Abstract
Objectives
The Single Ventricle Reconstruction trial randomized patients with single right ventricle lesions to a modified Blalock-Taussig or right ventricle-to-pulmonary artery shunt at the Norwood. This analysis describes outcomes at the stage II procedure and factors associated with a longer hospital length of stay (LOS).
Methods
We examined the association of shunt type with stage II hospital outcomes. Cox regression and bootstrapping were used to evaluate risk factors for longer LOS. We also examined characteristics associated with in-hospital death.
Results
There were 393 subjects in the analytic cohort. Median stage II procedure hospital LOS (8 days, IQR (6,14)), hospital mortality (4.3%), transplantation (0.8%), median ventilator time (2 days, IQR (1,3)), median intensive care unit LOS (4 days (IQR (3,7)), number of additional cardiac procedures or complications and serious adverse events did not differ by shunt type. Longer LOS was associated (R2=0.26) with center, longer post-Norwood LOS (HR 1.93 per log day, P<0.001), non-elective timing of the stage II procedure (HR 1.78, P<0.001) and pulmonary artery (PA) stenosis (HR 1.56, P<0.001). By univariate analysis, non-elective stage II (65% vs. 32%, P=0.009), ≥ moderate atrioventricular valve (AVV) regurgitation (75% vs. 24%, P<0.001) and AVV repair (53% vs. 9%, P<0.001) were among the risk factors associated with in-hospital death.
Conclusions
Norwood LOS, PA stenoses and non-elective stage II procedure, but not shunt type, are independently associated with longer LOS. Non-elective stage II, >moderate AVV regurgitation and need for AVV repair are among the risk factors for death.
Introduction
Staged surgical repair for hypoplastic left heart syndrome (HLHS) and other single right ventricle anomalies usually includes a stage II procedure (superior cavopulmonary connection) between the Norwood and Fontan procedures (total cavopulmonary connection). This approach reduces morbidity and mortality at the time of the Fontan procedure 1,2 and is well tolerated Several series suggest the median length of hospital stay (LOS) is 5–7 days and there are relatively few complications3–8. Despite the generally excellent results there are a number of preoperative risk factors associated with prolonged LOS, hospital death, or failure of the cavopulmonary circulation to provide adequate oxygenation and/or acceptable hemodynamics. The most commonly identified risks include stenosis or distortion of the pulmonary arteries (PA) or increased pulmonary vascular resistance (PVR)1,9–11, atrioventricular valve (AVV) regurgitation 10,12–14 and younger age at the time of the stage II13,15,16.
The primary outcome in the Single Ventricle Reconstruction (SVR) trial was transplant-free survival at 12 months post-randomization between subjects with single right ventricle anomalies palliated with a right ventricle-to-pulmonary artery shunt (RVPAS) versus a modified Blalock-Taussig shunt (MBTS) at the Norwood procedure17. Transplant-free survival 12 months after randomization was higher with the RVPAS, but the trial showed several significant differences between the two groups of Norwood survivors with regard to potential risk factors for the stage II procedure. Specifically, PA size by angiography before the stage II procedure as measured by the Nakata index was significantly smaller in the RVPAS group (median 99 [IQR 63–150] vs. 125 [88–166]), as was the diameter of the distal right PA (5.2±1.7 vs. 6.3±2.0 mm). PA interventions during the study period were more common in the RVPAS group (49% vs. 28%)17.
The primary purpose of this analysis was to report hospital outcomes following the stage II procedure in the SVR cohort and compare these outcomes between subjects who received the RVPAS vs. the MBTS. Because of the low expected mortality of the stage II procedure, the analyses focused predominantly on morbidity rather than mortality. Hospital LOS was the primary outcome with the hypothesis that LOS would be longer in the RVPAS group based on the increased incidence of pulmonary artery problems previously reported in this cohort. A secondary aim of this analysis was to identify risk factors for longer LOS and inhospital death during the stage II hospitalization.
Methods
Subjects
Details of the SVR trial design have been reported18. The Institutional Review Board or Research Ethics Board at each participating center approved the study protocol and written informed consent was obtained from parents prior to trial enrollment. Subjects were recruited from 15 centers in North America participating in the National Heart, Lung, and Blood Institute-funded Pediatric Heart Network between May 2005 and July 2008. Inclusion criteria consisted of a diagnosis of HLHS or other single right ventricle anomaly and planned Norwood procedure. Exclusion criteria were preoperative identification of anatomy rendering either a MBTS or RVPAS technically impossible, and any major congenital or acquired extracardiac abnormality that could independently affect the likelihood of the subject meeting the primary outcome of transplant-free survival at 12 months post-randomization. Only subjects who survived to the stage II procedure are included in this analysis.
Study Design and Measurements
Shunt type was defined for this analysis as the shunt in place at the end of the Norwood procedure (non-intention to treat). Other than random assignment of shunt, all participants were managed according to the standard practices at their clinical centers.
The outcome variables for the primary analysis included hospital LOS (primary outcome), days receiving mechanical ventilation, intensive care unit (ICU) LOS, performance of concurrent surgical or interventional procedures at the time of the stage II procedure or during postoperative hospitalization that are not routinly part of the stage II operation, number of serious adverse events (SAEs) and complications, and death. Those subjects who died during the stage II hospitalization were excluded from the analyses of LOS. We then analyzed potential risk factors that might influence hospital LOS or in-hospital death. The factors considered in the risk factor analysis were restricted to those judged to be clinically relevant. A full list of candidate predictors can be found in the online appendix, Table 1. For the purpose of this analysis, unplanned postoperative procedures were limited to major cardiovascular procedures and did not include procedures such as thoracentesis or chest tube placement, sternal debridement or gastrostomy tube insertion. To examine the impact of two potentially important confounders, namely missing data with regard to PVR and subjects who were not discharged after the Norwood and before the stage II procedure, alternative risk factor models were created.
Appendix Table 1.
