Abstract
Biorepositories are collections of surgically obtained human tissues for current and future investigations of disease mechanisms, therapeutics, and diagnostics. In ophthalmology, a critical challenge is how to interface the operating room with the laboratory. To attain standards required for basic research, clinical and research teams must cooperate to collect, annotate, and store specimens that yield consistent results required for advanced molecular techniques. We developed an efficient platform for obtaining vitreous and other eye tissues from the operating room and transferring them to the lab. The platform includes a mobile lab cart for on-site tissue processing, a multi-user, web-based database for point-of-care phenotypic capture, and an integrated data tracking system for long-term storage. These biorepository instruments have proven essential for our studies in ophthalmic disease proteomics. This system can be implemented in other operating rooms and laboratories for a variety of biological tissues.
1 Introduction
Human tissue repositories of ophthalmic surgical specimens are necessary for scientific investigations into disease mechanisms [1]. Biorepositories serve individual laboratories or large, collaborative efforts to study a wide range of biological traits in a broad spectrum of sample types [2–4]. In order to take advantage of modern molecular research techniques, specimens must be collected in a manner amenable to protein, DNA, RNA, and cellular analyses. This is especially important for high-throughput or “omics” experiments, where numerous samples undergo thousands of sensitive, simultaneous measurements. It is also important to have collections of specimens that are phenotypically curated and easy to track. Reaching these goals promises to provide an invaluable resource for current and future scientific research methods.
A major barrier to this type of translational research is the absence of appropriate infrastructure in the operating room and laboratory. The operating room is designed to perform safe and efficient surgery, not to collect experiment-ready tissues. The clinical pathology lab interfaces with the operating room, but tissue preservation is largely limited to formalin, which is suboptimal for many scientific methods. The collection of frozen tissues for the clinical pathology lab is labor intensive and costly, but justified where the primary focus is on clinical diagnostics. For a research focus, the ideal situation would be to have the scientific lab adjacent to the operating room, but this is rarely the case. Instead, research laboratories are often physically separated from the operating rooms and employ personnel with different goals and different work cultures.
A second barrier to translational research is the collection of adequate phenotypic details. Specimens that reach the clinical pathology lab have careful tracking systems that link a specimen to a patient identifier. However, clinical and surgical phenotypes with details relevant to research investigations are absent. Phenotypic information can be distributed across multiple electronic record systems that are not designed for research or completely inaccessible to the research personnel. Much of the associated clinical terminology is recorded to fit diagnostic billing codes, which can be too general, misleading, or lacking details necessary for good experimental design. Thus, the rate-limiting step for medical research breakthroughs may not be the science, but rather the availability of high quality tissue specimens with adequate phenotype details.
We encountered these issues when we initiated studies into vitreous proteomics, where it is essential that all samples incorporated into an experiment are phenotypically comparable and treated exactly the same to minimize the number of variables [5–7]. To study proliferative vitreoretinopathy (PVR), for example, five patients were prospectively selected since they shared the exact same disease stage, and phenotypic data was collected from the paper chart by the surgeon. Vitreous biopsies were collected by two different surgeons and applied to antibody arrays to measure hundreds of cytokines. While the cytokine expression pattern from four different patient samples collected by a single surgeon were nearly identical, the one sample collected from the second surgeon was different (Figure 1). It is possible that this was due to patient-patient variability. However, investigation showed the samples were handled differently in the operating room. Without standardized procedures, the proteomic composition of the specimen can change leading to the study of an experimental artifact rather than the study of a disease mechanism. Sample to sample variation is a common problem when collecting surgical specimens in nonophthalmologic disciplines as well. Without precise collection methods, proteomic profiles may be altered [8, 9]. In the case of collecting vitreous, we found that specimens were contaminated with cells, because there was no microfuge to spin down the samples. When collecting membranes (scar tissues), several samples were lost during routine handling because these small, delicate, transparent tissues could not be processed on site.
Figure 1. Variance in vitreous core cytokinomics.
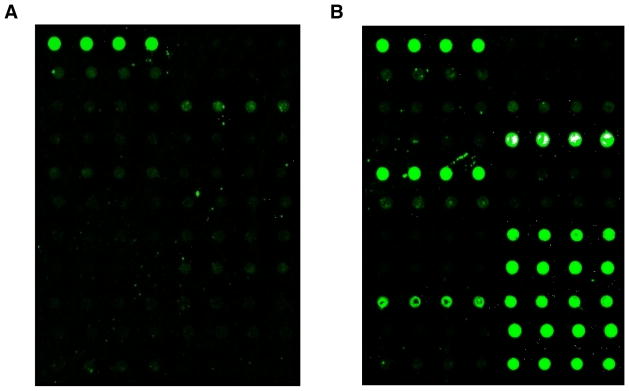
A. Cytokine profile of vitreous core collected without MORLI processing protocol. Fluorescent green dots depict cytokine protein expression in quadruplicate repeats. B. Cytokine profile of vitreous core collected from a patient with clinically identical disease as shown in (A), however processed with MORLI system. The relative cytokine expression was much higher in this sample, indicated by the higher intensity of the fluorescence on the slide.
