Abstract
γ-Aminobutyric acid type A receptors (GABAA-Rs) are considered to be the primary molecular targets of injectable anesthetics such as propofol, etomidate and the neurosteriod, alphaxalone. A number of studies have sought to understand the specific GABAA-R subtypes involved in the mechanism of action of these three drugs. Here, we investigated the role of α4-subunit containing GABAA-Rs in the neurobehavioral responses to these drugs. Drug responses in α4 subunit knockout (KO) mice were compared to wild type littermate controls. While etomidate and propofol are currently used as injectable anesthetics, alphaxalone belongs to the class of neurosteroid drugs having anesthetic effects. Low dose effects of etomidate and alphaxalone were studied using an open field assay. The moderate and high dose effects of all three anesthetics were measured using the rotarod and loss of righting reflex assays, respectively. The locomotor stimulatory effect of alphaxalone was reduced significantly in α4 KO mice compared to WT controls. Neither the low dose sedating effect of etomidate, nor the moderate/high dose effect of any of the drugs differed between genotypes. These results suggest that α4 subunit-containing GABAA-Rs are required for the low dose, locomotor stimulatory effect of alphaxalone but are not required for the sedating effect of etomidate or the moderate/high dose effects of etomidate, propofol or alphaxalone on motor ataxia and loss of righting reflex.
Keywords: γ-aminobutyric acid type A receptors, knockout mice, anesthetics
Background
Despite their wide clinical usage, the mechanism of action of injectable anesthetic drugs such as etomidate, propofol and alphaxalone is not completely known. Of prime importance for mediating the effects of anesthetics are γ-aminobutyric acid type A receptors (GABAA-Rs). GABAA-Rs are pentameric ligand gated chloride ion channels that mediate the majority of inhibitory neurotransmission in the brain. A functional receptor is assembled from amongst 19 distinct subunits, α1-6, β1-3, γ1-3, δ, ε, π, θ and ρ1-3 [1]. Most GABAA-Rs are composed of 2α, 2β and a γ or δ subunit [2,3]. This diversity in GABAA-R subunit composition results in substantial anatomical, functional and pharmacological heterogeneity. The contributions of particular GABAA-R subtypes to the behavioral endpoints produced by injectable anesthetics are gradually being understood- largely through studies using genetically engineered mouse lines. For example, GABAA-R N265M β3 subunit knockin (KI) studies revealed that β3-containing receptors are required for etomidate and propofol –induced immobility, respiratory depression and hypnosis (in part) [4],[5]. Similar studies on β2 N265S KI mice revealed that β2-containing GABAA-Rs mediate etomidate and propofol-induced sedation, hypothermia, heart rate depression, and hypnosis [6], [7,8]. These results are consistent with the studies of β2 and β3 knockout (KO) mice [9,10],[11]. Similarly, GABAA-R δ subunit KO mice revealed that δ-containing receptors are required for many neurosteroid-induced behavioral effects [12].
However, the role of the obligate α subunits partnering with β and δ subunits in the mechanisms of anesthetic action is largely unknown. δ subunit containing receptors are mainly extrasynaptic and are uniquely sensitive to low levels of GABA [13-15]. Because they are sensitive to non-saturating levels of GABA, extrasynaptic receptors are capable of greater potentiation in the presence of anesthetics [16]. In their extrasynaptic location, δ subunits pair with β2 or β3 subunits and α4 or α6 subunits [12,17-19]. Thus, α4βδ and α6βδ receptor combinations are good candidates for modulation by anesthetics like etomidate and propofol.
While α6-containing receptors show restricted localization in the cerebellum, α4-containing receptors are robustly expressed in brain regions that likely contribute to anesthetic-induced behaviors such as the thalamus, hippocampus, and cortex. On the subject of endogenous modulators, studies have also shown that fluctuating levels of endogenous steroids such as that encountered during the estrous cycle, pregnancy or stress influence the levels of α4-δ containing receptors [20,21]. Further, deficiency in GABAergic signaling during or after pregnancy can predispose individuals to mood instabilities during the post-partum period [22]. Consistent with this, δ KO mice showed abnormal maternal behaviors and depressive tendencies during the post-partum phase [23]. Such changes in receptor levels are considered important for maintaining normal neuronal excitability in the presence of cycling neurosteroid levels. Thus, α4 containing receptors may also be considered important in the actions of neurosteroid-based drugs.
A 2009 study by Meera et al. showed that etomidate, propofol and the neurosteroid, 3α, 21-dihydroxy-5α-pregnan-20-one (THDOC) increased peak currents with GABA similarly on α4β3 and in α4β3δ recombinant receptors indicating that increases in GABA efficacy are not solely due to δ subunit incorporation [24]. Evidence from other studies also supports the notion that anesthetic actions of GABAA-Rs may be determined by binding sites formed between the interface of αβ subunits [25].
