK (18), has repeatedly shown in vitro and in vivo anticancer effects both alone and when used in combination with chemotherapeutic agents such as carboplatin, cisplatin, and 5-Flurouracil (18–20). Other potential mechanisms responsible for the anticancer effects of ALA may involve antioxidant and anti-inflammatory activities (4, 5) or inhibition of nuclear factor-kappa B (NF-κB) (21) and activator protein-1 (AP-1) (22).
The common designation ‘alpha lipoic acid’ refers to a 50/50 racemic mixture of R- and S-alpha lipoic acid. The only reason for the presence of S-alpha lipoic acid in ALA preparations is an achiral manufacturing process. Although previously believed to be physiologically inactive, recent studies have suggested that S-alpha lipoic acid may actually compete with and inhibit the functions and effects of R-alpha lipoic acid, such as R-alpha lipoic acid’s inhibition of PDK, modulation of mitochondrial activity, and interaction with proteins, enzymes, and genes (23). For these reasons, we chose the more metabolically potent R-enantiomer (23–25) to investigate the effect of ALA on chemotherapy-induced cytotoxicity. In addition, shall sodium-R-alpha lipoate display promising effects in vitro, this form of ALA will be far more superior in terms of in vivo application due to greater water solubility and a much more favorable pharmacokinetic profile (23).
Camptothecin is a cytotoxic quinoline alkaloid isolated from the bark and stem of Camptotheca Acuminata. Camptothecin’s primary mechanism of action involves inhibition of topoisomerase I, which results in prevention of DNA unwinding and leads to cell death. Although camptothecin showed remarkable therapeutic potential in preliminary clinical trials, the drug subsequently failed due to toxicity, poor oral bioavailability, poor solubility in biological fluids, inappropriate pharmacokinetics, and lack of efficacy within a tolerable dose range (26). However, topotecan, a water-soluble derivative of camptothecin, has previously been investigated for the treatment of NSCLC (27). Paclitaxel is a plant alkaloid and taxane isolated from Taxus Brevifolia. Paclitaxel’s mechanism of action involves a hyperstabilization of microtubule polymers, preventing disassembly and causing cell death. Paclitaxel (trade name Taxol®), in combination with cisplatin, is indicated for the first-line treatment of NSCLC in patients who are not candidates for curative surgery and/or radiation therapy.
Camptothecin, paclitaxel, and analogues thereof have been used in the treatment of NSCLC, and our laboratory has extensive experience with these compounds both from a pharmacological and a drug delivery perspective (28–32). In particular, our laboratory has previously shown that lung targeting of a camptothecin prodrug (camptothecin-norvaline), via passive entrapment of rigid microparticles can reduce therapeutic doses of camptothecin 10-fold in a rat orthotopic lung cancer model (29). A synergy approach using sodium-R-alpha lipoate is proposed in order to further minimize the dose of camptothecin and reduce the dose of microparticles, thus minimizing the toxic potential of either the drug or the delivery system or both. In the current study, the cytotoxic effects of sodium-R-alpha lipoate, camptothecin, paclitaxel, camptothecin/sodium-R-alpha lipoate, and paclitaxel/sodium-R-alpha lipoate were evaluated on A549 human NSCLC adecnocarcinoma cells. An automated computer simulation of the combinatorial effects of sodium-R-alpha lipoate with camptothecin or paclitaxel was applied with CompuSyn® 3.01 software to characterize the interactions (synergy, additivity, or antagonism) and determine the potential for camptothecin or paclitaxel dose reduction.
MATERIALS & METHODS
Cell Culture
A549 human NSCLC adenocarcinoma cells (American Type Culture Collection, Rockville, MD, USA) were cultured and passaged in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100U/ml penicillin-100 μg/ml streptomycin. BEAS-2B human lung epithelial cells (ATCC) were cultured and passaged in serum-free bronchial epithelial growth medium (BEGM; Lonza Walkersville Inc., Walkersville, MD, USA) supplemented with 0.4% bovine pituitary extract (BPE), 0.1 % hydrocortisone, 0.1 % human EGF (hEGF), 0.1 % epinephrine, 0.1 % transferrin, 0.1 % insulin, 0.1 % retinoic acid, 0.1 % triiodothyronine, 0.1 % gentamycin amphoterecin-B (GA-1000), and 100U/ml penicillin-100 μg/ml streptomycin. Both cell lines were maintained in an incubator at 37°C with 5% CO2.