1.1 Biorepository Logistics
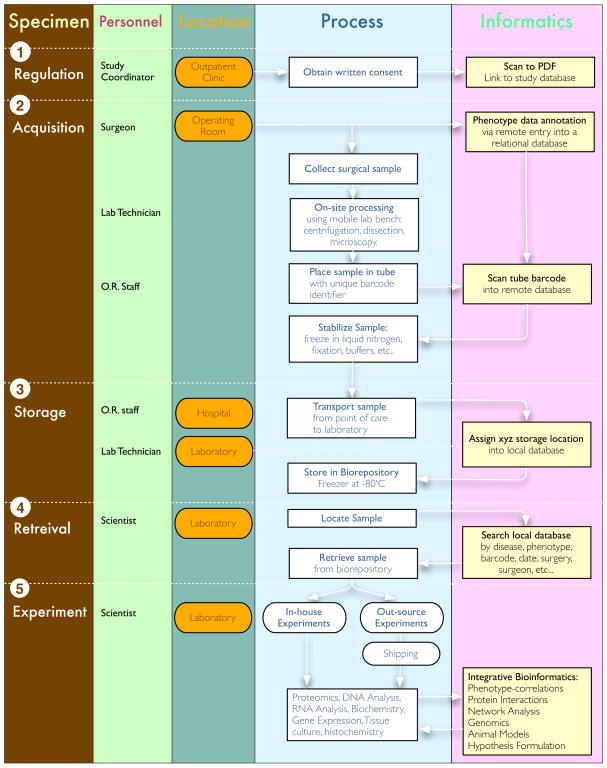
The collection of surgical specimens for laboratory proteomics is complex. Our goal was to optimize and standardize procedures starting in the operating room [10]. At the same time, we needed an informatics platform accessible to clinical and lab personnel that would track the specimens and capture phenotypic details. As in other biorepositories, there were additional obstacles to overcome, such as regulatory boundaries, costs, personnel training, sample security, and preparation for unknown future experimental techniques [10–14]. To accomplish these goals, we created a biorepository and relational database for vitreous samples as well as other eye tissues. We collected samples from both surgical and postmortem human eyes for proteomics, cell biology, and bioinformatics experiments. There are a number of steps involved in managing the tissue specimen before any proteomics experiment. A flow diagram outlines these steps and their relationships (Figure 2). Below we describe how our laboratory implemented our biorepository.
Figure 2. Biorepository flow diagram.
Process of sample acquisition to sample analysis in detail. All steps are outlined 1–5, including sample regulation, acquisition, storage, retrieval, and experimentation. The involved personnel, location, protocol details and associated bioinformatics processing are specified for each step.
2 Regulation of patient associated data
Before a sample can be collected for a biorepository, approval by the local Institutional Review Board for Human Subjects Research is required. The design of biorepositories typically focuses on providing tissue samples without personal identifiers so that anonymous samples can be broadly shared. Special software has even been developed to remove personal identifiers from medical records. Approval for use of anonymous tissue samples is relatively simple, but there are important limitations to this approach. First, from a practical standpoint, it is difficult to collect sufficiently detailed patient histories and phenotypic features in exam rooms and surgical operating rooms due to the premium placed on high patient volume and limited encounter times. Second, researchers cannot retrospectively data mine patient charts for relevant systemic conditions. This may be especially important as new hypotheses are formed and additional clinical data is required to select comparable specimens. Third, anonymization prevents the collection of prospective clinical data that can include critical clinical outcomes not observed until years after the specimen was collected.
For these reasons, we developed patient consents and protection measures that allow us to collect and maintain tissue samples linked to patient identifiers. The consent includes permission to mine data from clinic charts in the future. Samples analyzed by researchers outside the university are not given direct access to patient data but are required to collaborate with local researchers that can access clinical data relevant to the study. A study coordinator explains this to subjects using a detailed consent form during the preoperative evaluation.
3 Acquisition of surgical specimens
In the operating room, the personnel required for specimen acquisition include the surgeon, operating room staff, and lab technician. Standardization and optimization of specimen collection is a first step for downstream molecular studies. Equally important are well-annotated phenotypes associated with the samples. We found on-site processing within the operating room helped to maintain specimen integrity, since it was not possible to immediately or easily transfer specimens to the lab during or between cases. Capturing point-of-care phenotypic data in the operating room just after surgery was the most efficient method of recording this information, while it was fresh in the surgeon’s mind. To achieve this we developed a mobile laboratory bench and remote access database Samples collected from outside laboratories and surgeons required local IRB approval, informed consent, and then were collected, shipped, logged, and tracked as outlined below.