Evidence supporting the role of α4 subunit-containing receptors in the actions of injectable anesthetics and neurosteroids has also been obtained from α4 KO studies. α4 KO mice were largely insensitive to cellular and behavioral responses to the sedative-hypnotic THIP [26] and were also resistant to isoflurane-induced amnesia but not immobility [27]. Similarly, the role of α4-containing receptors in the effects of neurosteroids was substantiated by cellular characterization of the α4 KO. Electrophysiological recordings from the dentate gyrus granule neurons of the hippocampus of α4 KO mice revealed markedly reduced cellular responses to the synthetic neurosteroid anesthetic, alphaxalone [28].
Therefore, we hypothesized that α4-containing receptors play a role in the actions of etomidate, propofol and the neurosteroid, alphaxalone and sought to understand behavioral effects of these classes of drugs in the α4 KO. In the work described below, we tested this hypothesis by comparing wild type (WT) control and α4 KO mice on different behavioral endpoints in response to the injectable anesthetics etomidate, propofol and the prototypical neurosteroid drug, alphaxalone.
Methods
Subjects
The committee on animal care and use at the University of Pittsburgh approved all experimental protocols. Homozygous α4 KO and WT littermates were created by interbreeding breeding heterozygous mice that possessed one functional and one nonfunctional copy of the GABAA-R α4 subunit allele as described previously [26]. Genotyping was performed by Southern Blot analysis of tail DNA as previously described [26]. Mice were of a mixed genetic background of C57BL/6J and Strain 129S1/X1 and belonged to the F3/4 generations. Mice were group housed, kept on a twelve hour alternating light-dark cycle and allowed ad libitum access to food and water. Due to limited animal numbers, analysis of results by gender was not performed unless specified below. In most cases, cohorts of mice were naïve to drug treatment before each experiment. However, in some experiments, the same cohort of mice was subjected to two experiments but with a clearance period of at least 2 weeks between experiments. A 2-week interval between drug experiments is thought to return any transient expression changes back to baseline levels.
Drug Solutions
Etomidate (Amidate, 2 mg/ml, Hospira Inc., Lake Forest, IL), propofol (Diprivan, 10 mg/ml, Astra Zeneca, Wilmington, DE), and alphaxalone (Cat. No. P5052, Sigma Aldrich, St.Louis, MO) were used. Alphaxalone was solubilized in 22.5% β-hydroxycyclodextrin (HBC) (Cat. No. H107, Sigma Aldrich, St.Louis, MO) and sonicated to arrive at a stock concentration of 7 mg/ml. All injections were administered intraperitoneally (i.p.) except propofol, which was administered by the retro-orbital (r.o) route. The different doses of each drug were chosen based on prior experience or preliminary testing where applicable.
Anesthetic-induced changes in locomotor behavior
Alphaxalone was diluted to 1.5 mg/ml. Following preliminary testing, the locomotor stimulatory effect of alphaxalone (15 mg/kg) or vehicle (22.5% HBC in saline) was tested by placing mice in the center of an open field 10 min after injection. The open field testing chambers were plexiglass walled arenas (43.2 cm × 43.2 cm × 30.5 cm) located within sound attenuating cubicles in which mouse activity was automatically tracked (Med Associates, St. Albans, VT). Locomotor activity was assessed by measuring total distance covered. Anxiety-like behavior was assessed by comparing the percentage of distance traveled in the center zone over total distanced traveled. Mice that are more anxious would be expected to spend less time in the center zone compared to the periphery. The center zone was demarcated as the central square of the open field (11.25 × 11.25 cm). Both males and females were tested. Data were analyzed by two-way ANOVA with genotype and treatment as effects followed by Fisher’s Protected Least Significant Difference (PLSD) post hoc tests.
With etomidate, a low dose locomotor stimulatory effect was not observed. Instead, pilot studies with numerous doses of etomidate indicated that a 3 mg/kg dose reliably produced a measurable sedative effect that could be studied using the open field setup. Etomidate was diluted with saline to a concentration of 0.3 mg/ml. The sedative effects of a 3 mg/kg dose of etomidate were evaluated for 10 minutes. Male mice were placed in the testing room one day before the experiment. On the morning of the experiment, mice were weighed, then injected with etomidate (i.p., 0.01 ml/g body weight) and placed separately in clean cages. Five minutes later, mice were placed in the open field chambers for automated recording of distance traveled over a 10 minute period. Because vehicle control (i.e. saline) scores did not differ between genotypes in this assay [26], only drug-induced behavior was measured between genotypes. Data were analyzed by unpaired t-test.