MTT Cytotoxicity Assay
Camptothecin (Sigma Aldrich, St. Louis, MO, USA), paclitaxel (LC Laboratories, Woburn, MA, USA), and S-lipoic acid (Geronova Research Inc., Carson City, NV, USA) stock solutions were made using 100 % dimethyl sulfoxide (DMSO) and sodium-R-alpha lipoate (Geronova Research Inc., Carson City, NV, USA) solutions were prepared in sterile distilled water. Camptothecin stock solutions were always made fresh to ensure stability for the duration of the studies. A549 cells were plated at a density of 1 × 103 cells per well in 96-well plates and cultured for 24 hours, followed by treatment with the indicated compound(s) or DMSO solvent controls for camptothecin, paclitaxel, and S-lipoic acid treatment (0.01 % to 0.1 % DMSO). The concentrations used were, sodium-R-alpha lipoate, 0–16 mM; camptothecin, 0–25 nM; paclitaxel, 0–0.06 nM; S-lipoic acid, 0–10 mM; camptothecin/sodium-R-alpha-lipoate, 0–12 nM camptothecin and 0–8 mM sodium-R-alpha-lipoate; paclitaxel/sodium-R-alpha-lipoate, 0–0.03 nM paclitaxel and 0–8 mM sodium-R-alpha-lipoate. Mixtures were made by simply mixing the working solutions used for the individual compound treatments. After 2, 4, and 6 days of incubation, 3-(4,5-methylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT; Sigma Aldrich, St. Louis, MO, USA) was added and incubated for 2 hours at 37°C. Formazan products were solubilized with 100 % DMSO, and the optical densities were measured in a plate reader (Tecan; Mannedorf, Switzerland) at 570 nm. The effects of sodium-R-alpha lipoate, camptothecin/sodium-R-alpha lipoate, and paclitaxel/sodium-R-alpha lipoate on BEAS-2B cell viability were investigated in the same manner.
CompuSyn® 3.01 Analysis
Dose-response curves, combination indices, and dose reduction indices were generated for all treatments and time points with CompuSyn® 3.01 software (Paramus, NJ, USA) according to the manufacturer’s instructions. CompuSyn® 3.01 software uses an algorithm based on mass-action law to simulate the interaction of two or more compounds on the investigated pharmacological effect. Briefly, concentrations and corresponding effects levels (the effect of a treatment expressed as a decimal between 0 and 1, 0 being no effect and 1 being 100% effect) for all data points were input to generate a complete report of analytical results. The combination index (CI) is a parameter that indicates whether the interaction of 2 or more drugs is synergistic, additive, or antagonistic (additive (CI=1); synergistic (CI<1); antagonistic (CI>1)). The CI is calculated using the following equation, ((D1/Dx1) + (D2/Dx2)) = CI, where, at a certain effect level, D1 is the dose of drug 1 when used in combination with drug 2, Dx1 is the dose of drug 1 when used alone, D2 is the dose of drug 2 when used in combination with drug 1, and Dx2 is the dose of drug 2 when used alone. The dose reduction index (DRI) is a parameter that indicates the degree to which a drug dose can be reduced when used in combination with another drug and maintain an equivalent effect level. The DRI is calculated using the following equation, Dn/Dxn = DRIn, where, at a certain effect level, Dxn is the dose of the drug required to exert the aforementioned effect level when used alone and Dn is the dose of the drug required to exert the same effect level when used in combination with another drug.
Statistical Analysis
All statistical analyses were performed with GraphPad Prism® 4.0c software. Raw data for all treatments and time points was normalized and fitted with non-linear regression using GraphPad Prism® 4.0c software (La Jolla, CA, USA) to generate dose-response curves and calculate the half-maximal effective concentrations (EC50s) for all treatments and time points. Individual points within dose-response curves were expressed as mean ± standard error of mean. Comparisons between dose-response curves within each plot were made using the F-test. The limit for statistical significance was set at P<0.05.
RESULTS
Individual effect of camptothecin, paclitaxel, sodium-R-alpha lipoate, or S-lipoic acid on A549 cells
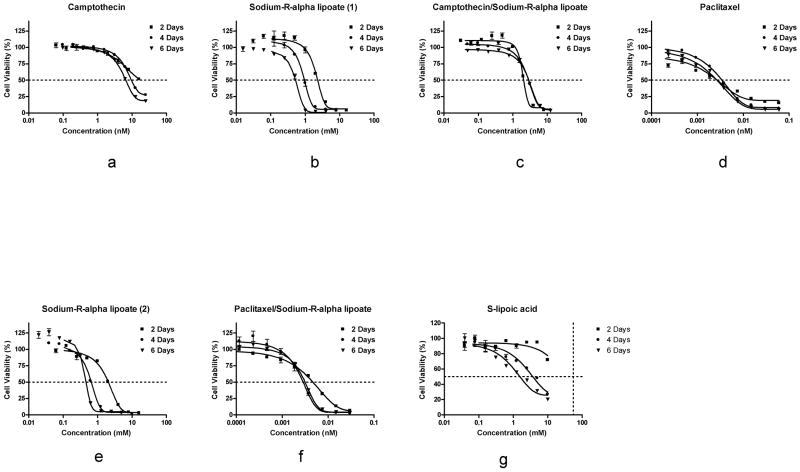
In order to determine the effects of the individual compounds on A549 cells, dose-response curves were constructed for camptothecin, paclitaxel, sodium-R-alpha lipoate, and S-lipoic acid after 2, 4, and 6 days of treatment (Figure 1). As hypothesized, sodium-R-alpha lipoate (Figure 1b and 1e) was found to be more potent than S-lipoic acid (Figure 1g). Paclitaxel was found to be the most cytotoxic compound (Figure 1d). The EC50s for camptothecin were 23.68, 14.76, 7.69 nM after 2, 4, and 6 days of treatment, respectively (Table 1). The EC50s for paclitaxel were 0.0031, 0.0034, and 0.0024 nM after 2, 4, and 6 days of treatment, respectively (Table 1). The EC50s for sodium-R-alpha lipoate when used individually in the camptothecin/sodium-R-alpha lipoate arm were 3.12, 2.21, and 0.40 mM after 2, 4, and 6 days of treatment, respectively (Table 1). The EC50s for sodium-R-alpha lipoate when used individually in the paclitaxel/sodium-R-alpha lipoate arm were 1.77, 0.70, and 0.52 mM after 2, 4, and 6 days of treatment, respectively (Table 1). The EC50s for S-lipoic acid were 3.56 and 1.54 mM after 4 and 6 days of treatment, respectively. An EC50 value for S-lipoic acid after 2 days of treatment could not calculated from the observed data.