Cox Regression Model for Predictors of Length of Hospital Stay
| N | Hazard ratio (95% CI) | P | |
|---|---|---|---|
| Center | 372 | 0.004 | |
| Demographics | |||
| Gender | |||
| Male | 238 | 1.05 (0.85, 1.30) | 0.62 |
| Female | 135 | Ref grp | |
| Hispanic | |||
| Yes | 69 | 1.27 (0.97, 1.65) | 0.08 |
| No | 300 | Ref grp | |
| Race | 0.27 | ||
| White | 304 | Ref grp | |
| Black | 51 | 0.79 (0.59, 1.06) | |
| Other | 14 | 1.09 (0.63, 1.88) | |
| SES score | 360 | 0.98 (0.96, 1.00) | 0.09 |
| Genetic syndrome | 0.02 | ||
| Yes | 91 | Ref grp | |
| No | 196 | 0.79 (0.59, 1.06) | |
| Unknown | 85 | 1.09 (0.63, 1.88) | |
| Anatomy | |||
| HLHS | 0.88 | ||
| Yes | 333 | 1.03 (0.74, 1.43) | |
| No | 40 | Ref grp | |
| Clinical diagnoses at time of stage II procedure | |||
| Branch PA stenosis | 0.16 | ||
| Yes | 97 | 1.18 (0.94, 1.49) | |
| No | 274 | Ref grp | |
| AVVR | 0.003 | ||
| Yes | 37 | 1.69 (1.20, 2.39) | |
| No | 334 | Ref grp | |
| Aortic arch obstruction | 0.53 | ||
| Yes | 28 | 1.13 (0.77, 1.67) | |
| No | 343 | Ref grp | |
| Norwood Hospitalization | |||
| Shunt type at the end of Norwood | 0.14 | ||
| MBTS | 164 | Ref grp | |
| RVPAS | 208 | 1.17 (0.95, 1.44) | |
| Log transformed Norwood LOS | 372 | 1.80 (1.53, 2.13) | <.001 |
| Discharge after Norwood operation | <.001 | ||
| No | 13 | 4.13 (2.29, 7.44) | |
| Yes | 359 | Ref grp | |
| On ECMO | 0.03 | ||
| Yes | 25 | 1.64 (1.07, 2.52) | |
| No | 348 | Ref grp | |
| Inter-stage Interventions | |||
| ECMO | |||
| Yes | 5 | 1.42 (0.58, 3.43) | 0.44 |
| No | 368 | Ref grp | |
| Inter-stage intervention on Aorta: | 0.543 | ||
| Yes | 62 | 1.09 (0.83, 1.43) | |
| No | 310 | Ref grp | |
| Inter-stage intervention on PA: | 0.95 | ||
| Yes | 10 | 1.02 (0.54, 1.91) | |
| No | 362 | Ref grp | |
| Inter-stage intervention on atrial septum: | 0.66 | ||
| Yes | 10 | 0.87 (0.46, 1.63) | |
| No | 362 | Ref grp | |
| Other Inter-stage intervention: | 0.89 | ||
| Yes | 31 | 1.03 (0.71, 1.48) | |
| No | 341 | Ref grp | |
| Pre-stage II Cath/Echo | |||
| RVFAC (range 0 to 1) | 344 | 0.93 (0.87, 1.003) per .05 unit increase | 0.06 |
| ≥Moderate Tricuspid valve regurgitation | 365 | ||
| Yes | 86 | 1.52 (1.19, 1.95) | 0.001 |
| No | 279 | Ref grp | |
| Pre-stage II Catheterization Hemodynamics | |||
| Ventricular end diastolic pressure from Cath, mmHg | 348 | 1.03 (1.00, 1.06) | 0.08 |
| Pulmonary vascular resistance, Wood units | 253 | 1.21 (1.07, 1.35) | 0.001 |
| Pre-Stage II Angiography | |||
| Severity of LPA stenosis | 325 | 1.00 (0.99, 1.00) | 0.64 |
| <15% | 196 | 1.16 (0.72, 1.89) | 0.73 |
| 15–<35% | 75 | 1.04 (0.62, 1.74) | |
| 35–<50% | 36 | 1.25 (0.71, 2.21) | |
| ≥50% | 18 | Ref grp | |
| Severity of RPA stenosis | 325 | 1.16 (0.72, 1.89) | 0.13 |
| <15% | 175 | 1.04 (0.62, 1.74) | 0.21 |
| 15–<35% | 80 | 1.25 (0.71, 2.21) | |
| 35–<50% | 40 | 1.16 (0.72, 1.89) | |
| ≥50% | 30 | Ref grp | |
| Total lower lobe index, mm2/M2 1 | 322 | 0.94 (0.89, 0.989) per a 50-unit increase | 0.02 |
| Nakata Index | 322 | 0.96 (0.90, 1.03) Per a 50-unit increase | 0.27 |
| At stage II | |||
| Reasons for timing of stage II procedure | <.001 | ||
| Elective | 252 | 0.61 (0.49, 0.77) | |
| Non-elective | 120 | Ref grp | |
| Age at stage II | 0.96 (0.90, 1.01) | 0.11 | |
| Weight –age-z on pre-stage II Echo | 368 | 0.89 (0.81, 0.97) | 0.009 |
| Type of stage II | 0.81 | ||
| Bidirectional cavopulmonary shunt | 234 | 0.85 (0.40, 1.80) | |
| Hemi-Fontan | 91 | 0.84 (0.39, 1.81) | |
| Bidirectional cavopulmonary anastomoses | 40 | 0.73 (0.33, 1.64) | |
| Other | 7 | Ref grp | |
| Total CPB time, per 10 min increase | 370 | 1.05 (1.02, 1.08) | <.001 |
| On DHCA | 371 | 0.56 | |
| Yes | 1.07 (0.85, 1.35) | ||
| No | Ref grp | ||
| Concurrent procedures: PA repair | 372 | 0.01 | |
| Yes | 133 | 1.32 (1.06, 1.63) | |
| No | 239 | Ref grp | |
| Concurrent procedures: Aortic Arch repair | 372 | 0.09 | |
| Yes (n=22) | 22 | 1.46 (0.95, 2.25) | |
| No (n=350) | 350 | Ref grp | |
| Concurrent procedures: AV valve repair | 372 | 0.03 | |
| Yes | 34 | 1.49 (1.04, 2.12) | |
| No | 338 | Ref grp | |
| Concurrent procedures: Aortic valve repair | 372 | 0.48 | |
| Yes | 9 | 0.79 (0.41, 1.53) | |
| No | 363 | Ref grp | |
| Concurrent: ASD | 372 | 0.73 | |
| Yes | 16 | 0.92 (0.55, 1.51) | |
| No | 356 | Ref grp |
SES: socio-economic status; HLHS: hypoplastic left heart syndrome; PA: pulmonary artery; AVVR: atrioventricular valve regurgitation; MBTS: modified Blalock-Taussig shunt; RVPAS: right ventricle-to-pulmonary artery shunt; LOS: length of hospital stay; ECMO: extracorporeal membrane oxygenation; Cath: catheterization; Echo: echocardiogram; RVFAC: right ventricle fractional area change; LPA: left pulmonary artery; RPA: right pulmonary artery; CPB: cardiopulmonary bypass; DHCA: deep hypothermic circulatory arrest; AV: atrioventricular; ASD: atrial septal defect; Ref grp: reference group; CI: confidence interval
Total lower lobe index was defined as (3.14*[right lower lobe diameter/2]2 + 3.14*[left lower lobe diameter/2]2)/BSA
Protocol-driven echocardiograms were obtained at a scheduled outpatient visit before the stage II procedure. The echocardiograms were interpreted at a core laboratory (Medical College of Wisconsin) to assess the degree of AVV regurgitation (none/mild/moderate/severe), right ventricular end-systolic volume (RVESV), end-diastolic volume (RVEDV), fractional area change (RVFAC), and right ventricular ejection fraction (RVEF). RVFAC was the primary measurement of right ventricular function used in further analyses. The rationale and detailed methods for echocardiographic assessment of RV volume and function in the SVR cohort has been detailed elsewhere19.