Recovery from anesthetic-induced motor ataxia
Next, mice were tested for their ability to recover from motor ataxia induced by alphaxalone, etomidate, and propofol. All training and testing was performed on a fixed speed rotarod (Ugo Basile 7650, Varese, Italy) at 8 RPM. With each drug, the degree and duration of ataxia were expected to differ based on the pharmacologic and pharmacokinetic profile of each drug. Therefore, while the fixed speed rotarod format was chosen, the time criteria and the inter-trial duration were modified for each drug based on pilot trials. For each experiment, area under the curve (AUC) was measured as the area between baseline performance and the impairment produced by the drug over the time course of the experiment. AUC’s were compared by one-way ANOVA with genotype as the main effect.
Alphaxalone
On the day of the experiment, male and female mice were subjected to training trials on a fixed speed rotarod. At least 4 training trials were administered at 10 min intervals so that mice achieved a criterion of 180 sec on the rotarod. Mice that did not achieve the criterion at least three consecutive times were excluded from the assay. Alphaxalone was solubilized in 22.5% β-hydroxycyclodextrin and injected at 50 mg/kg (i.p., 0.01 ml/g body weight). Post-injection, mice were tested every 10 min until recovery to baseline performance (180 sec) was achieved.
Etomidate
Male mice were trained on the day of the test to a criterion of 120 sec on fixed speed rotarod. Training trials were 20 min apart and at least 4 such trials were administered. Mice that did not achieve the criterion at least three consecutive times were excluded from the assay. Following training, etomidate was injected (i.p., 0.01 ml/g body weight) at 20 mg/kg. Mice lost their righting reflex briefly and recovered between 20-25 minutes. One KO mouse that was unimpaired with etomidate injection was excluded from the analysis. After injection, mice were tested on the rotarod every 20 mins for 3 hours.
Propofol
Mice of both sexes were trained on the previous day of the experiment to a criterion of 100 sec. Because propofol is a short acting drug, each mouse was given at least 4 trials, spaced 5 mins apart, to ensure that the task was learned. Once training was complete, mice were housed overnight in the testing room in their home cages. On the test day, mice were subjected to 2-3 trials to confirm compliance to criteria. Subjects that failed this test were excluded from the assay. Following this, propofol was administered at 25 mg/kg, (r.o.; 0.005 ml/g body weight) by diluting the clinical preparation with saline to a concentration of 5 mg/ml. Mice briefly lost their righting reflex. Mice were placed on the rotarod at intervals of 5 minutes until recovery to baseline performance (100 sec) was regained.
Anesthetic-Induced Loss of Righting Reflex (LORR)
Age-matched mice (8-20 weeks) of both sexes were injected with alphaxalone (70 mg/kg, i.p., 0.01 ml/g body weight,), etomidate (20 mg/kg, i.p., 0.01 ml/g body weight), or propofol (40 mg/kg, r.o., 0.004 ml/g body weight) for the LORR assay. Immediately after they lost their righting reflex, mice were placed supine on a V-shaped plexiglass trough until recovery. A heat lamp was used to ensure normothermia throughout the experiment. Endpoint of time to return of righting reflex was determined by ensuring that a mouse was able to right itself consecutively three times within 30 seconds. For etomidate-induced LORR, data were analyzed by two-way ANOVA with genotype and sex as main effects. For other LORR results, data were analyzed by unpaired t-test.
Results
A low dose of alphaxalone has no effect in α4 KO mice
To understand the role of the GABAA-R α4 subunit in anesthetic-induced changes in locomotor activity, WT and KO mice were compared for response to low doses of alphaxalone (15 mg/kg) or etomidate (3 mg/kg) in an open field assay.
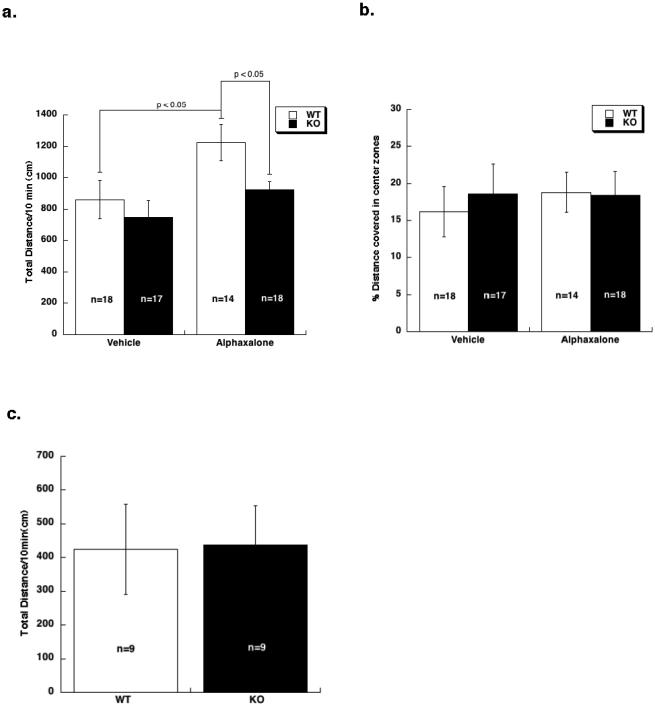
With alphaxalone, analysis of total distance traveled during the 10 min test period (Fig. 1A) revealed a significant main effect of treatment [two-way ANOVA; F(1,63) = 7, p < 0.05] and genotype [F(1,63) = 4, p<0.05], but no interaction between treatment and genotype. Subsequent pair-wise comparisons revealed that baseline activity in response to vehicle injections did not differ between genotypes. In contrast, alphaxalone stimulated locomotor activity in WT (p< 0.05) but not KO mice. Additionally, distance traveled by WT mice in response to alphaxalone was greater than the distance traveled by KO mice (p< 0.05). Thus, alphaxalone produced a locomotor stimulatory effect in WT but not KO mice.