FIG. 1.
Dose-response curves of the treatments. Effect of camptothecin, paclitaxel, sodium-R-alpha lipoate, S-lipoic acid, camptothecin/sodium-R-alpha lipoate, and paclitaxel/sodium-R-alpha lipoate on A549 cell viability after 2, 4, and 6 days of treatment. Cell viability was assessed using the MTT assay. Individual points within dose-response curves were expressed as mean ± standard error of mean. Differences between dose-response curves within each plot were found to be statistically significant (P<0.05).
TABLE 1.
EC50s camptothecin, paclitaxel, sodium-R-alpha lipoate, S-lipoic acid, camptothecin/sodium-R-alpha lipoate, and paclitaxel/sodium-R-alpha lipoate after treatment of A549 NSCLC cells for 2, 4, and 6 days of treatment.
| 2 Days | 4 Days | 6 Days | |
|---|---|---|---|
| Camptothecin (nM) | 23.68 | 14.76 | 7.69 |
| Sodium-R-alpha lipoate (1) (mM) | 3.12 | 2.21 | 0.40 |
| Paclitaxel (nM) | 0.0031 | 0.0034 | 0.0024 |
| Sodium-R-alpha lipoate (2) (mM) | 1.77 | 0.70 | 0.52 |
| S-lipoic acid (mM) | —— | 3.56 | 1.54 |
Sodium-R-alpha lipoate (1) and sodium-R-alpha lipoate (2) represent treatments where sodium-R-alpha lipoate was used individually in the camptothecin and paclitaxel arms of the MTT assay, respectively.
Effect of camptothecin/sodium-R-lipoate and paclitaxel/sodium-R-alpha lipoate combinations on A549 cells
In order to determine the effects of the camptothecin/sodium-R-alpha lipoate and paclitaxel/sodium-R-alpha lipoate combinations on A549 cells, dose-response curves were constructed after treatment for 2, 4, and 6 days (Figure 1). Figure 2 shows the CIs of the camptothecin/sodium-R-alpha lipoate and paclitaxel/sodium-R-alpha lipoate combinations at all effect levels after treatment for 2, 4, and 6 days. It was observed that the camptothecin/sodium-R-alpha lipoate combination had markedly lower CIs than the paclitaxel/sodium-R-alpha lipoate combination. The CIs for the camptothecin/sodium-R-alpha lipoate combination ranged from ~ 0.71 to 1.5, whereas the CIs for the paclitaxel/sodium-R-alpha lipoate ranged from ~0.8–9.9. The camptothecin/sodium-R-alpha lipoate combination showed several CIs in the synergistic or additive range (CI≤1), especially after 2 and 4 days of treatment. On the other hand, all combinations of paclitaxel and sodium-R-alpha lipoate were antagonistic (CI>1). Figure 3 shows the DRIs of the camptothecin/sodium-R-alpha lipoate and paclitaxel/sodium-R-alpha lipoate combinations as a function of sodium-R-alpha lipoate concentrations after treatment for 2, 4, and 6 days. It was found that the camptothecin/sodium-R-alpha lipoate combination had markedly higher DRIs than the paclitaxel/sodium-R-alpha lipoate combination. The DRIs for the camptothecin/sodium-R-alpha lipoate combination ranged from ~2.2–22.6, whereas the DRIs for the paclitaxel/sodium-R-alpha lipoate ranged from ~0.10–5.8. The camptothecin/sodium-R-alpha lipoate combination had several DRIs greater than 10, especially after 2 days of treatment. On the other hand, almost all DRIs for the paclitaxel/sodium-R-alpha lipoate combination were less than 5.
FIG. 2.
