Summary
Proteomic analysis is important in the examination of complex extracellular matrices such as the vitreous where several tissues both inside the eye and remote to the eye contribute to the diseased state. In these cases, genomic analysis of local tissue gene expression may be insufficient or misleading. By switching the emphasis from diagnostic biomarkers to biomarkers with therapeutic potential, we can create rationale therapeutic strategies for blinding vitreoretinal diseases. The same strategy can extend to other ocular tissues. We are just beginning to understand the molecular constituents of the vitreous in health and disease, and translational proteomics may more effectively direct efforts to cure blindness.
The vitreous is an extracellular matrix gel that covers the retina, ciliary body, and lens and fills 80% of the inner eye [1]. It is optically clear and estimated to be over 98% water [2]. The remaining molecular constituents include proteins, polysaccharides, proteoglycans, and metabolites, but the composition and function of this fraction is poorly understood [3]. In utero, the vitreous undergoes major tissue changes important for normal eye development [2]. After birth, the physiologic function is thought to be comparatively less important. The vitreous undergoes a natural, age-related liquefaction and is routinely removed during surgery to treat various retinal diseases [4].
The physical interaction between the vitreous and retina has been studied extensively, since many retinal diseases involve the vitreoretinal interface [2]. Surgical therapy for vitreomacular traction, macular hole, retinal detachment, epiretinal membrane, proliferative diabetic retinopathy, and proliferative vitreoretinopathy has benefited from the rapid advances in surgical instrumentation and techniques to dissect vitreous from its intraocular interfaces. Medical therapy for these diseases has lagged but could benefit from recent advances in proteomic analyses if applied to the vitreous. The recent approval of intravitreal ocriplasmin injections to relieve vitreomacular traction and close macular holes through proteolytic cleavage of vitreous proteins supports further study of pharmacological targeting of vitreous proteins. [5]. In this article we discuss challenges and opportunities in translational vitreous proteomics. The necessary infrastructure to collect and store vitreous specimens in biorepositories is described in our accompanying review.
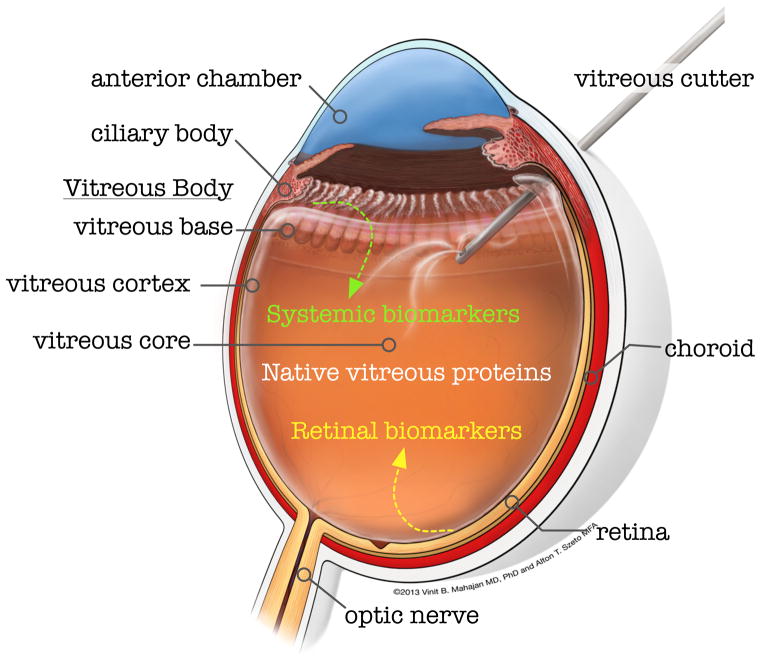
Normal vitreous is a non-homogenous tissue that must be divided into distinct anatomical regions for therapeutic dissection during surgery (Figure 1). The vitreous base, for example, is impossible to separate from the ciliary body, while the vitreous cortex can be peeled off the retina. The vitreous cortex is elastic and more adherent at the optic nerve, within the macula and along blood vessels. Many diseases are specifically localized to the vitreous cortex or base. However, only the vitreous core is sampled in proteomic studies, because it is more liquid and less adherent to the surrounding structures. To address this issue, our lab dissected the vitreous substructures in normal postmortem eyes and found protein compositional differences [6]. Obtaining samples from these structures in diseased eyes will be more complex, but it is likely to yield enriched samples for proteomic analysis of disorders such as pars planitis, retinal detachment, and epiretinal membrane.