Fig 1. The low dose effect of alphaxalone but not etomidate was absent in α4 KO mice.
a. Total distance traveled by WT and α4 KO mice in response to a low dose of alphaxalone (15 mg/kg) over a 10 min period. Genotypes did not differ in response to vehicle injection. Alphaxalone stimulated locomotor activity compared to vehicle in WT (p < 0.05) but not KO mice. b. Percent distance covered in the center zone of the open field by WT and α4 KO mice. No differences were observed between vehicle-treated and alphaxalone-treated WT and α4 KO animals. c. Total distance traveled in response to a low dose of etomidate (3 mg/kg). No differences were observed between WT and KO mice. Data are expressed as mean ± SEM.
The distance covered in the center zone of the activity chambers was also compared between genotypes (Fig. 1B). Mice that are more anxious would be expected to spend less time in the center zone compared to the periphery. This parameter is regarded as an indicator of anxiety-like behavior in rodents [29,30]. Two-way ANOVA did not reveal an effect of treatment, genotype, or an interaction. Thus, a 15 mg/kg dose of alphaxalone altered ambulatory behavior but did not alter anxiety-like behavior on this assay.
Next, to understand if low doses of etomidate produced similar differences as alphaxalone between WT and α4 KO mice, we conducted several pilot experiments using low doses of etomidate on an open field assay. Pilot experiments revealed that a 3 mg/kg dose of etomidate produced a significant sedating effect in the open field assay. Because saline-treated mice did not differ by genotype on total distance travelled in previous experiments with this strain of mice [26], only etomidate-treated WT and KO were tested. As shown in Fig. 1C, total distance covered in response to etomidate did not differ between genotypes.
Moderate dose effects of anesthetics or of the neurosteroid alphaxalone are unchanged in the α4 KO
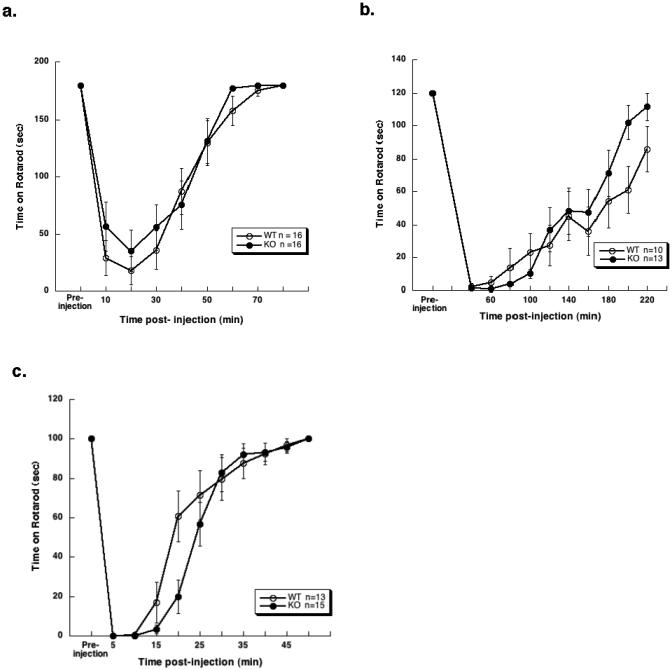
To test if the GABAA-R α4 subunit was required for the motor ataxic effects of injectable anesthetics, WT and KO mice were compared for motor impairment induced by a moderately high dose of alphaxalone (50 mg/kg), etomidate (20 mg/kg), or propofol (25 mg/kg) on a fixed speed rotarod (Fig. 2A-C, respectively). In all experiments, a one-way ANOVA analysis of AUC for performance on rotarod with etomidate, propofol, and alphaxalone showed no differences between the genotypes.