3.1 Mobile Operating Room Lab Interface (MORLI)
It is straightforward to determine the sample processing method for current scientific protocols, but it is impossible to know which protocols will be used in the future. Our studies with eye tissues showed that immediate freezing to −20 or −80 was the optimum and most versatile preservation method for downstream analysis of proteins, RNA, and DNA. Delayed freezing can otherwise result in degradation of these key molecules. In most surgical specimen collecting schemes, there is a delay in sample processing since the specimen needs to be carried from the operating room to the laboratory. In order to immediately process specimens in the operating room, a mobile cart containing all the elements of a laboratory bench was designed to simulate a laboratory bench with all the required instruments and devices.
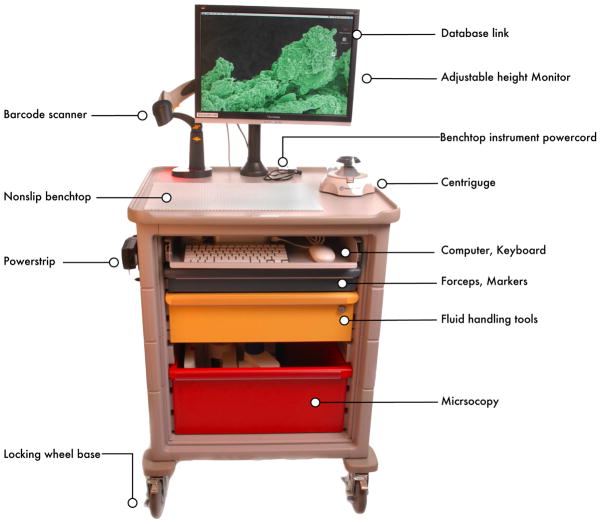
MORLI has three main components: a frame, computer, and lab supplies (Table). The frame is a medium-sized cart on wheels with a flat lab bench surface (Figure 3). There are three color-coded drawers to hold lab supplies that include pipettors, disposable pipette tips, 1.5 mL centrifuge tubes, a centrifuge, a small liquid nitrogen dewer or other cryofreezer, and a binocular dissecting microscope (Figure 4). It carries a sliding shelf for a keyboard, mouse, and Mac-mini with secure wireless access to a server database (Figure 4B). The computer connects to a mounted barcode scanner.
Table 1.
Mobile operating room laboratory bench parts.
| Category | Parts |
|---|---|
| Cart | Herman Miller Medical Cart 20-inch × 36-inch, raised edge top; Keyboard, 3, 6, 9- inch drawers; Power Strip; Flip-up Shelf |
| Microscopy | Zeiss Stereo Microscope; Surgical Instruments (micro forceps, backflush brush), Nunc Lab-Tek culture |
| Supplies | slides, Whatman filter paper |
| Biochemistry | Microcentrifuge; Microfuge Tubes; Ranin Pipettors; Dry Ice Containers, liquid nitrogen dewer (Nalgene, |
| Hardware | Rochester, NY) |
| Biological Reagents | Dry-ice bath, Cell culture media; 50-cc vials of fixatives |
Figure 3. MORLI.
The mobile operating room lab interface (MORLI) unit provides the necessities for sample processing in the operating room. These items include a Macintosh mini computer equipped with a scanner for reading tube barcodes as well as patient chart barcodes. It is also equipped with sterile pipette tips, pipetors, extra tubes, and a centrifuge for eliminating cellular debris from the samples immediately after they are extracted.
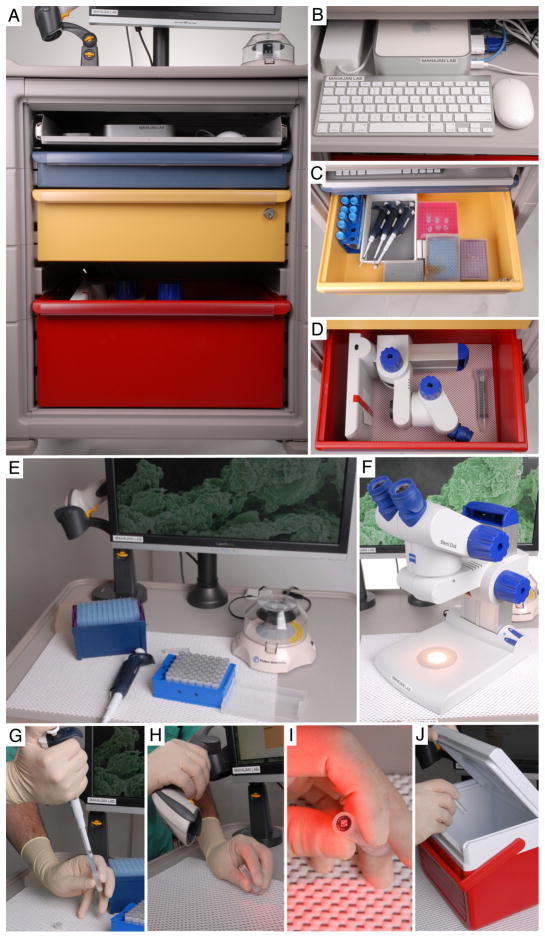
Figure 4. On-site specimen processing.