Figure 1. Cross sectional image of the human eye.
The vitreous is optically transparent but displays different physical properties in different locations. A vitreous cutter instrument is shown inserted through a pars plana incision into the liquid-gel vitreous core to biopsy the vitreous core. The elastic vitreous cortex forms the vitreoretinal interface and the dense vitreous base interfaces with the ciliary body. The proteomic profiles of these distinct vitreous regions are unique, explaining their unique mechanical properties and regional disease susceptibility. Proteins in the vitreous core can contain native vitreous proteins, systemically derived biomarkers, or proteins secreted by the retina. Proteomic profiling of the vitreous core collected during vitrectomy surgery has given insight into disease mechanisms and provided potential therapeutic targets.
Sampling the vitreous core has, nevertheless, yielded important proteomic insights that indicate the vitreous is not a passive tissue limited to structural space filling. Instead, it is a physiologically active, complex tissue containing diverse proteins that originate from both within and outside the eye. The ciliary body, lens, and retina are known to synthesize vitreous proteins, but a large fraction seems to be synthesized elsewhere before being trafficked to the vitreous [7, 8]. A clinically interesting fact that has received little attention is the vitreous’s high levels of immunoglobulin and albumin (approximately 80% of the protein composition) [1]. Careful analysis of vitreous immunoglobulin should help refine our concept of immune privilege in the eye [9]. Extracellular albumin exerts oncontic pressure that may be important in conditions with retinal edema and breakdown of the blood-retinal barrier such as diabetes. Albumin also carries a number of other proteins [10], which studies have overlooked since albumin is typically removed prior to proteomic analysis to identify lower abundant proteins.
Vitreous proteomic studies revealed expression of functionally related groups of proteins. Numerous immune system effectors, for example, are present. These include complement cascade proteins, macrophage migration inhibitory factor (MIF), and matrix metalloproteinases (MMPs) [11–13]. These proteins may be especially important since the vitreous does not normally contain cells and the eye is immune privileged. The vitreous also contains groups of oxidative stress and anti-inflammatory proteins that are important for maintaining the balance of local free radicals and preventing tissue damage. Some of these enzymes may be differentially localized to vitreous substructures [6, 14]. A surprising finding has been the abundance of intracellular proteins in this extracellular space, such as crystallins and metabolic intermediates [15]. These proteins may either be functionally active or degraded protein fragments released from the surrounding tissues. Some vitreous crystallins might represent retinal disease biomarkers [15]. Degraded retinal proteins secreted into the vitreous make possible indirect retinal biopsy through vitreous biopsies.
Human vitreous biopsies are typically preformed in the operating room with a vitreous cutter instrument. Most proteomic studies have used vitreous obtained with this technique, so they are limited to the investigation of surgical diseases. Needle biopsies can be performed in outpatient clinics, but the use of this technique is infrequent. We found that the proteomic vitreous composition did not vary significantly with either biopsy technique using LC-MS/MS [16]. The use of needle vitreous biopsies was also validated in protein microarrays [17]. A needle biopsy of the anterior chamber fluid is straightforward and has been suggested as a surrogate to vitreous biopsies. A comparison between anterior chamber and vitreous proteins, however, indicated there were significant differences in specific protein levels [18]. Needle biopsies of the vitreous make it possible to study numerous nonsurgical retinal diseases, such as age-related macular degeneration [19, 20]. With the high volume of intravitreal injections, insertion of needles into the vitreous has become routine. Needle vitreous biopsies could significantly expand the application of vitreous proteomics to important ophthalmic disorders.