Cardiac catheterization was performed in the majority of subjects before the stage II procedure as part of routine clinical care, and angiograms were interpreted at a core lab (Duke University). Hemodynamic measurements such as right ventricular end diastolic pressure (RVEDP) and calculations such as PVR were reported by the enrolling center. Anatomic measurements including PA size and stenoses were determined by the core lab. Because data needed to calculate PVR were missing for a large number of subjects it was excluded from the primary multivariable analysis. Total lower lobe index was calculated as a measure of total PA size from direct measurements of the right and left lower lobe PA diameters. This measurement has been shown to be the best measure of PA growth and is less affected by surgery than other indexed measures of PA size20 and was therefore used as the measurement of PA size for consideration of entry into the multivariable model.
In addition to the echocardiographic and catheterization based diagnoses of AVV regurgitation and PA stenosis we recorded the clinical diagnoses of these conditions, which were based on the overall impression of the treating cardiology teams.
Socioeconomic status at the time of randomization was assigned in two ways: 1) using a U.S. census-based score derived from six measures based on income, housing, and occupational-related features of the subject’s census block tract21, and 2) the percentage below the federal poverty level in the subject’s census block tract.
Statistical Methods
Hospital LOS was defined by days from stage II procedure date (day 1) to discharge date. Descriptive statistics are presented as mean ± standard deviation (SD), medians with interquartile range (IQR) and percentages as appropriate. Differences between those subjects who died vs. those who survived to hospital discharge were determined with t test, Wilcoxon rank sum test or a Fisher exact test. A univariate Cox regression model was used to identify associations between clinical factors and time to hospital discharge following the stage II procedure. Only those that were significant at P≤0.20 with respect to outcome were considered for inclusion in the multivariable model. The linearity of the covariates and the proportional hazards assumption were evaluated both graphically and statistically. A stepwise Cox regression selection was employed in conjunction with bootstrapping (1000 samples) to obtain reliability estimates for each of the predictors. All terms in the final multivariable model have a P< 0.05 and have a reliability >50%. The primary multivariable analysis was constructed using all cases with complete data. Two additional models were created; the first excluded 13 subjects who were not discharged between the Norwood and stage II operation and have been previously described22, and the second included PVR from the pre-stage II catheterization as a potential predictor. All analyses were conducted using SAS version 9.2 (Statistical Analysis System, Cary, NC) and SAS macros for bootstrapping estimates of reliability.
Results
Patient Population and Diagnoses
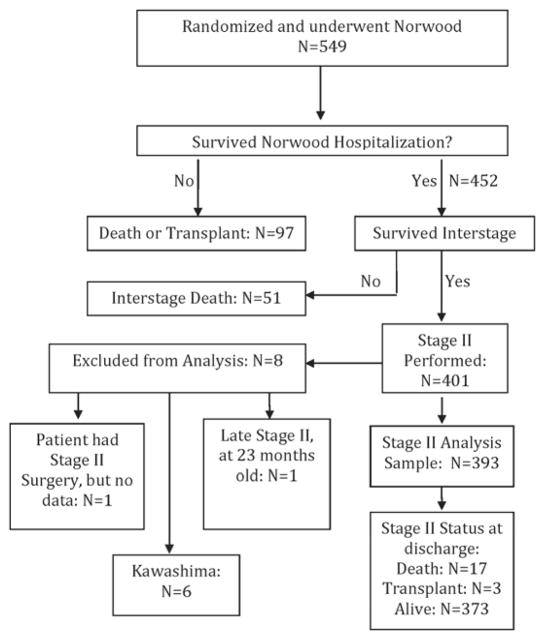
There were 549 subjects who were randomized in the SVR trial and underwent the Norwood procedure (Figure 1). Subjects who died or were transplanted before the stage II procedure, those with interrupted inferior vena cava resulting in a Kawashima procedure as a second stage, those subjects with missing data and one with an uncharacteristically late stage II procedure at 23 months of age were excluded from this analysis. The final cohort consisted of 393 subjects, and because of differential rates of attrition between randomization and the stage II procedure, 172 subjects had a MBTS and 221 had a RVPAS.
Figure 1.
Flow diagram showing the SVR trial cohort from inception through stage II hospitalization. SVR: Single Ventricle Reconstruction trial
The cohort was 64% male and 81% white. Anatomy was predominantly HLHS (88%). The most common associated diagnoses prior to the stage II procedure as reported by the clinical teams were branch PA stenosis (26%) and AVV regurgitation (12%). Mean right (n=189) and left (n=212) PA pressures at pre-stage II catheterization were 14.4±3.4 and 14.7±3.8 mm Hg respectively, and mean PVR (n=264) was 2.1±1.1 Wood Units × m2. Pre-stage II angiography revealed 17% of subjects to have 35% or greater stenosis of the left PA and 21% to have similar stenosis of the right PA. Echocardiography demonstrated at least moderate AVV regurgitation in 26% and a mean RVFAC of 34±8%. Further details can be found in the online appendix, Table 2.
Appendix Table 2.
