Abstract
Peripartum cardiomyopathy (PPCM) represents new heart failure in a previously heart-healthy peripartum patient. It is necessary to rule out all other known causes of heart failure before accepting a diagnosis of PPCM. The modern era for PPCM in the United States and beyond began with the report of the National Institutes of Health PPCM Workshop in 2000, clarifying all then-currently known aspects of the disease. Since then, hundreds of publications have appeared, an indication of how devastating this disease can be to young mothers and their families and the urgent desire to find solutions for its cause and better treatment. The purpose of this review is to highlight the important advances that have brought us nearer to the solution of this puzzle, focusing on what we have learned about PPCM since 2000; and what still remains unanswered. Despite many improvements in outcome, we still do not know the actual triggers that initiate the pathological process; but realize that cardiac angiogenic imbalances resulting from complex pregnancy-related immune system and hormonal changes play a key role.
Keywords: Peripartum cardiomyopathy, Pregnancy, Heart failure
Core tip: The purpose of this review is to highlight the important advances that have brought us nearer to the solution of this puzzle, focusing on what we have learned about peripartum cardiomyopathy (PPCM) since 2000; and what still remains unanswered. There have been many improvements in outcome. Increased understanding of the pathogenesis of PPCM is detailed herein; however, we still do not know the actual triggers that initiate the pathological process; but realize that cardiac angiogenic imbalances resulting from complex pregnancy-related immune system and hormonal changes play a key role.
INTRODUCTION
Peripartum cardiomyopathy (PPCM) represents new heart failure in a previously heart-healthy peripartum patient[1]. It is necessary to rule out all other known causes of heart failure before accepting a diagnosis of PPCM. Specific echocardiographic criteria define the requirement of systolic heart dysfunction with a left ventricular ejection fraction (LVEF) less than 0.45[2]. Even if the heart failure has its onset slightly out of the historic definition of time range from one month before delivery to 5 mo postpartum, the process is similar, designated as pregnancy-associated cardiomyopathy[3].
The modern era for PPCM in the United States began with the report of the NIH PPCM Workshop Group[1] in 2000, describing currently known aspects of the disease; including definition, incidence, potential etiologies, risk factors, diagnosis and management. Since then hundreds of publications have appeared, an indication of the pressing nature of the disease and the desire to find solutions for its cause and better treatment. There have been numerous excellent recent reviews[4-8], so this review is not designed to cover the broad basic facets of PPCM. Instead, the purpose of this review is to highlight the important advances that have brought us nearer to the solution of this puzzle; and to identify those key areas that remain without definitive answers. The summarized points of emphasis are listed in Table 1, and discussed individually below.
Table 1.
Summary of current state of knowledge about peripartum cardiomyopathy
| What do we know about PPCM? | What remains unknown about PPCM? |
| Awareness is important for making an earlier diagnosis with less dysfunction | Actual “triggers” that initiate the process |
| Hypertension in pregnancy increases risk for development of PPCM | Role of virus in pathogenesis |
| Most serious complications can be decreased or avoided | Why higher incidence and more severe disease in those with African heritage |
| Full recovery occurs more frequently than with any other cardiomyopathy | How important role cardiac autoantibodies play in pathogenesis |
| Autoimmunity (or immune system dysfunction) a part of pathogenesis | The extent and details of genetic factors |
| Inflammatory cardiomyopathy is common | Importance of the role of prolactin and prolactin inhibition treatment |
| Higher incidence and more severe disease in those of African heritage | Importance of the role of sFLT1 in pathogenesis |
| There can be a genetic predisposition | Why do some recovered have a relapse of heart failure with subsequent pregnancy |
| Effective evidence-based treatment guidelines available | Role of micronutrients and trace metals in pathogenesis |
| Most recovered do not have a relapse of heart failure in subsequent pregnancy | |
| Occurs globally, but with geographic variations for incidence and unique characteristics |
PPCM: Peripartum cardiomyopathy.
INCREASING AWARENESS OF PPCM
We know that it helps to have a high index of suspicion that pregnancy-associated heart failure could occur in a previously heart-healthy young woman. Although it is possible that a fulminant myocarditis/cardiomyopathy can suddenly appear without prior warning and awareness, almost all of these women, upon reflection, can recognize that they experienced signs and symptoms earlier by days and weeks. My incessant theme is this: Physicians, nurses and patients must be alert to the possibility that a young woman, despite the lack of any type of heart problem in her medical history, may develop a serious cardiomyopathy with acute onset of heart failure in the setting of pregnancy[9].