Comparing normal and diseased vitreous proteomes is used to identify disease biomarkers. Due to ethical issues, normal vitreous is not sampled from healthy individuals. The collection of normal vitreous from brain-dead individuals during donor organ harvesting is an alternative, but it has not been explored. Instead, vitreous from autopsy eyes of clinically milder conditions, such as macular hole and epiretinal membranes, are used as comparative controls for more severe conditions. In diabetic retinopathy and proliferative vitreoretinopathy, the vitreous gel composition undergoes physical changes suggesting proteins may be differentially expressed [21–23]. Frequently identified biomarkers of diabetic retinopathy include vascular endothelial growth factor (VEGF), interleukin-6 (IL-6), phosphatidylinositol-glycan biosynthesis class F protein (PIGF), pigment epithelium derived factor (PEDF), angiotensin II (Ang II), transforming growth factor beta (TGF-beta), intercellular adhesion molecule 1 (ICAM-1), hepatocyte growth factor (HGF), monocyte chemotactic protein 1 (MCP-1), complement component C3, angiostatin, endostatin, and angiogenin [11, 15, 24–27]. Although elevated VEGF was not originally identified through proteomics studies, proteomic findings would have strongly supported testing of anti-VEGF-therapy, which has emerged as a highly effective treatment for diabetic macular edema [28, 29]. PEDF, along with prostaglandin-D2 synthase and interphotoreceptor retinoid-binding protein [30], was identified in an alternative proteomic strategy to compare vitreous and serum proteomes [30]. Proliferative vitreoretinopathy is a poorly understood fibrotic reaction following retinal detachment and is difficult to treat surgically. Frequently identified biomarkers for proliferative vitreoretinopathy include VEGF, IL-6, interleukin-8 (IL-8), ICAM-1, MCP-1, PDGF, fibroblast growth factor (FGF), HGF, PEDF, TGF-beta, and tumor necrosis factor (TNF) [24, 31]. The overlap of proteomes and biomarkers between PVR, diabetic retinopathy, and other nonophthalmic fibrotic diseases may allow for transfer of biologic therapies between seemingly unrelated diseases.
There are highly advanced bioinformatic algorithms to effectively match proteins to the thousands of peptide mass-spectral data generated in experiments. Meaningful organization of these protein lists into the context of disease remains a challenge. It is relatively simple to focus on a handful of single, differentially expressed proteins. Systems analyses, however, can identify global relationships between groups of proteins. Current software can perform gene ontology categorization, unbiased cluster analysis, and pathway representation. We and others found with gene ontology that the vitreous contains numerous intracellular proteins that might originate from the retina. Pathway analysis identified glucose metabolism enzymes. Identifying groups of proteins that physically interact is another important relationship, such as the proteins that combine to form the membrane attack complex from the complement cascade. However, interactome databases are largely based on prior intracellular protein interaction studies, so there is a paucity of data for extracellular protein interactions relevant to the vitreous. Only now are methods being developed to identify extracellular protein interactomes [32]. So, it will be important to reanalyze vitreous proteomics data in the future as these interactome databases develop.
Vitreous proteomic studies have generated lists of potential biomarkers, but few of these have been validated beyond experimental replicates; hence, several validation studies are required. To overcome experimental and statistical bias, biomarkers require prospective validation in secondary populations. Similar to genetic studies with multiple measurements in large datasets, many of the markers will not pass stringent cutoffs. Biomarkers that repeatedly emerge in different studies are more convincing. Since many proteomic platforms identify only peptides, detection of whole proteins can be validated using ELISA, Western blot, or immunohistochemistry of diseased tissues. Even if a biomarker is convincingly expressed during disease, functional validation is necessary to determine its role in disease. Functional validation of extracellular carbonic anhydrase-I (CA-I) is an important example. This molecule was identified through proteomic differential expression of surgically obtained vitreous from humans with diabetic retinopathy [33]. Evaluation of rat models with elevated levels of CA-I in the vitreous demonstrated it caused an increase in retinal vascular leakage and intraretinal edema similar to that found in proliferative diabetic retinopathy and diabetic macular edema. Because of the diverse cellular origin of vitreous proteins, acceptable human tissue culture models are not well-established. The use of animal models remains an underdeveloped resource for functional validation.
The prototypic animal model for the study of human retinal degenerations is the mouse[34], because of well established protocols for genetic manipulation [34, 35]. Histopathological features of some inherited human vitreoretinopathies have been observed in the mouse [36], suggesting that molecular manipulation of mouse vitreous proteins might be possible. The vitreous volume is significantly smaller in the mouse relative to the human due to the much larger size of the mouse lens. To overcome this perceived limitation, we developed an evisceration technique to isolate sufficient mouse vitreous suitable for proteomic analyses [37]. Our preliminary proteome of the normal mouse vitreous indicates many of the same proteins found in human vitreous are present, including collagens, albumin, IgG, oxidative stress enzymes, cytokines, and others [38]. This suggests that potential vitreous biomarkers could be analyzed in genetically manipulated mouse models.