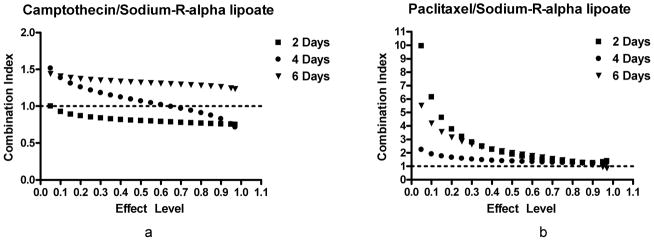
CIs of combination treatments. CIs as a function of effect level of the campothecin/sodium-R-alpha lipoate and paclitaxel/sodium-R-alpha lipoate combinations after treatment of A549 cells for 2, 4, and 6 days. CIs determine the type of interaction [Additive (CI=1); Synergistic (CI<1); Antagonistic (CI>1)].
FIG. 3.
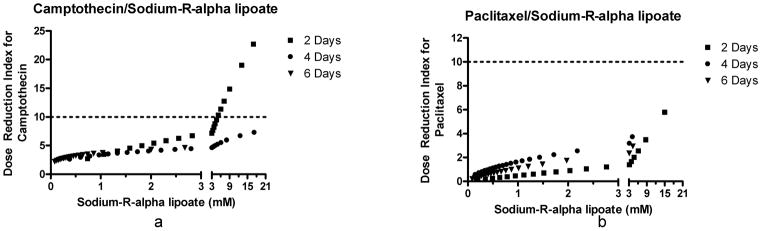
DRIs of the combination treatments. DRIs for camptothecin and paclitaxel when combined with sodium-R-alpha lipoate. The DRI is a parameter that indicates the degree to which a drug dose can be reduced when used in combination with another drug and maintain an equivalent effect level.
Effect of sodium-R-lipoate, camptothecin/sodium-R-lipoate, and paclitaxel/sodium-R-alpha lipoate on BEAS-2B lung epithelial cells
In order to determine the effects of sodium-R-alpha lipoate on ‘normal’ BEAS-2B lung epithelial cells, dose-response curves were constructed after 2, 4, and 6 days of treatment. Concentrations of sodium-R-alpha lipoate were the same as those used for A549 NSCLC adenocarcinoma cells. It was found that sodium-R-alpha lipoate had no effect BEAS-2B cell viability (Figure 4). However, longer treatment times showed that sodium-R-alpha lipoate may have exerted an anti-proliferative effect since, although the results of the MTT assay showed lower absorbance (data not shown), examination by microscopy indicated a lack of cytotoxicity (intact, adherent colonies, as opposed to single, floating cells). To further confirm the lack of toxicity of sodium-R-alpha lipoate towards BEAS-2B cells, a ‘rescue experiment’ was conducted in which BEAS-2B cells that had been incubated in sodium-R-alpha lipoate were subsequently incubated in control DMEM medium. It was found that upon subsequent incubation in control DMEM medium, BEAS-2B proliferated at rates observed in normal cell culture (data not shown). Representative data shown in Figure 4 highlight the differential effect of sodium-R-alpha lipoate on A549 NSCLC cells versus BEAS-2B ‘normal’ lung epithelial cells.
FIG. 4.
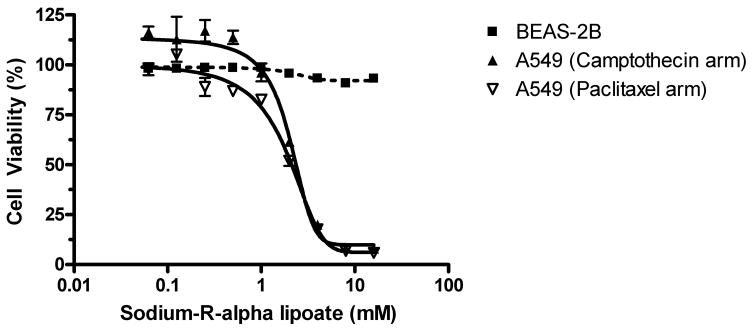
Effect of sodium-R-alpha lipoate on BEAS-2B cells. Representative data of the effect of sodium-R-alpha lipoate on A549 NSCLC and BEAS-2B ‘normal’ lung epithelium cell viability after treatment for 2 days.
Since the data suggested that sodium-R-alpha lipoate is selective for A549 NSCLC cells vs. BEAS-2B ‘normal’ lung epithelial cells, the camptothecin/sodium-R-alpha lipoate and paclitaxel/sodium-R-alpha lipoate combinations were also evaluated on BEAS-2B cells. Concentrations for the three compounds were as previously used. It was found that sodium-R-alpha lipoate moderately protected BEAS-2b cells from the cytotoxic effect of camptothecin (Figure 5a). For example, exposure to 4 nM campothecin alone resulted in ~68% cell death, whereas exposure to 4 nM camptothecin with sodium-R-lipoate resulted in only ~10% cell death. However, sodium-R-alpha failed to exert a chemoprotective effect on paclitaxel-induced cytotoxicity (Figure 5b).
FIG. 5.