Shematic representation of sample processing with MORLI equipment. A. MORLI overview showing cart organization. B. Computer working station including keyboard, iMac mini, and mouse. C. Drawer for the storage of pipettes, tips, and tubes of various sizes for different applications. D. Microscope storage drawer. E. Benchtop working station for the processing of samples. Also serves as storage space for computer barcode scanner and mini centrifuge. F. Microscope for the processing of small samples, for example epiretinal membrane tissues. G. After a sample has been collected, it is centrifuged and then transferred to microfuge tube imprinted with a barcode. H. Next the sample barcode is scanned into the database using the scanner on the bench-top. I. Two-dimensional barcode on bottom of tube. J. The sample is flash frozen in liquid nitrogen and transported to the laboratory in a biohazard cooler.
For tissue or cellular studies, the portable lab microscope allows a surgeon to further dissect the specimen away from the surgical field (Figure 4F). Tissue can be oriented and processed for downstream molecular studies. Specimens can also be placed directly into culture dishes. For a liquid vitreous sample, we collect specimens using a cutter and manual aspiration into a three cc syringe. This is passed to the scrub nurse and then to the circulating nurse or lab technician. The sample is immediately transferred into a sterile microfuge tube and spun down in the micro-centrifuge to eliminate cellular debris. Specifics about equipment on MORLI are listed in Table 1. MORLI components can be changed to fit the needs of any surgical sample collection. For example, surgical tissues can be placed into RNA preservative solution for RNA sequencing studies, specific buffers for enzymatic assays, or embedding cassettes to accommodate larger surgical tissues.
3.2 Database Design
A database was created using MySQL, HTML, PHP, and phpMyAdmin software. The site was then deployed using web-based Wordpress modules on a secure internal website. There was an open source relational management system (RDBMS). The relational database was designed to hold phenotypic data and formatted to allow data export to bioinformatics software to identify relationships with proteomic data. A key feature was accessibility to the identical database by lab personnel and operating room personnel at different times and locations. The user-interface for each group was modified to accommodate their specific tasks.
3.3 Specimen tracking system
An electronic database (LIMS, laboratory information management system) is an efficient instrument for logging sample information and retrieving samples quickly [15]. It is also essential to track samples that have been used and no longer exist. For specimen tracking, we use an alphanumeric bar-coding system where two-dimensional DataMatrix barcodes are permanently etched onto cryovials. This eliminates the need for patient identifiers on the tube and the possibility of losing a label while in extended cold-storage, which can be problematic in labeling systems [16]. The clarified vitreous is transferred to a new 1.5 mL cryovial (Figure 4G). A scanner is used to capture and log the tiny unique barcode on the cryovial into the database on the MORLI computer (Figure 4H, I). The sample is frozen in liquid nitrogen and placed into a transport cooler (Figure 4J).
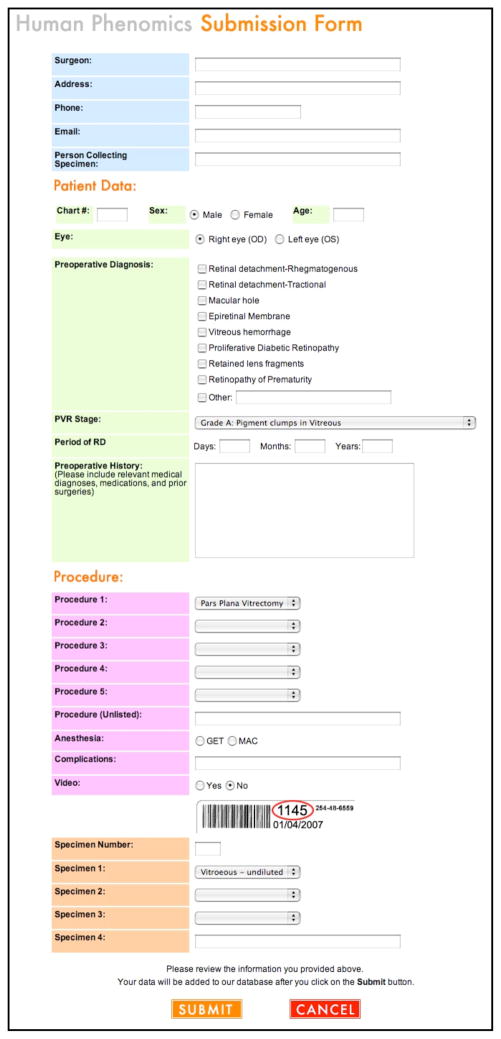
While in the operating room, patient information on the specimen is entered into the database. The user interface is designed for efficient entry of patient demographics, phenotype data, and sample features in a format familiar to surgeons and clinical personnel (Figure 5). The fields include name, medical record number, age, sex, diagnosis, pre-operative history, and the operating procedure. When surgery has concluded, the surgeon enters any unique features of the surgery that could not be ascertained preoperatively. The specimen is submitted to the database and assigned a new laboratory-record tracking number. Commercially available database software, such as Filemaker Pro (www.filemaker.com) and Microsoft Access (http://office.microsoft.com/en-us/access/), are alternatives to the custom software we developed.