Personalized medicine is a new paradigm that changes the therapeutic approach from “generic treatment ladders” to patient-specific therapies [39]. We feel that personalized proteomes may have more value than personalized genomes in directing vitreoretinal disease therapy. Uveitis denotes intraocular inflammation, and in more than 50% of cases, the exact cause is not known. Noninfectious uveitis is first treated with oral and periocular corticosteroids, then intraocular steroids, and finally with oral steroid-sparing immunosupressives. In the case of inherited CAPN5-associated uveitis [40, 41], this treatment paradigm was insufficient. For example, CAPN5 patients failed treatment with infliximab, a monoclonal antibody directed against tumor necrosis factor-alpha (TNF-alpha). In preliminary studies using a personalized vitreous proteomics strategy, we discovered there was no TNF-alpha in a patient’s vitreous, which explained why targeting TNF-alpha was misguided. Instead, we found elevated levels of VEGF, IL-6, and IL-12, which are the next logical therapeutic targets [24]. As a proof of principal, we found that intravitreal injections of an anti-VEGF agent reversed features of CAPN5-associated uveitis. This example points to the potential importance of profiling vitreous proteins to determine the best therapy, especially to avoid targeting proteins that are absent. Other potential biomarkers for uveitides have been identified [42–44].
Acknowledgments
The authors are supported by NIH Grant K08EY020530 and Research to Prevent Blindness (VBM) and NIH Grant 1F32EY022280 - 01A1 (JMS).
Footnotes
Financial Disclosure: The authors have declared no financial or commercial conflicts of interest.
References
- 1.Grus FH, Joachim SC, Pfeiffer N. Proteomics in ocular fluids. Proteomics Clin Appl. 2007;1:876–888. doi: 10.1002/prca.200700105. [DOI] [PubMed] [Google Scholar]
- 2.Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog Retin Eye Res. 2000;19:323–344. doi: 10.1016/s1350-9462(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 3.Aliyar HA, Foster W, Hamilton PD, Ravi N. Towards the development of an artificial human vitreous. Polymer Preprints, American Chemical Society, Division of Polymer Chemistry. 2004;45:469–470. [Google Scholar]
- 4.Sebag J. Molecular biology of pharmacologic vitreolysis. Trans Am Ophthalmol Soc. 2005;103:473–494. [PMC free article] [PubMed] [Google Scholar]
- 5.Hong J, Deng SX, Xu J. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012;367:2053. doi: 10.1056/NEJMc1211068. author reply 2054. [DOI] [PubMed] [Google Scholar]
- 6.Skeie JM, Mahajan VB. Dissection of human vitreous body elements for proteomic analysis. J Vis Exp. 2011;(47):2455. doi: 10.3791/2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funaki H, Sawaguchi S, Yaoeda K, Koyama Y, et al. Expression and localization of angiogenic inhibitory factor, chondromodulin-I, in adult rat eye. Invest Ophthalmol Vis Sci. 2001;42:1193–1200. [PubMed] [Google Scholar]
- 8.Halfter W, Dong S, Dong A, Eller AW, Nischt R. Origin and turnover of ECM proteins from the inner limiting membrane and vitreous body. Eye (Lond) 2008;22:1207–1213. doi: 10.1038/eye.2008.19. [DOI] [PubMed] [Google Scholar]
- 9.Zhou R, Caspi RR. Ocular immune privilege. F1000 Biol Rep. 2010:2. doi: 10.3410/B2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou M, Lucas DA, Chan KC, Issaq HJ, et al. An investigation into the human serum “interactome”. Electrophoresis. 2004;25:1289–1298. doi: 10.1002/elps.200405866. [DOI] [PubMed] [Google Scholar]
- 11.Gao BB, Chen X, Timothy N, Aiello LP, Feener EP. Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J Proteome Res. 2008;7:2516–2525. doi: 10.1021/pr800112g. [DOI] [PubMed] [Google Scholar]