Fig 2. Anesthetic-induced motor ataxia assessed with a fixed speed (8 rpm) rotarod did not differ between WT and α4 KO mice.
No differences between genotypes were observed in response to any of the drugs tested on this assay. a. Alphaxalone (50 mg/kg)- b. etomidate (20 mg/kg)- and c. propofol (25 mg/kg)-induced motor ataxia. Each point represents mean time on rotarod ± SEM. One KO mouse that was not impaired with etomidate treatment was eliminated from analysis. Similarly, for the alphaxalone assay, two WT mice that were unimpaired with alphaxalone treatment were eliminated from the analysis.
High dose effects of anesthetics or of the neurosteroid alphaxalone are unchanged in the α4 KO
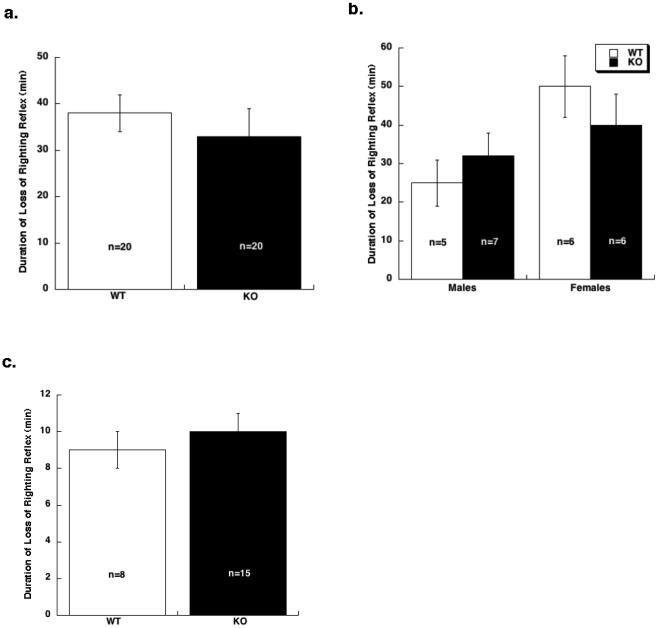
Finally, the role of GABAA-R α4 subunit in the sedative/hypnotic response induced by high doses of injectable anesthetics, was studied using the LORR assay. WT and KO mice were compared for the duration of LORR induced by alphaxalone (70 mg/kg), etomidate (20 mg/kg), or propofol (40 mg/kg). Responses to alphaxalone and propofol, respectively, did not differ between genotypes (Figure 3A and 3C). The duration of LORR in response to etomidate was influenced by sex (two-way ANOVA; F (1,20) = 5.4; p < 0.05). Hence, males and females were analyzed separately (Fig. 3B). However, genotypes did not differ in response to etomidate within either sex.
Fig 3. Anesthetic-induced LORR did not differ between WT and α4 KO mice.
a. Alphaxalone (70 mg/kg)-induced LORR did not differ between WT and α4 KO mice. b. Etomidate (20 mg/kg) –induced LORR was influenced by sex (two-way ANOVA; p < 0.05). Therefore, male and females were analyzed separately. However, no differences were observed between genotypes within either sex. c. Propofol (40 mg/kg)-induced LORR did not differ between genotypes. Data are expressed as mean ± SEM.
Discussion
The goal of these studies was to understand the contribution of α4-containing GABAA-Rs in mediating the behavioral effects of the injectable anesthetics alphaxalone, etomidate and propofol. Anesthetic-induced changes in locomotor activity, motor impairment, and LORR were compared between genotypes. The main finding from these studies was that KO mice were insensitive to the locomotor stimulatory effect of a low dose of alphaxalone compared to WT. All other behavioral responses measured in response to alphaxalone, etomidate and propofol did not differ between genotypes.
Neurosteroids are thought to exert their behavioral effects largely via δ-containing GABAA-Rs [12,31]. Studies of δ subunit KO mice demonstrated that this subunit was required for alphaxalone- and pregnanolone-induced LORR. Additionally, the anxiolytic and pro-absence seizure effects of ganaxalone were reduced in δ KO mice. However, the importance of individual α subunits for clinically relevant behavioral effects of neurosteroids has not been previously examined. It is well established that δ-containing GABAA-Rs almost always include α4 or α6 subunits [12,17-19], although there are rare populations of δ-containing receptors in specific brain regions devoid of α4 and α6 [32]. Because α6 subunit-containing receptors are restricted to cerebellar granule cells, we hypothesized that the more widely expressed α4 receptors are key α subunits of the δ-containing receptors mediating neurosteroid-induced behavioral effects. In support of this hypothesis we also previously reported that hippocampal dentate gyrus neurons from α4 KO mice showed reduced current potentiation with alphaxalone compared to WT controls [28]. Therefore, we predicted that KO of α4 would reduce behavioral sensitivity to alphaxalone.