Characteristics of the Analytic Cohort of Subjects Undergoing the Stage II Procedure
| Overall (n=3931) | Stage II Mortality (n=17) | Non-Transplant Survivors (n=373) | P* | |
|---|---|---|---|---|
| Mean±SD/N (%) | Mean±SD/N (%) | Mean±SD/N (%) | ||
| Demographics | ||||
| Male | 250 (63.6%) | 11 (64.7%) | 238 (63.8%) | 1.00 |
| Hispanic | 0.32 | |||
| Yes | 70 (18.1%) | 1 (6.7%) | 69 (18.7%) | |
| No | 317 (81.9%) | 14 (93.3%) | 300 (81.3%) | |
| Unknown | 6 | 2 | 4 | |
| Race | 0.10 | |||
| White | 317 (81.3%) | 11 (64.7%) | 304 (82.2%) | |
| Black | 58 (14.9%) | 5 (29.4%) | 52 (14.1%) | |
| Other | 15 (3.9%) | 1 (5.9%) | 14 (3.8%) | |
| Unknown | 3 | 0 | 3 | |
| SES score | 0.4±5.1 | 0.4±3.6 | 0.4±5.1 | 0.97 |
| Genetic Syndrome/other anomalies | 0.005 | |||
| Yes | 93 (23.7%) | 1 (5.9%) | 91 (24.4%) | |
| No | 204 (51.9%) | 6 (35.3%) | 196 (52.5%) | |
| Unknown | 96 (24.4%) | 10 (58.8%) | 86 (23.1%) | |
| Anatomy | ||||
| HLHS | 346 (88.0%) | 11 (64.7%) | 333 (89.3%) | 0.009 |
| Clinical diagnoses at time of stage II procedure2 | ||||
| Branch PA stenosis present | 102 (26.1%) | 4 (25.0%) | 98 (26.3%) | 1.00 |
| AVVR | 45 (11.5%) | 8 (50.0%) | 37 (10.0%) | <.001 |
| Aortic arch obstruction | 30 (7.7%) | 1 (6.3%) | 28 (7.5%) | 1.00 |
| Norwood Hospitalization | ||||
| Shunt type at the end of Norwood | 1.00 | |||
| MBTS | 172 (43.8%) | 7 (41.2%) | 164 (44.0%) | |
| RVPAS | 221 (56.2%) | 10 (58.8%) | 209 (56.0%) | |
| Length of Norwood hospitalization, days | 33.8±30.8 Median (IQR) 24 (17, 39) | 72.2±51.7 Median=61 | 31.9±28.3 Median=24 | <.001 |
| Not discharged after Norwood | 22 (5.6%) | 8 (47.1%) | 13 (3.5%) | <.001 |
| ECMO during Norwood hospitalization | 32 (8.1%) | 6 (35.3%) | 25 (6.7%) | 0.001 |
| Inter-stage interventions | ||||
| ECMO | 5 (1.3%) | 0 | 5 (1.3%) | 1.00 |
| Intervention on Aorta | 68 (17.3%) | 4 (23.5%) | 63 (16.9%) | 0.51 |
| Intervention on PA | 10 (2.5%) | 0 (0.0%) | 10 (2.7%) | 1.00 |
| Intervention on atrial septum | 10 (2.5%) | 0 (0.0%) | 10 (2.7%) | 1.00 |
| Other interventions | 32 (8.1%) | 1 (5.9%) | 31 (8.3%) | 1.00 |
| Pre-stage II Echocardiography | ||||
| RVFAC | 0.34±0.08 (n=363) | 0.31±0.06 (n=16) | 0.34±0.08 (n=344) | 0.17 |
| ≥ Moderate tricuspid valve regurgitation | <.001 | |||
| Yes | 100 (26.0%) | 12 (75.0%) | 86 (23.6%) | |
| No | 284 (74.0%) | 4 (25.0%) | 279 (76.4) | |
| Catheterization Hemodynamics | ||||
| RVEDP (mmHg) | 8.3±3.3 (n=366) | 10.8±3.5 (n=16) | 8.1±3.2 (n=348) | 0.001 |
| PVR (Woods units) | 2.1±1.1 (n=264) | 1.9±0.7 (n=10) | 2.1±1.2 (n=253) | 0.61 |
| Transpulmonary gradient (mmHg) | 4.9±3.1 (n=159) | 5.2±2.4 (n=7) | 4.8±3.1 (n=150) | 0.76 |
| Angiography | ||||
| Percent LPA stenosis at site of maximal stenosis (relative to proximal left lower diameter) | 14.4±18.9 (n=336) Median (IQR) 2.0 (0.0, 26.5) | 18.2±21.9 (n=11) | 14.3±18.8 (n=325) | 0.50 |
| <15% | 202 (60.1%) | 6 (54.5%) | 196 (60.3%) | 0.62 |
| 15 – ≤ 35% | 77 (22.9%) | 2 (18.2%) | 75 (23.1%) | |
| 35 – ≤ 50% | 38 (11.3%) | 2 (18.2%) | 36 (11.1%) | |
| >50% | 19 (5.7%) | 1 (9.1%) | 18 (5.5%) | |
| Percent RPA stenosis at site of maximal stenosis (relative to proximal right lower diameter) | 18.2±20.6 (n=337) Median (IQR) 10.0 (0.0, 33.0) | 22.0±19.1 (n=11) | 18.1±20.6 (n=325) | 0.54 |
| <15% | 180 (53.4%) | 4 (36.4%) | 175 (53.8%) | 0.45 |
| 15 – ≤ 35% | 85 (25.2%) | 5 (45.5%) | 80 (24.6%) | |
| 35 – ≤ 50% | 41 (12.2%) | 1 (9.1%) | 40 (12.3%) | |
| >50% | 31 (9.2%) | 1 (9.1%) | 30 (9.2%) | |
| Total lower lobe index, mm2/M2,3 | 167.0±90.0 (n=333) | 169.5±72.2 (n=11) | 167.0±90.6 (n=322) | 0.93 |
| Nakata index | 128.5±81.0 (n=333) | 114.3±53.7 (n=11) | 129.0±81.8 (n=322) | 0.56 |
P-values obtained from t-test, Wilcoxon rank sum test and Fisher exact test. Test of significant differences between in-hospital deaths and survivors but not the transplants
Overall cohort includes survivors (n=373), non-survivors (n=17) and transplants prior to discharge (n=3)
As designated by overall clinical evaluation
HLHS: hypoplastic left heart syndrome; PA: pulmonary artery; AVVR: atrioventricular valve regurgitation; MBTS: modified Blalock-Taussig shunt; RVPAS: right ventricle-to-pulmonary artery shunt; ECMO: extracorporeal membrane oxygenation; RVFAC: right ventricular fractional area change; RVEDP: right ventricular end-diastolic pressure; PVR: pulmonary vascular resistance; LPA: left pulmonary artery; RPA: right pulmonary artery; SD: standard deviation
Total lower lobe index was defined as (3.14*[right lower lobe diameter/2]2 + 3.14*[left lower lobe diameter/2]2)/BSA
Mean age at the time of the stage II procedure was 5.3±1.7 months. The stage II procedure was elective, as reported by the individual site investigators, in 66%. The subjects who underwent stage II non-electively were younger (4.6±1.7 vs. 5.6±1.5 months, P<.001) and the average number of concurrent procedures was higher (1.2±1.5 vs. 0.8±1.0, P<.001). Timing of the stage II procedure was not associated with shunt type (29% MBTS vs. 37.5% RVPAS were non-elective, P=0.86). The most common reason for non-elective timing of the stage II procedure was progressive hypoxemia (n=107), with ventricular dysfunction (n=33), shunt stenosis or occlusion (n=17), aortic arch obstruction (n=13), failure to thrive (n=12), AVV regurgitation (n=12) and PA stenosis (n=5) accounting for almost all remaining non-elective stage II procedures.
A right bidirectional cavopulmonary anastomosis was the most common type of stage II procedure and was performed in 64% of subjects, with a right hemi-Fontan being the next most common at 24%. Cardiopulmonary bypass time averaged 93±43 minutes and 73% of cases were performed without deep hypothermic circulatory arrest. Concurrent procedures not otherwise part of a usual stage II procedure or unplanned cardiovascular interventions before stage II hospital discharge were performed in 56% of subjects. The most common concurrent procedures were those involving PA plasty (36%) followed by atrioventricular valve repair (11%) and aortic arch repair (6%) (Table 1).
Table 1.