- 12.Takano A, Hirata A, Inomata Y, Kawaji T, et al. Intravitreal plasmin injection activates endogenous matrix metalloproteinase-2 in rabbit and human vitreous. Am J Ophthalmol. 2005;140:654–660. doi: 10.1016/j.ajo.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Mitamura Y, Takeuchi S, Matsuda A, Tagawa Y, et al. Macrophage migration inhibitory factor levels in the vitreous of patients with proliferative vitreoretinopathy. Am J Ophthalmol. 1999;128:763–765. doi: 10.1016/s0002-9394(99)00368-2. [DOI] [PubMed] [Google Scholar]
- 14.Skeie JM, Wagner BA, Teoh ML, Ross J, et al. Functional validation of proteomics screening: superoxide dismutase-3 regulates oxidative stress in human eyes with diabetic disease. HUPO (Human Proteome Organisation) 11th Annual World Congress; Boston, MA. September 9–13 2012; Poster # 223. [Google Scholar]
- 15.Wang H, Feng L, Hu J, Xie C, Wang F. Differentiating vitreous proteomes in proliferative diabetic retinopathy using high-performance liquid chromatography coupled to tandem mass spectrometry. Exp Eye Res. 2013;108:110–119. doi: 10.1016/j.exer.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Skeie JM, Brown EN, Martinez HD, Russell SR, et al. Proteomic analysis of vitreous biopsy techniques. Retina. 2012;32:2141–2149. doi: 10.1097/IAE.0b013e3182562017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfahler SM, Brandford AN, Glaser BM. A prospective study of in-office diagnostic vitreous sampling in patients with vitreoretinal pathology. Retina. 2009;29:1032–1035. doi: 10.1097/IAE.0b013e3181a2c1eb. [DOI] [PubMed] [Google Scholar]
- 18.Ecker SM, Hines JC, Pfahler SM, Glaser BM. Aqueous cytokine and growth factor levels do not reliably reflect those levels found in the vitreous. Mol Vis. 2011;17:2856–2863. [PMC free article] [PubMed] [Google Scholar]
- 19.Davuluri G, Espina V, Petricoin EF, 3rd, Ross M, et al. Activated VEGF receptor shed into the vitreous in eyes with wet AMD: a new class of biomarkers in the vitreous with potential for predicting the treatment timing and monitoring response. Arch Ophthalmol. 2009;127:613–621. doi: 10.1001/archophthalmol.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ecker SM, Pfahler SM, Hines JC, Lovelace AS, Glaser BM. Sequential in-office vitreous aspirates demonstrate vitreous matrix metalloproteinase 9 levels correlate with the amount of subretinal fluid in eyes with wet age-related macular degeneration. Mol Vis. 2012;18:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 21.Los LI, van der Worp RJ, van Luyn MJ, Hooymans JM. Age-related liquefaction of the human vitreous body: LM and TEM evaluation of the role of proteoglycans and collagen. Invest Ophthalmol Vis Sci. 2003;44:2828–2833. doi: 10.1167/iovs.02-0588. [DOI] [PubMed] [Google Scholar]
- 22.Walia S, Clermont AC, Gao BB, Aiello LP, Feener EP. Vitreous proteomics and diabetic retinopathy. Semin Ophthalmol. 2010;25:289–294. doi: 10.3109/08820538.2010.518912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Liu F, Cui SJ, Liu Y, et al. Vitreous proteomic analysis of proliferative vitreoretinopathy. Proteomics. 2008;8:3667–3678. doi: 10.1002/pmic.200700824. [DOI] [PubMed] [Google Scholar]
- 24.Funatsu H, Yamashita T, Yamashita H. Vitreous fluid biomarkers. Adv Clin Chem. 2006;42:111–166. doi: 10.1016/s0065-2423(06)42004-7. [DOI] [PubMed] [Google Scholar]
- 25.Loukovaara S, Robciuc A, Holopainen JM, Lehti K, et al. Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFbeta1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol (Copenh) 2012;91:531–539. doi: 10.1111/j.1755-3768.2012.02473.x. [DOI] [PubMed] [Google Scholar]