Figure 5. Specimen submission page.
Screenshot of the specimen submission form. This form can be accessed remotely through the webpage anywhere in the world so samples can be entered into the system as soon as they are collected. Fields to be filled out at the time of entry include information about the person collecting the sample, information about the patient and procedure, as well as the information about the sample type. Tracking information including the patient hospital number and the sample barcode are recorded at this time to ensure the sample is not lost after it leaves the operating room.
4 Storage of surgical specimens
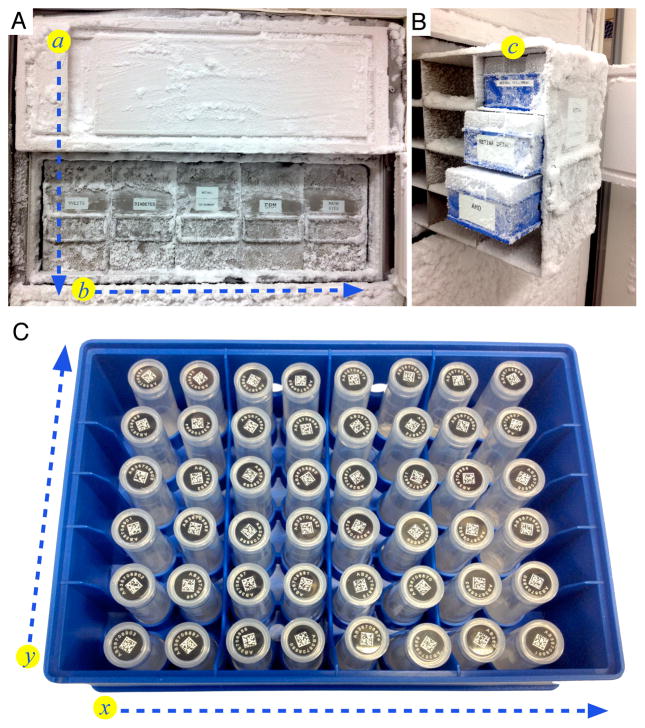
The next challenge was the transfer of the specimen from the operating room to the laboratory before the specimen thaws. This was delegated on the day of surgery to clinical or laboratory personnel. Upon arrival to the lab, specimens are transferred to a −80° C freezer for long term storage. Emergency sources of electricity or liquid nitrogen are accessible in case of an emergency power outage. The specimen was identified by searching the database using its barcode on a computer adjacent to the storage freezer. The boxes and shelves are organized by general disease category. Each tube location can be located by Cartesian coordinates that correspond to the shelf (a), rack (b), box (c), and location within the box (x, y) (Figure 7). Lab personnel enter the storage coordinates into the database. The freezer is connected to an emergency back up power supply in order to prevent any thawing during a power outage [17]. Another precaution includes an alert system programmed into the freezer for notifications of power outages delivered directly to e-mail or telephone. A similar system can be created to store any sample type at any temperature by using barcode stickers on tissue blocks or jars rather than a tube.
Figure 7. Freezer Organization.
A. The specimens are stored in a −80°C freezer containing 5 separate shelves holding 5 disease specific racks, which are denoted as spatial coordinates a and b. B. Each rack contains 20 boxes, organized to hold unique specimen subsets in coordinate. C. The specimen boxes hold 48 two-dimensional barcoded tubes within a Cartesian coordinate system where (x, y) is denoted with (A–F, 1–8). The exact spatial location of a sample within the freezer is tracked in the database according to shelf #, rack #, box #, and then (A–F, 1–8).