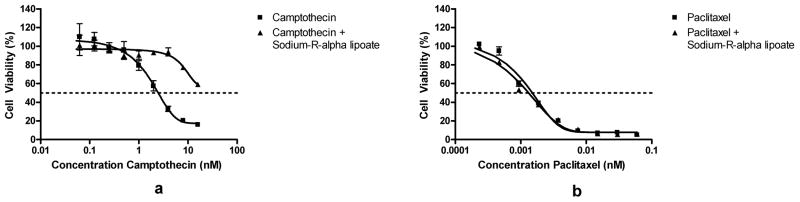
Effect of campothecin/sodium-R-alpha lipoate and paclitaxel/sodium-R-alpha lipoate on BEAS-2B cells. Cell viability of BEAS-2B ‘normal’ lung epithelial cells after treatment with campothecin/sodium-R-alpha lipoate or paclitaxel/sodium-R-alpha lipoate for 2 days.
DISCUSSION
Lung cancer is among the most common and lethal cancers in the United States and worldwide. As the most prevalent type of lung cancer, NSCLC kills more people than breast, colon, and prostate cancers combined and remains one of the deadliest forms of cancer in the world. Currently used treatments for the disease include surgery, chemotherapy, radiotherapy, biological therapy, laser therapy, photodynamic therapy, and combinations thereof. Innovative therapies targeting specific pathways associated with apoptosis, cell proliferation, and angiogenesis are also under investigation. Despite these treatment options, the general prognosis of NSCLC remains extremely poor, with a 5-year survival rate of only ~15%. With chemotherapy being one of the most commonly recommended treatments in late stage disease, during which most patients are diagnosed, the search continues for more effective and less toxic chemotherapeutic strategies. One potentially viable option is combination therapy. Combination therapy can have major advantages as a chemotherapeutic strategy, including higher potency, lower toxicity, and a potential for greatly reduced therapeutic doses.
Our previous experience with camptothecin and paclitaxel prompted us to investigate novel, minimally-toxic combination therapy for NSCLC involving the use of these agents. We hypothesized that ALA may be an ideal agent for combination therapy of NSCLC for several reasons, including, 1) it has shown in vitro and in vivo evidence of anticancer activity, 2) it has already been used clinically in a number of conditions and has shown a lack of toxicity at fairly high doses, 3) it has shown selective cytotoxic effects against cancer cells (i.e. no effect on ‘normal’ cell viability), 4) it has favorable pharmacokinetic parameters (e.g. high bioavailability after oral administration), and 5) it has potent antioxidant and anti-inflammatory activities which may exert a chemoprotective effect on normal tissues during toxic chemotherapy. In addition, the sodium salt of the R-(+) enantiomer of ALA was selected for the following reasons: 1) only the R-(+) enantiomer is endogenously synthesized and obtained through the diet, 2) the R-(+) enantiomer is more pharmacologically active and metabolically potent both in vitro and in vivo, (24, 25, 33) and 3) sodium-R-alpha lipoate is likely to be more advantageous than other forms of ALA in terms of in vivo application due to greater water solubility and a much more favorable pharmacokinetic profile (23). In this study, the effect of sodium-R-alpha lipoate on camptothecin- and paclitaxel-induced cytotoxicity was evaluated in A549 human NSCLC cells. Dose-response curves after treatment with the individual compounds for 2, 4, and 6 days revealed that, as hypothesized, sodium-R-alpha lipoate (Figure 1b, 1e) is more potent than S-lipoic acid (Figure 1g). The superiority of sodium-R-alpha lipoate cytotoxicity towards A549 NSCLC cells lends further credence to a metabolic cytotoxic mechanism of alpha lipoic acid as suggested by Wenzel et al. (9). In other words, the higher potency of sodium-R-alpha lipoate in increasing mitochondrial respiration (and increasing superoxide production) resulted in higher levels of cell death. It was also found that paclitaxel was the most cytotoxic compound (Figure 1d). CompuSyn® 3.01 analysis of the camptothecin/sodium-R-alpha lipoate and paclitaxel/sodium-R-alpha lipoate combinations revealed that interactive effects vary and depend on both the doses of the compounds and the treatment times. Comparison of the CIs at the various effect levels showed that camptothecin/sodium-R-alpha lipoate combination is more favorable due to markedly lower CIs and a greater number of CIs indicative of additivity and/or synergy (CI≤1), especially after 2 and 4 days of treatment. All CIs for the paclitaxel/sodium-R-alpha lipoate combination were indicative of antagonistic effects (CI>1). DRIs for the camptothecin/sodium-R-alpha lipoate combination (~2.2–22.6) were much greater than those for the paclitaxel/sodium-R-alpha lipoate combination (~0.10–5.8). Since the DRI is a parameter that indicates the degree to which a drug dose can be reduced when used in combination with another drug and maintain an equivalent effect level, these results show that the dose of camptothecin can be reduced up to 22-fold when used in combination with sodium-R-alpha lipoate. This favorable data for the camptothecin/sodium-R-alpha lipoate combination suggests that the combination deserves further evaluation. More specifically, since the lowest CIs and the highest DRIs for the camptothecin/sodium-R-alpha-lipoate combination occurred at 2 and 4 days and at higher effect levels (i.e., higher cytotoxicity), it will be interesting to investigate whether there exists a schedule-dependency (simultaneous treatment vs. pre- or post-treatment) for this combination and/or whether the most favorable combinatorial effects occur when the maximum level of cytotoxic induction is attempted. It should also be noted that, according to Chou, synergy at higher effect levels, as in the case of the camptothecin/sodium-R-alpha lipoate combination, is more relevant to anticancer therapy (34). This adds even further significance to the current results.