Subject and Surgical Characteristics at the Time of Stage II Procedure
| Overall (n=3931) | Stage II Mortality (n=17) | Survivors (n=373) | P*** | |
|---|---|---|---|---|
| Mean±SD/N (%) | Mean±SD/N (%) | Mean±SD/N (%) | ||
| Age at stage II, months | 5.3±1.7 | 4.9±2.2 | 5.3±1.6 | 0.33 |
| Weight-for-age z score* | −1.8±1.2 | −2.5±1.6 (n=15) | −1.8±1.2 (n=368) | 0.02 |
| Timing of stage II procedure | 0.009 | |||
| Elective | 260 (66.2%) | 6 (35.3%) | 252 (67.6%) | |
| Non-elective | 133 (33.8%) | 11 (64.7%) | 121 (32.4%) | |
| Type of stage II procedure | 0.65 | |||
| Bidirectional cavopulmonary shunt | 250 (63.6%) | 13 (76.5%) | 235 (63.0%) | |
| Hemi-Fontan | 94 (23.9%) | 2 (11.8%) | 91 (24.4%) | |
| Bilateral bidirectional cavopulmonary shunts | 42 (10.7%) | 2 (11.8%) | 40 (10.7%) | |
| Other | 7 (1.8%) | 0 (0.0%) | 7 (1.9%) | |
| Operative Support | ||||
| Total CPB time (min) | 92.7±43.4 | 134.1±43.7 (n=16) | 90.4±42.1 (n=371) | <.001 |
| DHCA | 0.15 | |||
| Yes | 105 (26.9%) | 7 (43.8%) | 97 (26.1%) | |
| No | 286 (73.1%) | 9 (56.3%) | 275 (73.9%) | |
| Concurrent procedures** | ||||
| Pulmonary artery repairs | 141 (35.9%) | 8 (47.1%) | 133 (35.7%) | 0.44 |
| Atrioventricular valve repairs | 44 (11.2%) | 9 (52.9%) | 34 (9.1%) | <.001 |
| Aortic arch repairs | 24 (6.1%) | 1 (5.9%) | 22 (5.9%) | 1.00 |
| Other vascular repairs | 19 (4.8%) | 1 (5.9%) | 17 (4.6%) | 0.56 |
| Atrial septal interventions | 17 (4.3%) | 1 (5.9%) | 16 (4.3%) | 0.54 |
| Aortic/neo-aortic valve repair | 9 (2.3%) | 0 (0.0%) | 9 (2.4%) | 1.00 |
| ECMO | 9 (2.3%) | 4 (23.5%) | 4 (1.1%) | <.001 |
| EFE resection | 9 (2.3%) | 0 | 9 (2.4%) | 1.00 |
| Arrhythmia intervention | 8 (2.0%) | 1 (11.8%) | 5 (1.3%) | 0.03 |
| Airway procedure | 5 (1.3%) | 0 | 5 (1.3%) | 1.00 |
| Intervention for ICU complication | 4 (1.0%) | 1 (5.9%) | 3 (0.8%) | 0.16 |
| Atrial reduction | 3 (0.8%) | 3 (17.7%) | 0 | <.001 |
| Other | 7 (1.8%) | 1 (5.9%) | 6 (1.6%) | 0.27 |
Overall cohort includes survivors (n=373), non-survivors (n=17) and transplants prior to discharge (n=3)
From pre-stage II echocardiogram
Includes post-operative procedures during stage II hospitalization
P-values obtained from t-test, Wilcoxon rank sum test and Fisher exact test. Test of significant differences between in-hospital deaths and survivors but not the transplants
CPB: cardiopulmonary bypass; DHCA: deep hypothermic circulatory arrest; ECMO: extracorporeal membrane oxygenation; EFE: endocardial fibroelastosis; ICU: intensive care unit; SD: standard deviation
Stage II Morbidity Outcomes
Outcomes by shunt type are summarized in Table 2. Median hospital LOS was 8 days (IQR: 6, 14). There were no differences in hospital LOS, ventilator days, ICU LOS, number of concurrent procedures, serious adverse events or complications between subjects with a RVPAS and those with a MBTS.
Table 2.
Outcomes by Shunt Type1
| Overall (N=393) | MBTS (N=172) | RVPAS (n=221) | P* | ||||
|---|---|---|---|---|---|---|---|
| Mean±SD/N (%) | Median (IQR) | Mean±SD/N (%) | Median (IQR) | Mean±SD/N (%) | Median (IQR) | ||
| Number of inhospital deaths | 17 (4.3%) | 7 (4.1%) | 10 (4.5%) | 1.00 | |||
| Number of inhospital transplants | 3 (0.8%) | 1 (0.6%) | 2 (0.9%) | 1.00 | |||
| Length of hospital stay, days*** | 13.9±17.0 | 8 (6, 13) | 12.5±13.9 | 8 (6, 11.5) | 15.0±19.1 | 8 (6, 15.0) | 0.07 |
| Total ventilated days*** | 4.0±12.3 | 2 (1, 3) | 3.1±5.7 | 2 (1, 3) | 4.8±15.7 | 2 (1, 3.5) | 0.34 |
| Total ICU days*** | 8.0±14.3 | 4 (3, 7) | 7.2±10.8 | 4 (3, 6) | 8.6±16.6 | 4 (3, 8) | 0.11 |
| Number of SAEs or complications | 1.2±2.5 | 0 (0, 1) | 1.1±1.8 | 0 (0, 2) | 1.3±3.0 | 0 (0, 1) | 0.84 |
| 0 | 210 (53.4%) | 91 (52.9%) | 119 (53.8%) | 0.96 | |||
| 1 | 85 (21.6%) | 37 (21.5%) | 48 (21.7%) | ||||
| ≥ 2 | 98 (24.9%) | 44 (25.6%) | 54 (24.4%) | ||||
| Number of concurrent procedures** | 0.9±1.2 | 1 (0, 1) | 0.9±1.1 | 1 (0, 1) | 0.9±1.3 | 1 (0,1) | 0.77 |
| 0 | 174 (44.3%) | 76 (44.2%) | 98 (44.3%) | 0.58 | |||
| 1 | 147 (37.4%) | 68 (39.5%) | 79 (35.8%) | ||||
| ≥ 2 | 72 (18.3%) | 28 (16.3%) | 44 (19.9%) | ||||
Refers to shunt type in place at the end of the Norwood procedure
P-values obtained from Wilcoxon rank sum test and Fisher exact test
Includes post-operative procedures during stage II hospitalization
Excluding deaths and transplants
MBTS: modified Blalock-Taussig shunt; RVPAS: right ventricle-to-pulmonary artery shunt; ICU: intensive care unit; SAE: serious adverse event; SD: standard deviation; IQR: interquartile range
Univariate Analysis for LOS
The analysis for the primary outcome was performed on a cohort of 372 subjects, which excluded 17 in-hospital deaths, 3 in-hospital heart transplants and 1 subject with no information regarding discharge in the data set. The list of candidate predictors for hospital LOS following the stage II procedure and the univariate modeling results can be found in the online appendix Table 2.