5 Retrieval of surgical specimens
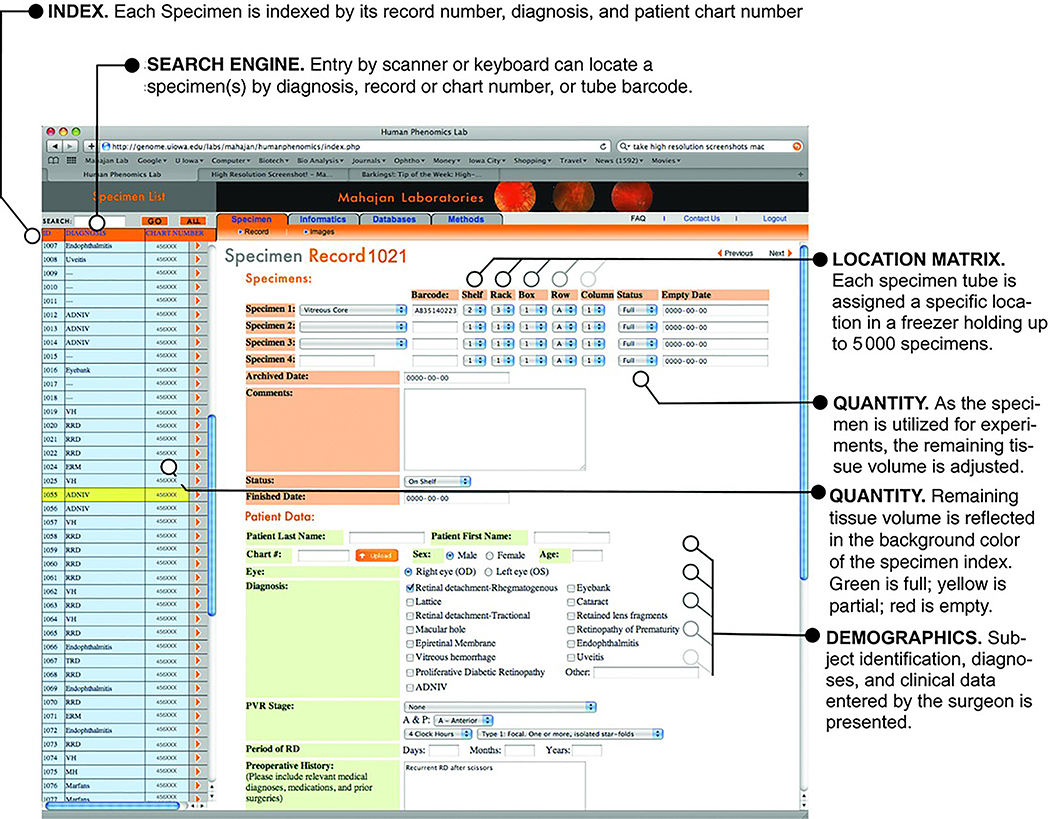
Specimens are often stored for many years before they are required for experiments. Locating old samples in a freezer can be one of the most frustrating trials a scientist faces. The barcoded cryovial and database system makes finding multiple or rare specimens trivial work. Specimens can be located using a search engine by record number, diagnosis, tube barcode, or patient chart number. Each sample has its own record page in the database, which includes its location matrix, availability, and patient demographics. The location matrix information is beneficial as it provides the exact freezer location of the sample, so it can be retrieved quickly. The relational aspect of the database allows the specification of multiple criteria during a search. For example, specimens from male diabetics with tractional detachments and no prior laser surgery can be identified. Once samples are consumed, their availability status is changed on the record page, which is also visible on the main specimen list by color coding: green for full samples, yellow for partially used samples, and red for depleted samples (Figure 6). In cases where sufficient samples are not available for experiments, surgeons can be alerted to collect these.
Figure 6. Specimen record page.
Image of sample record page showing sample information including patient demographics and specific location in the biorepository. This has a search function on the left side of the page for sample searching by disease, chart number, sample barcode, or omics ID number. The list reflects the status of the sample, i.e. if the sample is new, has been previously thawed or if it is empty/removed. The sample background color indicates one of these three statuses (green for new, yellow for used, and red for empty). At the top of the page, there is a sample location matrix key, allowing the user to locate the sample before opening the freezer. Details regarding patient information are also on the page.
6 Experiment
After retrieving specimens, scientists in the lab can perform in-house proteomics experiments or out-source lab studies. Because the phenotypes are detailed and carefully ascertained, statistically significant data can be obtained with fewer specimen numbers. These phenotype annotations allow integrative bioinformatic expansion of proteomic data into other disease database analysis. Careful collection of specimens in the operating room also creates the opportunity for the application of direct tissue culture, biochemical, and nucleic acid techniques. Although our main focus was to freeze unfixed vitreous, we also carried aliquots of 4% paraformaldehyde in MORLI to fix retinal membranes. Fixed tissues were later used for standard histology and immunohistochemistry.”
Since adoption of our platform, processing with MORLI resulted in samples with consistent and robust protein composition collected from different surgical teams. Phenotypic annotation is more complete. Using sample handling and storage validation assays allow for quick and reliable testing of sample processing [18]. In the case of the vitreous biorepository, it became clear that protein decomposition can occur soon after vitreous aspiration, and therefore it was essential to freeze specimens as soon as possible. Performing these steps on-site and at the point-of-care helped create a high-quality biorepository.
7 Discussion and conclusions
Proteomics will identify new molecules involved in ophthalmic disease that may serve as diagnostic markers or therapeutic targets. In the ophthalmology operating room, we routinely perform surgery to remove diseased eye tissues and discard them. These discarded tissues are wasted in terms of their scientific potential. Although these tissues serve no diagnostic purpose today, they are extremely valuable for scientific studies that will lead to future diagnostic tests and novel treatments for blinding disease.