Consistent with our hypothesis, α4 KO mice did not respond to the locomotor stimulatory effect of a low dose of alphaxalone in an open field assay. This result indicates that α4-containing receptors are critical for the low dose locomotor stimulatory effect of alphaxalone. In contrast to our expectations, the moderate/high dose effects of alphaxalone on motor incoordination and LORR were unchanged between WT and KO mice suggesting that α4-containing receptors are not required for these effects. This result is especially surprising considering the reduction in sensitivity of δ subunit KO mice to neurosteroid-induced LORR.
Recent photoaffinity labeling experiments with azietomidate indicate that etomidate binds to specific amino acids at the α-β interface [12,25]. In addition, a study comparing the effect of etomidate on recombinant α4β3 and α4β3δ receptors showed that etomidate-induced enhancement was similar in both kinds of receptors [24]. Thus, increases in GABA efficacy by anesthetics are not necessarily due to the δ subunit alone. Consistent with this observation, the δ KO mice did not display differences in response to etomidate or propofol in LORR assays [12]. To ascertain if the α4 subunit played a role in the behavioral effect of etomidate, we compared LORR, motor ataxia and sedation between WT and KO mice. These effects were unchanged between genotypes suggesting that α4 subunits are not critical for etomidate effects on behavior. In our hands, however, a sex difference was observed within each genotype in response to etomidate-induced LORR. This result is consistent with a previous study where α1 GABAA-R subunit KO female (but not male) mice were slightly resistant to etomidate-induced LORR [33]. However, others have not observed this difference [10]. Given that these effects were observed in WT as well as α4 KO mice, we speculate that differences between sexes in response to etomidate may be a result of underlying differences in levels of circulating neurosteroids or changes in GABAA-R subunit expression across the estrus cycle [e.g.,34]. Taking into account results from α5 KO mice, where etomidate-induced LORR, motor ataxia and sedation were also unchanged between genotypes [35], together, these data indicate that the major α subunit(s) responsible for mediating etomidate-induced LORR, motor ataxia, and sedation is likely α2, α3, and/or α6. Furthermore, since α2 and α3 frequently partner with β3 and γ2 subunits to form α2β3γ2 and α3β3γ2 receptor subtypes, and β3 N265M knockin mice demonstrate that β3 is required for etomidate-induced immobility and hypnosis [5], these receptor subtypes seem most likely to mediate these effects of etomidate.
Next, propofol-induced LORR and motor ataxia were compared in α4 KO and WT. Because WT and KO mice did not differ in their response to propofol, these studies indicate that α4-containing GABAA-Rs are not required for these effects of propofol. Previous work on the actions of propofol has centered on the β2 and β3 subunits. Sites N289, N290 and N265 of β2 and β3 subunits are thought to be critical for the effects of propofol through work on recombinant receptors expressed in xenopus laevis oocytes, HEK cells as well as in mutant mouse models [36-38,5]. We previously reported that α1 KO mice were normally sensitive to propofol-induced LORR [33]. The studies reported here on α4 KO mice indicate that α4-containing GABAA-Rs are also not required for the propofol-induced motor ataxia or LORR. Further studies on α2, α3 or α6 mutant mouse models will have to be conducted to ascertain if actions of propofol are dependent on a particular α subunit.
A noteworthy observation from studies on GABAA-R α4 subunit KO mice, is the emerging common theme that the α4 subunit is only required for response to very low doses/concentrations of some GABAergic drugs. α4-containing receptors have previously been referred to as the ‘one glass of wine’ receptors due to their unique sensitivity to low doses of ethanol [39,40]. As reported here, α4 KO mice were less sensitive to low dose neurosteroid-induced locomotor stimulation but were normally sensitive to moderate/high dose neurosteroid-induced motor ataxia and LORR. Similarly, for the volatile anesthetic isoflurane, α4 KO mice were substantially less sensitive to low concentrations that produced amnesia, slightly less sensitive to higher concentrations that produced LORR, and normally sensitive to very high concentrations that produced the equivalent of surgical immobility [27]. Finally, α4 KO mice were resistant to low dose ethanol-induced cognitive impairment but not to moderate/high doses of ethanol [41,42]. Given the sensitivity of extrasynaptic α4 containing receptors to ambient levels of GABA, it is not surprising to imagine that these receptors are more readily modulated at low doses of anesthetic drugs. Higher doses of the same drugs presumably activate synaptic receptors to enforce the full effects of the drug thus rendering the contribution of GABA α4 receptors, at higher concentrations, infinitesimal. The exception to these observations is the behavioral response to the selective extrasynaptic GABAA-R agonist, gaboxadol (a.k.a. THIP) [26]. α4 KO mice were largely insensitive to even high doses of this drug. These results indicate that for many (but not all) GABAergic drugs, α4-containing GABAA-R may only be important for low dose effects.