Multivariable Model for LOS
Longer hospital LOS was independently associated (R2=0.26) with center, longer post- Norwood LOS (HR 1.93 per log day, P<0.001), non-elective timing for the stage II procedure (HR 1.78, P<0.001) and a clinical diagnosis of branch PA stenosis at the time of the stage II procedure (HR 1.56, P<0.001) (Table 3A). Shunt type was not significant when added to this model (P=0.92). An alternative analysis that excluded the 13 subjects who were not discharged from the hospital following the Norwood procedure showed the same associations as the primary model and an additional inverse association between RVFAC and post-stage II LOS (Table 3B). A multivariable model (N=253) that excluded subjects in whom PVR could not be measured found the same significant risk factors as the primary model except that higher PVR (HR 1.24 per 1 Wood Unit × M2, P=0.004) instead of pulmonary artery stenosis was associated with longer hospital LOS (Table 3C). Of note, the incidence of ≥ 35% branch stenosis was similar between those subjects with PVR reported and those for whom that data was missing (data not shown). Median hospital LOS was calculated for subgroups defined by the presence vs. absence of all possible combination of the three risk factors identified in the multivariable model (Table 4). A cut point of 24 days was used to define prolonged LOS following the Norwood operation since this was the median LOS for transplant-free survivors who later underwent a Stage II procedure. The median LOS following the stage II procedure was 6 days when no risk factors were present and 13 days when all three risk factors were present.
Table 3A.
Final Model for Risk Factors Associated with Stage II Hospital Length of Stay. (N=371, generalized R2=0.26).
| Variable | Hazard ratio (95% CI) | P | Reliability |
|---|---|---|---|
| Site | 0.002 | 97% | |
| Log of Norwood Length of stay in days | 1.93 (1.60, 2.34) | <.001 | 99% |
| Non-elective reason for timing of stage II | 1.78 (1.38, 2.31) | <.001 | 78% |
| Associated clinical diagnosis with stage II: Branch PA stenosis present | 1.56 (1.20, 2.02) | <.001 | 64% |
Table 3B.
Final Model for Risk Factors Associated with Stage II Hospital Length of Stay Excluding 13 Subjects Not Discharged Between Norwood and Stage II Procedures. (N=331, generalized R2=0.24).
| Variable | Hazard ratio (95% CI) | P | Reliability |
|---|---|---|---|
| Site | <.001 | 98% | |
| Log of Norwood Length of stay in days | 1.56 (1.25, 1.96) | <.001 | 79% |
| Non-elective reason for timing of stage II | 1.88 (1.42, 2.50) | <.001 | 89% |
| Associated clinical diagnosis with stage II: Branch PA stenosis present | 1.60 (1.22, 2.10) | <.001 | 81% |
| Pre-stage II Echo: RV fractional area change (range 0 to 1)* | 0.91 (0.85, 0.98) Per 0.05 increase | 0.017 | 54% |
28 subjects had missing RV fractional area change on pre-stage II Echo.
Table 3C.
Final Model for Risk Factors Associated with Stage II Hospital Length of Stay Including Only Subjects for Whom PVR Calculations Were Available. (N=253, generalized R2=0.28).
| Variable | Hazard ratio (95% CI) | P | Reliability |
|---|---|---|---|
| Site | 0.003 | 95% | |
| Log of Norwood Length of stay in days | 1.84 (1.48, 2.30) | <.001 | 99% |
| Non-elective reason for timing of stage II | 1.51 (1.09, 2.09) | 0.013 | 62% |
| Pre-stage II cath PVR, Wood units | 1.24 (1.07, 1.44) | 0.004 | 69% |
PA: pulmonary artery; Echo: echocardiogram; RV: right ventricle: cath: catheterization; PVR: pulmonary vascular resistance; CI: confidence interval
Table 4.
Median Stage II Procedure LOS (days) by Risk Factor Group
| Number of Risk Factors | Norwood LOS > 24 Days | Reason for Timing of Stage II Procedure | Diagnosis of PA Stenosis | Median Stage II Procedure LOS |
|---|---|---|---|---|
| 0 (Best) | No | Elective | No | 6 (n=91) |
| 1 | Yes | Elective | No | 8 (n=97) |
| 1 | No | Non-Elective | No | 8 (n=38) |
| 1 | No | Elective | Yes | 8 (n=36) |
| 2 | Yes | Elective | Yes | 9 (n=27) |
| 2 | No | Non-Elective | Yes | 9 (n=18) |
| 2 | Yes | Non-Elective | No | 14 (n=48) |
| 3 (Worst) | Yes | Non-Elective | Yes | 13 (n=16) |
PA=pulmonary artery; LOS=length of stay, days
Mortality
Seventeen subjects (4%) died after the stage II procedure and before hospital discharge. An additional 3 subjects (1%) remained in the hospital until transplantation. Univariate comparison of those subjects who died or were transplanted during the stage II hospitalization with those subjects who survived to discharge without transplant is shown in Table 1. Characteristics associated with in-hospital mortality or transplantation included longer Norwood LOS, not being discharged between the Norwood and stage II procedures, extracorporeal membrane oxygenation (ECMO) after the Norwood, nonelective timing of the stage II procedure, a clinical diagnosis of AVV regurgitation at the time of the stage II procedure, higher RVEDP at pre-stage II catheterization, lower weightfor- age z score at the time of the pre-stage II echocardiogram, greater than or equal to moderate AVV regurgitation on pre-stage II echocardiogram, longer total cardiopulmonary bypass time at the stage II procedure, and surgical atrioventricular valve repair during the stage II hospitalization. Among those with elective timing of the stage II procedure, there was no statistically significant relationship between age at the stage II procedure and death or transplant (data not shown). Because of the overall low in-hospital mortality/transplant rate, multivariable modeling was not performed.
Discussion
Outcomes of stage II hospitalization for subjects enrolled in the Pediatric Heart Network sponsored SVR trial were favorable and not associated with the type of shunt placed at the initial Norwood procedure. The median hospital LOS was 8 days, consistent with several previous single center reports5–8,12,23–25, although some series have reported considerably longer LOS ranging from 11 to 21 days13,26. Given this wide variability in LOS in the published literature, it is not surprising that there was a significant association of LOS with the surgical center at which the stage II procedure was performed.
Both anatomical and physiological PA complications have been implicated as risk factors for worse stage II outcomes including pulmonary vascular resistance >3 Wood Units × M2 and PA distortion1, although others have reported that PA augmentation does not increase mortality risk2. More contemporary series suggest a greater adverse consequence of elevated PVR than PA stenosis, at least with respect to mortality before the Fontan operation9,11,27. With regard to hospital LOS, Gray et al. found that the need to place a stent in the RVPAS before the stage II procedure was associated with a 10-day increase in LOS26. The current study found that a clinical diagnosis of branch PA stenosis was the measurement most associated with increased hospital LOS, even more so than detailed angiographic measurements. It is important to note that this model did not include PVR because this measurement was obtained in only 253 of the 393 subjects in the cohort. When PVR was included in the model, branch PA stenosis was no longer associated with increased hospital LOS. It should be noted that PVR cannot be easily measured in subjects with significant branch PA stenosis and it is therefore likely that the latter model excluded the majority of subjects with significant branch PA stenosis. Furthermore, measures of total PA growth (Nakata index or the lower lobe index) were not associated with stage II mortality in a univariate analysis.