Biorepositories have been developed to provide an organized means to store biological samples over time so that they are available for scientific research in the future. There are several complications encountered in developing a biorepository that is efficient for sample retention over a period of time. These include sample collection, processing, and storage. MORLI provides a means to offer surgeons in the operating room a way to process samples in a consistent manner that minimizes sample degradation and contamination.
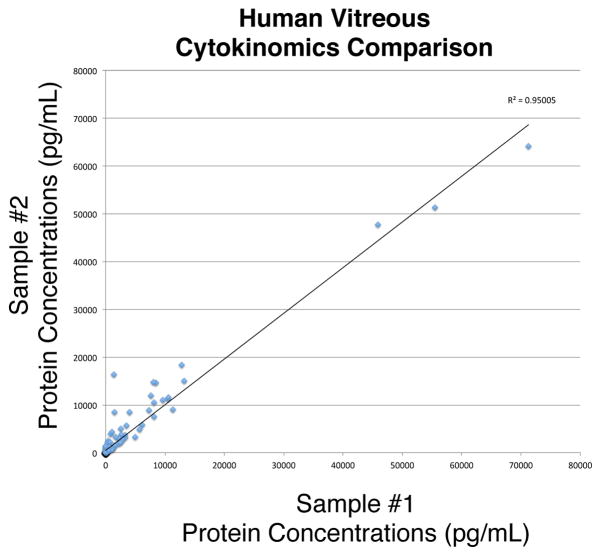
Since the development of MORLI, samples have been processed and frozen down within minutes of being collected. Previously, samples were collected, stored in the operating room until after the procedure was finished (sometimes on ice) and then brought to the laboratory. This process could result in substantial protein degradation. Samples from eyes with similar diagnoses had very different levels of protein, quantified by ELISA as described above (Figure 1). Following the new protocol using MORLI, two samples with highly correlated diagnoses were run on the same ELISA platform and the concentrations of 200 different proteins for each sample were compared on a scatter plot. The correlation coefficient was 0.95 (Figure 8). This correlation is very high, indicating the two samples were processed identically and share the same etiology. With this type of standardization, samples with different levels of RNA, DNA, or protein expression will indicate different disease mechanisms rather than processing error.
Figure 8. MORLI validation example.
Cytokinomics of vitreous samples collected and stored according to the protocols outlined in this manuscript. Two samples from patients with epiretinal membranes were collected and analyzed using multiplex ELISA cytokine arrays that measure the protein concentrations of over 200 different cytokines. The protein concentrations identified in the samples were graphed in this scatter plot. The two axes represent the two different samples. The linear regression correlation coefficient indicated that the two samples have similar protein concentrations as expected. If the samples had been collected from patients with poor phenotype data or with different protocols, the correlation would have been less.
There are three vital aspects in creating a biorepository to assure the quality of the samples it contains. First, sample tracking and storage are just as essential as sample quality control. The developed database and barcode based freezer location matrix make storing and retrieving samples straight-forward. Second, all surgeons in the operating room have access to the database on MORLI, so when a sample is collected its tube and the patients chart number are immediately scanned and stored into the system to prevent any possible identification mix-ups. Third, once placed into the freezer at the laboratory, the exact location of the tube is logged into the specimen record page. This eliminates the problem of searching through many tubes and boxes for a single sample. These methods of sample collection, processing, and storage allow for a reliable biorepository for current and future research.
With the establishment of several biorepositories for all sample types in different locations, scientific researchers will be able to collaborate in the effort to increase the existing list of knowledge sharing databases on the web [19]. The future of biobanking may include integrated centers, leading the collaboration of large research projects [20] as well as accreditation courses to ensure standard operating procedures and quality assurance of biorepositories [21]. To perform large collaborative projects in the future, samples will need to be collected in a consistent manner as well as processed and stored properly. By replicating aspects of the lab in the operating room with MORLI, we have provided a system for creating an efficient biorepository of human eye tissue samples, which can be applied to any type of biological sample collection.
Acknowledgments
The authors are supported by Research to Prevent Blindness and NIH 1K08EY020530-01A1 (VBM), and NIH 1F32EY022280 - 01A1 (JMS). The authors wish to thank Stephen R. Russell, M.D., James C. Folk, M.D., H. Culver Boldt, M.D., Elliott H. Sohn, M.D., Karen M. Gehrs M.D., Christine N. Kay M.D., Paul S. Tlucek M.D., Matthew A. Cunningham M.D., Ryan M. Tarantola M.D., Korianne Elkins M.D., Riz Somani M.D., A. Brock Roller M.D, and Andrew S. Davis M.D. for collecting vitreous specimens and Michael Golamco B.A. for software coding.
Footnotes
Financial Disclosure: The authors have declared no financial or commercial conflicts of interest.