An important finding in this study was that sodium-R-alpha lipoate showed a selective cytotoxic effect for A549 NSCLC adenocarcinoma cells. ‘Normal’ BEAS-2B lung epithelial cells were essentially unaffected after treatment with sodium-R-alpha lipoate (Figure 4). However, longer treatment of BEAS-2B lung epithelial cells with sodium-R-alpha lipoate induced an anti-proliferative effect, although examination of cell morphology indicated a lack of cytotoxicity. Also, replacement of sodium-R-alpha lipoate treatment medium with control DMEM medium resulted in restoration of normal rates of BEAS-2B cell proliferation (data not shown). Thus, the selective nature of sodium-R-alpha lipoate for A549 NSCLC adenocarcinoma cells vs. ‘normal’ BEAS-2B lung epithelial cells was twice confirmed.
Compounds displaying antioxidant and/or anti-inflammatory activities may exert a chemoprotective effect on normal cells against the toxicity of chemotherapeutic regimens (3). To gain some insight into a potential chemoprotective effect of sodium-R-alpha lipoate, BEAS-2B cells were also treated with camptothecin/sodium-R-alpha lipoate and paclitaxel/sodium-R-alpha lipoate combinations. It was found that sodium-R-alpha lipoate exerted a significant chemoprotective effect against the cytotoxicity of campothtecin towards BEAS-2B cells (Figure 5a). On the other hand, sodium-R-alpha lipoate failed to protect against paclitaxel-induced cytotoxicity (Figure 5b). The reasons for the difference in interaction between sodium-R-alpha lipoate and camptothecin or paclitaxel are beyond the scope of this manuscript. The implications of this finding are that not only can the dose of camptothecin be reduced up to 22-fold (corresponding to the highest DRI) and maintain an equivalent cytotoxic effect level, but that this dose reduction is accomplished with a selective agent that is benign to normal tissues and that may exert a chemoprotective effect on normal tissues exposed to the already reduced chemotherapy doses. To our knowledge, there are very few agents with this capability. Although natural compounds such as curcumin (35) from turmeric and EGCG (36) from green tea have been shown to induce a selective cytotoxic effect on cancer versus normal cells, it is well known that these compounds have critical disadvantages in terms of in vivo application, including extremely poor bioavailability, high rate of metabolism, inactive metabolic products, and rapid elimination and clearance from the body (37). Sodium-R-alpha lipoate, on the other hand, has high bioavailability (even after oral administration), favorable pharmacokinetic parameters (23), and has active intracellular metabolic products (dihydrolipoate) (38) that may also possess anticancer activity. In addition, as mentioned previously, our lab has several successful strategies for improving the delivery and/or reducing the toxicity of campothecin, including tumor-targeted bioconjugate-based and poly (ethylene glycol)-based delivery, membrane transport facilitation, and pro-drug approaches (28–32, 39, 40). It is likely, and future studies would confirm, that simultaneous use of sodium-R-alpha lipoate with other pharmacological or drug delivery approaches such as ours may result in even more efficient and less toxic therapeutic strategies.
In conclusion, in the current studies, it was shown that camptothecin/sodium-R-alpha lipoate is a more favorable combination than paclitaxel/sodium-R-alpha lipoate in terms of in vitro cytotoxic effects towards A549 human NSCLC adenocarcinoma cells due to a higher likelihood of synergy/additivity and a higher potential for chemotherapy dose reduction. Minimally-toxic combination therapy with camptothecin/sodium-R-alpha lipoate deserves further evaluation as a chemotherapeutic strategy against NSCLC.
Acknowledgments
The authors would like to thank Drs. Hilliard L. Kutscher, Yashveer Singh, and Manjeet Deshmukh for their assistance and helpful insight during the preparation of this manuscript.
FUNDING
This work was supported by National Cancer Institute/National Institutes of Health grant 1R01CA15506.