One caveat to these studies that cannot be ignored is that the mice studied were global, constitutive KOs that may harbor alterations to compensate for the lack of α4, which may mask the normal endogenous role of α4 in behavioral responses to anesthetics. Such compensatory changes are not uncommon in response to the constitutive lack of particular receptors, as is the case with δ KO mice [43]. In the α4 KO mice, as well, altered levels of other GABAA-R subunits have been observed [44]. Additionally, we have observed that while α4 KO reduces ethanol potentiation of tonic inhibition, synaptic responses to ethanol are surprisingly increased [45]. These studies are indicative of compensatory changes occurring in the α4 KO mice. However, changes in levels of all known GABA subunits in the α4 KO mice have not yet been quantified. Thus, how these and other yet to be discovered, examples of compensation in α4 KO mice impact responses to anesthetics remains to be determined.
In summary, we report that all injectable anesthetic-induced behavioral responses tested were similar between WT and GABAA-R α4 subunit KO mice with the exception that KO animals were less sensitive to low dose alphaxalone-induced locomotor stimulation. Therefore, we conclude that α4-containing receptors are not required for most injectable anesthetic-induced behavioral responses, but may selectively mediate low dose responses to neurosteroid anesthetics like alphaxalone.
Acknowlegements
The authors would like to thank Erik Bennet and Carolyn Ferguson for expert technical assistance.
References
- 1.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acid A receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 2.Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Mohler H. Five subtypes of type A γ-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci USA. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao M, Sonner JM, Husain SS, Miller KW, Jurd R, Rudolph U, Eger EI., 2nd R (+) etomidate and the photoactivable R (+) azietomidate have comparable anesthetic activity in wild-type mice and comparably decreased activity in mice with a N265M point mutation in the γ-aminobutyric acid receptor β3 subunit. Anesth Analg. 2005;101:131–135. doi: 10.1213/01.ANE.0000153011.64764.6F. [DOI] [PubMed] [Google Scholar]
- 5.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor β3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds DS, Rosahl TW, Cirone J, O’Meara GF, Haythornthwaite A, Newman RJ, Myers J, Sur C, Howell O, Rutter AR, Atack J, Macaulay AJ, Hadingham KL, Hutson PH, Belelli D, Lambert JJ, Dawson GR, McKernan R, Whiting PJ, Wafford KA. Sedation and anesthesia mediated by distinct GABAA receptor isoforms. J Neurosci. 2003;23:8608–8617. doi: 10.1523/JNEUROSCI.23-24-08608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeller A, Arras M, Lazaris A, Jurd R, Rudolph U. Distinct molecular targets for the central respiratory and cardiac actions of the general anesthetics etomidate and propofol. FASEB J. 2005;19:1677–1679. doi: 10.1096/fj.04-3443fje. [DOI] [PubMed] [Google Scholar]
- 8.Cirone J, Rosahl TW, Reynolds DS, Newman RJ, O’Meara GF, Hutson PH, Wafford KA. γ-Aminobutyric acid type A receptor β2 subunit mediates the hypothermic effect of etomidate in mice. Anesthesiology. 2004;100:1438–1445. doi: 10.1097/00000542-200406000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Quinlan JJ, Homanics GE, Firestone LL. Anesthesia sensitivity in mice that lack the β3 subunit of the γ-aminobutyric acid type A receptor. Anesthesiology. 1998;88:775–780. doi: 10.1097/00000542-199803000-00030. [DOI] [PubMed] [Google Scholar]
- 10.Blednov YA, Jung S, Alva H, Wallace D, Rosahl T, Whiting PJ, Harris RA. Deletion of the α1 or β2 subunit of GABAA receptors reduces actions of alcohol and other drugs. J Pharmacol Exp Ther. 2003;304:30–36. doi: 10.1124/jpet.102.042960. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson C, Hardy SL, Werner DF, Hileman SM, Delorey TM, Homanics GE. New insight into the role of the β3 subunit of the GABAA-R in development, behavior, body weight regulation, and anesthesia revealed by conditional gene knockout. BMC Neurosci. 2007;8:85. doi: 10.1186/1471-2202-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mihalek RM, Banjeree PK, Korpi E, Quinlan JJ, Firestone LL, Mi Z-P, Lagenaur C, Tretter V, Sieghart W, Anagnostaras S, Sage JR, Fanselow M, Guidotti A, Spigelman I, Li A, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in GABA type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophys. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- 16.Orser BA. Extrasynaptic GABAA receptors are critical targets for sedative-hypnotic drugs. J Clin Sleep Med. 2006;2:S12–18. [PubMed] [Google Scholar]