We hypothesized that the RVPAS group would have a longer hospital LOS due to smaller PA size and increased number of PA interventions between the Norwood and stage II procedures. Hospital LOS following the stage II procedure tended to be longer in the RVPAS group although this did not reach statistical significance (mean 15.0 vs. 12.5 days and identical medians of 8 days; Wilcoxon P=0.07 and Cox model for time to discharge P=0.14). Therefore, it appears that it is really pulmonary artery anatomy rather than shunt type that is associated with stage II outcomes. Smaller series have shown no difference between the RVPAS and MBTS on outcomes at the stage II procedure23,24 and Januszewska reported a shorter ICU LOS with the RVPAS compared to the MBTS28. While our data suggests that it is PA abnormalities, rather than shunt type alone that is associated with longer LOS, the RVPAS seems likely to be responsible for a larger number of PA abnormalities than the MBTS.
The finding that LOS following the stage II procedure is associated with a longer LOS following the Norwood procedure likely represents a surrogate for a multitude of other problems, such as ongoing heart failure from diminished ventricular function or AVV regurgitation, feeding difficulties, or other complications that result in morbidity. All of these variables were significantly associated with prolonged stage II hospital LOS in the univariate but not the multivariable analysis. Friedman and colleagues found that prolonged LOS following the Norwood procedure was associated with failure to complete the Fontan procedure14 suggesting that these complications have long-term consequences in this population.
Similarly, the association of non-elective timing of the stage II procedure with longer stage II hospital LOS may reflect several anatomic and physiologic complications. Although age at procedure and weight z-score were univariate predictors of LOS in the current study, they were not independent predictors of LOS. This finding is supported by the observations that subjects who underwent non-elective stage II were younger and more likely to have concurrent procedures. Several previous investigators have shown associations between younger age or smaller size and complications resulting in prolonged LOS8,15,24,29 or death16 following the stage II procedure. A comparison of those undergoing the stage II procedure at < 3 months of age to those who were 3 months or older did not identify an independent effect of age when other factors such as preoperative PA pressure and AVV regurgitation were taken into account10.
The stage II procedure in-hospital mortality rate in this multi-center cohort was 4%, with an additional 1% of subjects undergoing heart transplantation before discharge. The majority of reports from single centers have indicated mortality rates less than 2%, and frequently less than 1%4–7,12,25,28. Even those series limited to patients with HLHS6,28 and thus anatomically similar to the current cohort, have generally, although not universally11, demonstrated lower hospital mortality than in the SVR cohort, and Scheurer and colleagues found that HLHS was not associated with any additional risk at the stage II procedure in a series of single ventricle patients12. An important difference between the SVR trial cohort and others is that the SVR cohort is an incident cohort and might therefore be more inclusive than single center reports. Also, publication bias may favor lower mortality rates being published.
Several factors were associated with mortality in a univariate analysis. Of particular note are moderate or greater AVV regurgitation, whether noted on the pre-stage II echocardiogram or as a clinical diagnosis, AVV repair concurrent with the stage II procedure, and an increased RVEDP at the pre-stage II catheterization. AVV regurgitation has been identified as a risk for early10 and late12–14 mortality after the stage II procedure. Hypoplastic left heart syndrome patients with AVV regurgitation may be at higher risk than those with other types of single ventricle anatomy29 although there are also reports that AVV regurgitation is not associated with in-hospital mortality at the stage II2 or Fontan procedures30, unlike the current results. Longer-term follow up of the subjects in the SVR cohort who have undergone AVV repair will be important to determine if the strategy attempting repair of the moderate or severely regurgitant AVV at the time of the stage II procedure is ultimately successful.
There are several limitations to our investigation. Practice variation exists between centers, as was reflected by the significant association of center with hospital LOS. Furthermore, our multivariable model accounted for only 26% of the variance in LOS, suggesting that there are other important issues that impact individual patients and were not measured or analyzed here despite the extensive nature of the SVR database. As previously discussed, many variables evaluated in this study may be confounded with others, such as age at stage II and non-elective timing for stage II. Consequently, some variables deemed important in the multivariable models may only be surrogates for other unmeasured variables. By adjusting for site and using bootstrapping, we have tried to minimize bias and maximize reliability.
In conclusion, we found that the stage II procedure for HLHS and other single right ventricle anomalies is most commonly followed by a relatively short hospital course with a small number of clinically significant complications, and this is unrelated to the shunt type placed at the Norwood operation. Overall hospital mortality following the stage II procedure is low in this generally high-risk population, even though the need for additional surgical and interventional procedures at the time of the stage II hospitalization is relatively high. Patients who require a non-elective stage II procedure, those with a diagnosis of branch PA stenosis and those who had a prolonged LOS following the Norwood procedure are at significant risk for a prolonged hospital LOS following the stage II procedure.
Acknowledgments
Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057, HL109781, HL109737). This work is solely the responsibility of the authors and do not necessarily represent the official views of NHLBI or NIH.
Footnotes
ClinicalTrials.gov number, NCT00115934
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pridjian AK, Mendelsohn AM, Lupinetti FM, Beekman RH, Dick M, Serwer G, et al. Usefulness of the bidirectional Glenn procedure as staged reconstruction for the functional single ventricle. Am J Cardiol. 1993;71:959–62. doi: 10.1016/0002-9149(93)90914-x. [DOI] [PubMed] [Google Scholar]
- 2.Forbess JM, Cook N, Serraf A, Burke RP, Mayer JE, Jonas RA. An institutional experience with second- and third-stage palliative procedures for hypoplastic left heart syndrome: the impact of the bidirectional cavopulmonary shunt. J Am Coll Cardiol. 1997;29:665–70. doi: 10.1016/s0735-1097(96)00529-3. [DOI] [PubMed] [Google Scholar]
- 3.Reddy VM, McElhinney DB, Moore P, Haas GS, Hanley FL. Outcomes after bidirectional cavopulmonary shunt in infants less than 6 months old. J Am Coll Cardiol. 1997;29:1365–70. doi: 10.1016/s0735-1097(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 4.Douglas WI, Goldberg CS, Mosca RS, Law IH, Bove EL. Hemi-Fontan procedure for hypoplastic left heart syndrome: outcome and suitability for Fontan. Ann Thorac Surg. 1999;68:1361–7. doi: 10.1016/s0003-4975(99)00915-7. [DOI] [PubMed] [Google Scholar]