References
- 1.Founti P, Topouzis F, van Koolwijk L, Traverso CE, et al. Biobanks and the importance of detailed phenotyping: a case study--the European Glaucoma Society GlaucoGENE project. Br J Ophthalmol. 2009;93:577–581. doi: 10.1136/bjo.2008.156273. [DOI] [PubMed] [Google Scholar]
- 2.Fullerton SM, Anderson NR, Guzauskas G, Freeman D, Fryer-Edwards K. Meeting the governance challenges of next-generation biorepository research. Sci Trans Med. 2010;2:15cm13. doi: 10.1126/scitranslmed.3000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riegman PH, Morente MM, Betsou F, de Blasio P, Geary P. Biobanking for better healthcare. Mol Oncol. 2008;2:213–222. doi: 10.1016/j.molonc.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riegman PH, Dinjens WN, Oosterhuis JW. Biobanking for interdisciplinary clinical research. Pathobiology. 2007;74:239–244. doi: 10.1159/000104451. [DOI] [PubMed] [Google Scholar]
- 5.Shevde LA, Riker AI. Current concepts in biobanking: development and implementation of a tissue repository. Front Biosci (Schol Ed) 2009;1:188–193. doi: 10.2741/s18. [DOI] [PubMed] [Google Scholar]
- 6.Vaught JB. Biorepository and biospecimen science: a new focus for CEBP. Cancer Epidemiol Biomarkers Prev. 2006;15:1572–1573. doi: 10.1158/1055-9965.EPI-06-0632. [DOI] [PubMed] [Google Scholar]
- 7.Downey P, Peakman TC. Design and implementation of a high-throughput biological sample processing facility using modern manufacturing principles. Int J Epidemiol. 2008;37(Suppl 1):i46–50. doi: 10.1093/ije/dyn031. [DOI] [PubMed] [Google Scholar]
- 8.Ennis DP, Pidgeon GP, Millar N, Ravi N, Reynolds JV. Building a bioresource for esophageal research: lessons from the early experience of an academic medical center. Dis Esophagus. 2010;23:1–7. doi: 10.1111/j.1442-2050.2009.00969.x. [DOI] [PubMed] [Google Scholar]
- 9.Grizzle WE, Bell WC, Sexton KC. Issues in collecting, processing and storing human tissues and associated information to support biomedical research. Cancer Biomark. 2010;9:531–549. doi: 10.3233/CBM-2011-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell R. National Biobanks: Clinical Labor, Risk Production, and the Creation of Biovalue. Science Technology & Human Values. 2010;35:330–355. doi: 10.1177/0162243909340267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott P, Peakman TC. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37:234–244. doi: 10.1093/ije/dym276. [DOI] [PubMed] [Google Scholar]
- 12.Schrohl AS, Wurtz S, Kohn E, Banks RE, et al. Banking of biological fluids for studies of disease-associated protein biomarkers. Mol Cell Proteomics. 2008;7:2061–2066. doi: 10.1074/mcp.R800010-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson MG. Ethics and biobanks. Br J Cancer. 2009;100:8–12. doi: 10.1038/sj.bjc.6604795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhir R. Prostate cancer biobanking. Curr Opin Urol. 2008;18:309–314. doi: 10.1097/MOU.0b013e3282fb7cbe. [DOI] [PubMed] [Google Scholar]
- 15.Troyer D. Biorepository standards and protocols for collecting, processing, and storing human tissues. Methods Mol Biol. 2008;441:193–220. doi: 10.1007/978-1-60327-047-2_13. [DOI] [PubMed] [Google Scholar]
- 16.Kay AB, Estrada DK, Mareninov S, Silver SS, et al. Considerations for uniform and accurate biospecimen labelling in a biorepository and research environment. J Clin Pathol. 2011;64:634–636. doi: 10.1136/jcp.2010.080655. [DOI] [PubMed] [Google Scholar]
- 17.Fagan M, Ball P. Design and implementation of a large-scale liquid nitrogen archive. Int J Epidemiol. 2008;37(Suppl 1):i62–64. doi: 10.1093/ije/dym287. [DOI] [PubMed] [Google Scholar]
- 18.Peakman TC, Elliott P. The UK Biobank sample handling and storage validation studies. Int J Epidemiol. 2008;37(Suppl 1):i2–6. doi: 10.1093/ije/dyn019. [DOI] [PubMed] [Google Scholar]
- 19.Howe D, Costanzo M, Fey P, Gojobori T, et al. Big data: The future of biocuration. Nature. 2008;455:47–50. doi: 10.1038/455047a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morente MM, Fernandez PL, de Alava E. Biobanking: old activity or young discipline? Semin Diagn Pathol. 2008;25:317–322. doi: 10.1053/j.semdp.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 21.De Souza YG, Greenspan JS. Biobanking past, present and future: responsibilities and benefits. AIDS. 2013;27:303–312. doi: 10.1097/QAD.0b013e32835c1244. [DOI] [PMC free article] [PubMed] [Google Scholar]