- 17.Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maguire J, Mody I. Steroid hormone fluctuations and GABAAR plasticity. Psychoneuroendocrinology. 2009;34(Suppl 1):S84–90. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen H, Smith SS. Plasticity of the α4βδ GABAA receptor. Biochem Soc Trans. 2009;37:1378–1384. doi: 10.1042/BST0371378. [DOI] [PubMed] [Google Scholar]
- 22.Maguire J, Ferando I, Simonsen C, Mody I. Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci. 2009;29:9592–9601. doi: 10.1523/JNEUROSCI.2162-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maguire J, Mody I. GABAAR plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meera P, Olsen RW, Otis TS, Wallner M. Etomidate, propofol and the neurosteroid THDOC increase the GABA efficacy of recombinant α4β3δ and α4β3 GABAA receptors expressed in HEK cells. Neuropharmacology. 2009;56:155–160. doi: 10.1016/j.neuropharm.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li GD, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci. 2006;26:11599–11605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rau V, Iyer SV, Oh I, Chandra D, Harrison N, Eger EI, 2nd, Fanselow MS, Homanics GE, Sonner JM. γ-Aminobutyric acid type A receptor α4 subunit knockout mice are resistant to the amnestic effect of isoflurane. Anesth Analg. 2009;109:1816–1822. doi: 10.1213/ANE.0b013e3181bf6ae6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang J, Suryanarayanan A, Chandra D, Homanics GE, Olsen RW, Spigelman I. Functional consequences of GABAA receptor α4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol Clin Exp Res. 2008;32:19–26. doi: 10.1111/j.1530-0277.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 29.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 30.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 31.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- 33.Kralic JE, Wheeler M, Renzi K, Ferguson C, O’Buckley TK, Grobin AC, Morrow AL, Homanics GE. Deletion of GABAA receptor α1 subunit-containing receptors alters responses to ethanol and other anesthetics. J Pharmacol Exp Ther. 2003;305:600–607. doi: 10.1124/jpet.102.048124. [DOI] [PubMed] [Google Scholar]
- 34.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 35.Cheng VY, Martin LJ, Elliott EM, Kim JH, Mount HT, Taverna FA, Roder JC, Macdonald JF, Bhambri A, Collinson N, Wafford KA, Orser BA. α5 GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26:3713–3720. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegwart R, Jurd R, Rudolph U. Molecular determinants for the action of general anesthetics at recombinant α3β2γ2 γ-aminobutyric acid(A) receptors. J Neurochem. 2002;80:140–148. doi: 10.1046/j.0022-3042.2001.00682.x. [DOI] [PubMed] [Google Scholar]
- 37.Siegwart R, Krahenbuhl K, Lambert S, Rudolph U. Mutational analysis of molecular requirements for the actions of general anaesthetics at the γ-aminobutyric acidA receptor subtype, α1β2γ2. BMC Pharmacol. 2003;3:13. doi: 10.1186/1471-2210-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonsson Fagerlund M, Sjodin J, Krupp J, Dabrowski MA. Reduced effect of propofol at human α1β2(N289M)γ2 and α2β3(N290M)γ2 mutant GABAA receptors. Br J Anaesth. 2010;104:472–481. doi: 10.1093/bja/aeq023. [DOI] [PubMed] [Google Scholar]
- 39.Wallner M, Hanchar HJ, Olsen RW. Low-dose alcohol actions on α4β3δ GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci USA. 2006;103:8540–8545. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to α4/6β3δ GABAA receptors. Proc Natl Acad Sci USA. 2006;103:8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cushman JD, Moore MD, Jacobs NS, Olsen RW, Fanselow MS. Behavioral pharmacogenetic analysis on the role of the α4 GABAA receptor subunit in the ethanol-mediated impairment of hippocampus-dependent contextual learning. Alcohol Clin Exp Res. 2011;35:1948–1959. doi: 10.1111/j.1530-0277.2011.01546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandra D, Werner DF, Liang J, Suryanarayanan A, Harrison NL, Spigelman I, Olsen RW, Homanics GE. Normal acute behavioral responses to moderate/high dose ethanol in GABAA receptor α4 subunit knockout mice. Alcohol Clin Exp Res. 2008;32:10–18. doi: 10.1111/j.1530-0277.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- 44.Suryanarayanan A, Liang J, Meyer EM, Lindemeyer AK, Chandra D, Homanics GE, Sieghart W, Olsen RW, Spigelman I. Subunit compensation and plasticity of synaptic GABAA receptors induced by ethanol in α4 subunit knockout mice. Front Neurosci. 2011;5:110. doi: 10.3389/fnins.2011.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]