ROLE OF THE FUNDING SOURCE
The funding source had no involvement in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Dempke WC, Suto T, Reck M. Targeted therapies for non-small cell lung cancer. Lung Cancer. 2010;67:257–274. doi: 10.1016/j.lungcan.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Links M, Lewis C. Chemoprotectants, a review of their clinical pharmacology and therapeutic efficacy. Drugs. 1999;57:293–308. doi: 10.2165/00003495-199957030-00003. [DOI] [PubMed] [Google Scholar]
- 4.Ghibu S, Richard C, Vergely C, Zeller M, Cottin Y, et al. Antioxidant properties of an endogenous thiol, Alpha-lipoic acid, useful in the prevention of cardiovascular diseases. J Cardiovasc Pharmacol. 2009;54:391–398. doi: 10.1097/fjc.0b013e3181be7554. [DOI] [PubMed] [Google Scholar]
- 5.Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997;29:315–331. doi: 10.1016/s0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- 6.Vallianou N, Evangelopoulos A, Koutalas P. Alpha-lipoic Acid and diabetic neuropathy. Rev Diabet Stud. 2009;6:230–236. doi: 10.1900/RDS.2009.6.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bustamante J, Lodge JK, Marcocci L, Tritschler HJ, Packer L, et al. Alpha-lipoic acid in liver metabolism and disease. Free Radic Biol Med. 1998;24:1023–1039. doi: 10.1016/s0891-5849(97)00371-7. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki YJ, Aggarwal BB, Packer L. Alpha-lipoic acid is a potent inhibitor of NF-kappa B activation in human T cells. Biochem Biophys Res Commun. 1992;189:1709–1715. doi: 10.1016/0006-291x(92)90275-p. [DOI] [PubMed] [Google Scholar]
- 9.Wenzel U, Nickel A, Daniel H. alpha-Lipoic acid induces apoptosis in human colon cancer cells by increasing mitochondrial respiration with a concomitant O2-*-generation. Apoptosis. 2005;10:359–368. doi: 10.1007/s10495-005-0810-x. [DOI] [PubMed] [Google Scholar]
- 10.Na MH, Seo EY, Kim WK. Effects of alpha-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells. Nutr Res Pract. 2009;3:265–271. doi: 10.4162/nrp.2009.3.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dozio E, Ruscica M, Passafaro L, Dogliotti G, Steffani L, et al. The natural antioxidant alpha-lipoic acid induces p27(Kip1)-dependent cell cycle arrest and apoptosis in MCF-7 human breast cancer cells. Eur J Pharmacol. 2010;641:29–34. doi: 10.1016/j.ejphar.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Choi SY, Yu JH, Kim H. Mechanism of alpha-lipoic acid-induced apoptosis of lung cancer cells. Ann N Y Acad Sci. 2009;1171:149–155. doi: 10.1111/j.1749-6632.2009.04708.x. [DOI] [PubMed] [Google Scholar]
- 13.Abolhassani M, Guais A, Sanders E, Campion F, Fichtner I, et al. Screening of well-established drugs targeting cancer metabolism, reproducibility of the efficacy of a highly effective drug combination in mice. Invest New Drugs. 2012;30:1331–1342. doi: 10.1007/s10637-011-9692-7. [DOI] [PubMed] [Google Scholar]
- 14.Guais A, Baronzio G, Sanders E, Campion F, Mainini C, et al. Adding a combination of hydroxycitrate and lipoic acid (METABLOC) to chemotherapy improves effectiveness against tumor development, experimental results and case report. Invest New Drugs. 2012;30:200–211. doi: 10.1007/s10637-010-9552-x. [DOI] [PubMed] [Google Scholar]
- 15.Isidoro A, Martinez M, Fernandez PL, Ortega AD, Santamaria G, et al. Alteration of the bioenergetic phenotype of mitochondria is a hallmark of breast, gastric, lung and oesophageal cancer. Biochem J. 2004;378:17–20. doi: 10.1042/BJ20031541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papandreou I, Goliasova T, Denko NC. Anticancer drugs that target metabolism, Is dichloroacetate the new paradigm? Int J Cancer. 2011;128:1001–1008. doi: 10.1002/ijc.25728. [DOI] [PubMed] [Google Scholar]
- 17.Korotchkina LG, Sidhu S, Patel MS. R-lipoic acid inhibits mammalian pyruvate dehydrogenase kinase. Free Radic Res. 2004;38:1083–1092. doi: 10.1080/10715760400004168. [DOI] [PubMed] [Google Scholar]
- 18.Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99:989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong J, Xie G, He J, Li J, Pan F, et al. Synergistic antitumor effect of dichloroacetate in combination with 5-fluorouracil in colorectal cancer. J Biomed Biotechnol. 2011;2011:740564. doi: 10.1155/2011/740564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiebiger W, Olszewski U, Ulsperger E, Geissler K, Hamilton G. In vitro cytotoxicity of novel platinum-based drugs and dichloroacetate against lung carcinoid cell lines. Clin Transl Oncol. 2011;13:43–49. doi: 10.1007/s12094-011-0615-z. [DOI] [PubMed] [Google Scholar]
- 21.Goraca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, et al. Lipoic acid - biological activity and therapeutic potential. Pharmacol Rep. 2011;63:849–858. doi: 10.1016/s1734-1140(11)70600-4. [DOI] [PubMed] [Google Scholar]