- 5.Kogon BE, Plattner C, Leong T, Simsic J, Kirshbom PM, Kanter KR. The bidirectional Glenn operation: a risk factor analysis for morbidity and mortality. J Thorac Cardiovasc Surg. 2008;136:1237–42. doi: 10.1016/j.jtcvs.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Azakie A, Johnson NC, Anagnostopoulos PV, Akram SM, McQuillen P, Sapru A. Stage II palliation of hypoplastic left heart syndrome without cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2011;141:400–6. doi: 10.1016/j.jtcvs.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lapar DJ, Mery CM, Peeler BB, Kron IL, Gangemi JJ. Short and long-term outcomes for bidirectional glenn procedure performed with and without cardiopulmonary bypass. Ann Thorac Surg. 2012;94:164–70. doi: 10.1016/j.athoracsur.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Menon SC, McCandless RT, Mack GK, Lambert LM, McFadden M, Williams RV, et al. Clinical Outcomes and Resource Use for Infants With Hypoplastic Left Heart Syndrome During Bidirectional Glenn: Summary From the Joint Council for Congenital Heart Disease National Pediatric Cardiology Quality Improvement Collaborative Registry. Pediatr Cardiol. 2013;34:143–8. doi: 10.1007/s00246-012-0403-8. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra SP, Ivy DD, Mitchell MB, Campbell DN, Dines ML, Miyamoto S, et al. Performance of cavopulmonary palliation at elevated altitude: midterm outcomes and risk factors for failure. Circulation. 2008;118(14 Suppl):S177–81. doi: 10.1161/CIRCULATIONAHA.107.751784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrucci O, Khoury PR, Manning PB, Eghtesady P. Outcomes of the bidirectional Glenn procedure in patients less than 3 months of age. J Thorac Cardiovasc Surg. 2010;139:562–8. doi: 10.1016/j.jtcvs.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Alsoufi B, Manlhiot C, Awan A, Alfadley F, Al-Ahmadi M, Al-Wadei A, et al. Current outcomes of the Glenn bidirectional cavopulmonary connection for single ventricle palliation. Eur J Cardiothorac Surg. 2012;42:42–9. doi: 10.1093/ejcts/ezr280. [DOI] [PubMed] [Google Scholar]
- 12.Scheurer MA, Hill EG, Vasuki N, Maurer S, Graham EM, Bandisode V, et al. Survival after bidirectional cavopulmonary anastomosis: analysis of preoperative risk factors. J Thorac Cardiovasc Surg. 2007;134:82–9. doi: 10.1016/j.jtcvs.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Hansen JH, Uebing A, Furck AK, Scheewe J, Jung O, Fischer G, et al. Risk factors for adverse outcome after superior cavopulmonary anastomosis for hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 2011;40:e43–9. doi: 10.1016/j.ejcts.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Friedman KG, Salvin JW, Wypij D, Gurmu Y, Bacha EA, Brown DW, et al. Risk factors for failed staged palliation after bidirectional Glenn in infants who have undergone stage one palliation. Eur J Cardiothorac Surg. 2011;40:1000–6. doi: 10.1016/j.ejcts.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaquiss RDB, Ghanayem NS, Hoffman GM, Fedderly RT, Cava JR, Mussatto KA, et al. Early cavopulmonary anastomosis in very young infants after the Norwood procedure: impact on oxygenation, resource utilization, and mortality. J Thorac Cardiovasc Surg. 2004;127:982–9. doi: 10.1016/j.jtcvs.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 16.Nicolas RT, Hills C, Moller JH, Huddleston CB, Johnson MC. Early outcome after Glenn shunt and Fontan palliation and the impact of operation during viral respiratory season: analysis of a 19-year multi-institutional experience. Ann Thorac Surg. 2005;79:613–7. doi: 10.1016/j.athoracsur.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–92. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohye RG, Gaynor JW, Ghanayem NS, Goldberg CS, Laussen PC, Frommelt PC, et al. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136:968–75. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frommelt PC, Guey LT, Minich LL, Bhat M, Bradley TJ, Colan SD, et al. Does initial shunt type for the Norwood procedure affect echocardiographic measures of cardiac size and function during infancy?: the Single Ventricle Reconstruction trial. Circulation. 2012;125:2630–8. doi: 10.1161/CIRCULATIONAHA.111.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy VM, McElhinney DB, Moore P, Petrossian E, Hanley FL. Pulmonary artery growth after bidirectional cavopulmonary shunt: is there a cause for concern? J Thorac Cardiovasc Surg. 1996;112:1180–90. doi: 10.1016/S0022-5223(96)70131-9. [DOI] [PubMed] [Google Scholar]
- 21.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 22.Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, et al. Interstage mortality after the Norwood procedure: Results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906. doi: 10.1016/j.jtcvs.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballweg JA, Dominguez TE, Ravishankar C, Kreutzer J, Marino BS, Bird GL, et al. A contemporary comparison of the effect of shunt type in hypoplastic left heart syndrome on the hemodynamics and outcome at stage 2 reconstruction. J Thorac Cardiovasc Surg. 2007;134:297–303. doi: 10.1016/j.jtcvs.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 24.Lai L, Laussen PC, Cua CL, Wessel DL, Costello JM, del Nido PJ, et al. Outcomes after bidirectional Glenn operation: Blalock-Taussig shunt versus right ventricle-to-pulmonary artery conduit. Ann Thorac Surg. 2007;83:1768–73. doi: 10.1016/j.athoracsur.2006.11.076. [DOI] [PubMed] [Google Scholar]
- 25.Tanoue Y, Kado H, Boku N, Tatewaki H, Nakano T, Fukae K, et al. Three hundred and thirty-three experiences with the bidirectional Glenn procedure in a single institute. Interact Cardiovasc Thorac Surg. 2007;6:97–101. doi: 10.1510/icvts.2006.138560. [DOI] [PubMed] [Google Scholar]
- 26.Gray RG, Minich LL, Weng HY, Heywood MC, Burch PT, Cowley CG. Effect of Endovascular Stenting of Right Ventricle to Pulmonary Artery Conduit Stenosis in Infants With Hypoplastic Left Heart Syndrome on Stage II Outcomes. Am J Cardiol. 2012;110:118–23. doi: 10.1016/j.amjcard.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 27.Hussain A, Arfi AM, Hussamuddin M, Haneef AA, Jamjoom A, Al-Ata J, et al. Comparative outcome of bidirectional Glenn shunt in patients with pulmonary vascular resistance > or = 3.5 woods units versus. Am J Cardiol. 2008;102:907–12. doi: 10.1016/j.amjcard.2008.04.063. [DOI] [PubMed] [Google Scholar]
- 28.Januszewska K, Kołcz J, Mroczek T, Procelewska M, Malec E. Right ventricle-topulmonary artery shunt and modified Blalock-Taussig shunt in preparation to hemi- Fontan procedure in children with hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 2005;27:956–61. doi: 10.1016/j.ejcts.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Anderson JB, Beekman RH, Border WL, Kalkwarf HJ, Khoury PR, Uzark K, et al. Lower weight-for-age z score adversely affects hospital length of stay after the bidirectional Glenn procedure in 100 infants with a single ventricle. J Thorac Cardiovasc Surg. 2009;138:397–404. doi: 10.1016/j.jtcvs.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Kerendi F, Kramer ZB, Mahle WT, Kogon BE, Kanter KR, Kirshbom PM. Perioperative risks and outcomes of atrioventricular valve surgery in conjunction with Fontan procedure. Ann Thorac Surg. 2009;87:1484–8. doi: 10.1016/j.athoracsur.2009.02.059. [DOI] [PubMed] [Google Scholar]