- 26.Mohan N, Monickaraj F, Balasubramanyam M, Rema M, Mohan V. Imbalanced levels of angiogenic and angiostatic factors in vitreous, plasma and postmortem retinal tissue of patients with proliferative diabetic retinopathy. J Diabetes Complications. 2012;26:435–441. doi: 10.1016/j.jdiacomp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimura T, Sonoda KH, Sugahara M, Mochizuki Y, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS ONE. 2009;4:e8158. doi: 10.1371/journal.pone.0008158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fong AH, Lai TY. Long-term effectiveness of ranibizumab for age-related macular degeneration and diabetic macular edema. Clin Interv Aging. 2013;8:467–483. doi: 10.2147/CIA.S36811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen QD, Brown DM, Marcus DM, Boyer DS, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 30.Yamane K, Minamoto A, Yamashita H, Takamura H, et al. Proteome analysis of human vitreous proteins. Mol Cell Proteomics. 2003;2:1177–1187. doi: 10.1074/mcp.M300038-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Citirik M, Kabatas EU, Batman C, Akin KO, Kabatas N. Vitreous vascular endothelial growth factor concentrations in proliferative diabetic retinopathy versus proliferative vitreoretinopathy. Ophthalmic Res. 2012;47:7–12. doi: 10.1159/000324200. [DOI] [PubMed] [Google Scholar]
- 32.Ozkan E, Carrillo RA, Eastman CL, Weiszmann R, et al. An Extracellular Interactome of Immunoglobulin and LRR Proteins Reveals Receptor-Ligand Networks. Cell. 2013;154:228–239. doi: 10.1016/j.cell.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao BB, Clermont A, Rook S, Fonda SJ, et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med. 2007;13:181–188. doi: 10.1038/nm1534. [DOI] [PubMed] [Google Scholar]
- 34.Mahajan VB, Skeie JM, Assefnia AH, Mahajan M, Tsang SH. Mouse eye enucleation for remote high-throughput phenotyping. J Vis Exp. 2011;(57):3184. doi: 10.3791/3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung LSB, Voleti VB, Lin JH, Tsang SH. In: Genetic Diseases of the Eye. Traboulsi EI, editor. Oxford University Press; New York: 2012. pp. 356–372. [Google Scholar]
- 36.Utriainen A, Sormunen R, Kettunen M, Carvalhaes LS, et al. Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Hum Mol Genet. 2004;13:2089–2099. doi: 10.1093/hmg/ddh213. [DOI] [PubMed] [Google Scholar]
- 37.Skeie JM, Tsang SH, Mahajan VB. Evisceration of mouse vitreous and retina for proteomic analyses. J Vis Exp. 2011;(50):2795. doi: 10.3791/2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skeie JM, Tsang SH, Mahajan VB. Mouse Vitreoretinal Proteome [ARVO Abstract] Invest Ophthalmol Vis Sci. 2013;54:e-Abstract 2465. [Google Scholar]
- 39.Lee RW, Dick AD. Current concepts and future directions in the pathogenesis and treatment of non-infectious intraocular inflammation. Eye (Lond) 2012;26:17–28. doi: 10.1038/eye.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tlucek PS, Folk JC, Sobol WM, Mahajan VB. Surgical management of fibrotic encapsulation of the fluocinolone acetonide implant in CAPN5-associated proliferative vitreoretinopathy. Clinical ophthalmology. 2013;7:1093–1098. doi: 10.2147/OPTH.S43939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tlucek PS, Folk JC, Orien JA, Stone EM, Mahajan VB. Inhibition of neovascularization but not fibrosis with the fluocinolone acetonide implant in autosomal dominant neovascular inflammatory vitreoretinopathy. Arch Ophthalmol. 2012;130:1395–1401. doi: 10.1001/archophthalmol.2012.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauck SM, Dietter J, Kramer RL, Hofmaier F, et al. Deciphering membrane-associated molecular processes in target tissue of autoimmune uveitis by label-free quantitative mass spectrometry. Mol Cell Proteomics. 2010;9:2292–2305. doi: 10.1074/mcp.M110.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauck SM, Hofmaier F, Dietter J, Swadzba ME, et al. Label-free LC-MSMS analysis of vitreous from autoimmune uveitis reveals a significant decrease in secreted Wnt signalling inhibitors DKK3 and SFRP2. J Proteomics. 2012;75:4545–4554. doi: 10.1016/j.jprot.2012.04.052. [DOI] [PubMed] [Google Scholar]
- 44.Mao L, Yang P, Hou S, Li F, Kijlstra A. Label-free proteomics reveals decreased expression of CD18 and AKNA in peripheral CD4+ T cells from patients with Vogt-Koyanagi-Harada syndrome. PLoS ONE. 2011;6:e14616. doi: 10.1371/journal.pone.0014616. [DOI] [PMC free article] [PubMed] [Google Scholar]