- 22.Mervaala E, Finckenberg P, Lapatto R, Muller DN, Park JK, et al. Lipoic acid supplementation prevents angiotensin II-induced renal injury. Kidney Int. 2003;64:501–508. doi: 10.1046/j.1523-1755.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 23.Carlson DA, Smith AR, Fischer SJ, Young KL, Packer L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern Med Rev. 2007;12:343–351. [PubMed] [Google Scholar]
- 24.Smith JR, Thiagaraj HV, Seaver B, Parker KK. Differential activity of lipoic acid enantiomers in cell culture. J Herb Pharmacother. 2005;5:43–54. [PubMed] [Google Scholar]
- 25.Loffelhardt S, Bonaventura C, Locher M, Borbe HO, Bisswanger H. Interaction of alpha-lipoic acid enantiomers and homologues with the enzyme components of the mammalian pyruvate dehydrogenase complex. Biochem Pharmacol. 1995;50:637–646. doi: 10.1016/0006-2952(95)00175-y. [DOI] [PubMed] [Google Scholar]
- 26.Pizzolato JF, Saltz LB. The camptothecins. Lancet. 2003;361:2235–2242. doi: 10.1016/S0140-6736(03)13780-4. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien M, Eckardt J, Ramlau R. Recent advances with topotecan in the treatment of lung cancer. Oncologist. 2007;12:1194–1204. doi: 10.1634/theoncologist.12-10-1194. [DOI] [PubMed] [Google Scholar]
- 28.Deshmukh M, Chao P, Kutscher HL, Gao D, Sinko PJ. A series of alpha-amino acid ester prodrugs of camptothecin, in vitro hydrolysis and A549 human lung carcinoma cell cytotoxicity. J Med Chem. 2010;53:1038–1047. doi: 10.1021/jm901029n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao P, Deshmukh M, Kutscher HL, Gao D, Rajan SS, et al. Pulmonary targeting microparticulate camptothecin delivery system, anticancer evaluation in a rat orthotopic lung cancer model. Anticancer Drugs. 2010;21:65–76. doi: 10.1097/CAD.0b013e328332a322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lalloo A, Chao P, Hu P, Stein S, Sinko PJ. Pharmacokinetic and pharmacodynamic evaluation of a novel in situ forming poly(ethylene glycol)-based hydrogel for the controlled delivery of the camptothecins. J Control Release. 2006;112:333–342. doi: 10.1016/j.jconrel.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Paranjpe PV, Stein S, Sinko PJ. Tumor-targeted and activated bioconjugates for improved camptothecin delivery. Anticancer Drugs. 2005;16:763–775. doi: 10.1097/01.cad.0000172834.78068.7c. [DOI] [PubMed] [Google Scholar]
- 32.Paranjpe PV, Chen Y, Kholodovych V, Welsh W, Stein S, et al. Tumor-targeted bioconjugate based delivery of camptothecin, design, synthesis and in vitro evaluation. J Control Release. 2004;100:275–292. doi: 10.1016/j.jconrel.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 33.Zimmer G, Beikler TK, Schneider M, Ibel J, Tritschler H, et al. Dose/response curves of lipoic acid R-and S-forms in the working rat heart during reoxygenation, superiority of the R-enantiomer in enhancement of aortic flow. J Mol Cell Cardiol. 1995;27:1895–1903. doi: 10.1016/0022-2828(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 34.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 35.Yoon MJ, Kim EH, Lim JH, Kwon TK, Choi KS. Superoxide anion and proteasomal dysfunction contribute to curcumin-induced paraptosis of malignant breast cancer cells. Free Radic Biol Med. 2010;48:713–726. doi: 10.1016/j.freeradbiomed.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Das A, Banik NL, Ray SK. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer. 2010;116:164–176. doi: 10.1002/cncr.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manson MM, Farmer PB, Gescher A, Steward WP. Innovative agents in cancer prevention. Recent Results Cancer Res. 2005;166:257–275. doi: 10.1007/3-540-26980-0_17. [DOI] [PubMed] [Google Scholar]
- 38.Yamasaki M, Kawabe A, Nishimoto K, Madhyastha H, Sakakibara Y, et al. Dihydro-alpha-lipoic acid has more potent cytotoxicity than alpha-lipoic acid. In Vitro Cell Dev Biol Anim. 2009;45:275–280. doi: 10.1007/s11626-008-9164-3. [DOI] [PubMed] [Google Scholar]
- 39.Minko T, Paranjpe PV, Qiu B, Lalloo A, Won R, et al. Enhancing the anticancer efficacy of camptothecin using biotinylated poly(ethylene glycol) conjugates in sensitive and multidrug-resistant human ovarian carcinoma cells. Cancer Chemother Pharmacol. 2002;50:143–150. doi: 10.1007/s00280-002-0463-1. [DOI] [PubMed] [Google Scholar]
- 40.Lalloo AK, Luo FR, Guo A, Paranjpe PV, Lee SH, et al. Membrane transport of camptothecin, facilitation by human P-glycoprotein (ABCB1) and multidrug resistance protein 2 (ABCC2) BMC Med. 2004;2:16. doi: 10.1186/1741-7015-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]