One reason for the importance of this heightened awareness is that if the patient and her health care providers know about PPCM there is greater potential to recognize it earlier. An earlier detection means that the baseline or diagnostic echocardiographic LVEF is likely to be higher; and when it is in the range of 0.35 or above, the chances for full recovery are much greater (Table 2)[10-17]. At that level the mortality rate is essentially zero and the full recovery rate approaches 100%. When at-diagnosis LVEF is lower, rate of progression towards recovery is slower, particularly in those of African heritage (See Figure 1 and Table 2).
Table 2.
Echocardiographic parameters at diagnosis as predictors of recovery (Left ventricular ejection fraction ≥ 50%) for peripartum cardiomyopathy n (%)
| Study | Recovered | Non-recovered | P |
| Goland et al[10,11] (25% African-American) | 115 (61.5) | 72 (38.5) | |
| Diagnosis mean LVEF | 0.31 | 0.23 | < 0.0001a |
| Diagnosis mean LVEDd (cm) | 5.5 | 6.1 | 0.002a |
| Amos et al[12] (51% African-American) | 22 (44.9) | 27 (55.1) | |
| Diagnosis mean LVEF | 0.23 | 0.20 | 0.16 |
| Mean LVEF (%) at 2 mo | 43 | 24 | < 0.001a |
| Diagnosis mean LVEDd (cm) | 5.6 | 6.2 | 0.01a |
| Modi et al[13] (88.6% African-American) | 14 (35) | 26 (65) | |
| Diagnosis mean LVEF | 0.29 | 0.21 | 0.02a |
| Diagnosis mean LVEDd (cm) | 5.9 | 6.2 | 0.16 |
| Fett et al[14] (all African heritage) | 32 (27.6) | 84 (72.4) | |
| Diagnosis mean LVEF | 0.28 | 0.23 | 0.002a |
| Diagnosis mean LVEDd (cm) | 5.6 | 5.9 | 0.03a |
| Safirstein et al[15] (3.6% African-American) | 43 (78.2) | 12 (21.8) | |
| Diagnosis mean LVEF | 0.29 | 0.24 | 0.13 |
| Diagnosis mean LVEDd (cm) | 5.4 | 5.9 | 0.21 |
| Diagnostic LVEF > 0.35 | 25/25 | 0 | < 0.001a |
| 1Haghikia et al[16] | 45 (47) | 51 (53) | |
| Diagnosis mean LVEF | 0.28 | 0.17 | < 0.0001a |
| McNamara et al[17] (30% African-American) | 59 (65) | 32 (35) | |
| Diagnostic LVEF < 0.30 | 10/30 (33) | 20/30 (67) | 0.001a |
| Diagnostic LVEF ≥ 0.30 | 58/70 (82.9)2 | 21/70 (17.1)2 | 0.001a |
For this group, recovery defined as LVEF 0.55, mean LVEF shown for improved vs non-improved;
Pending last echo late data entry from 12 mo postpartum. LVEDd: Left ventricular end-diastolic diameter; LVEF: Left ventricular ejection fraction; Recovered: Last LVEF ≥ 0.50; Non-recovered: Last LVEF < 0.50;
P ≤ 0.05 vs non-recovered.
Figure 1.
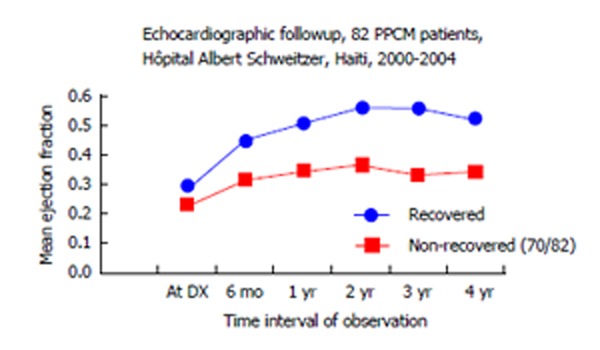
Lower systolic heart function at diagnosis of peripartum cardiomyopathy often means less recovery, “start low, stay low”[6,14,26]. PPCM: Peripartum cardiomyopathy.
Studies have shown that lower at-diagnosis LVEF is found when there are delays in diagnosis. This is well demonstrated in the study by Goland et al[10] of 182 United States PPCM patients. They looked at major adverse events, defined as either death or complications that were life threatening. “Delay in diagnosis” referred to patient estimate of time from onset of symptoms to time of confirming the diagnosis of PPCM. 136 PPCM patients who had no adverse events had a mean delay in diagnosis of 1.7 wk while 46 PPCM patients who did have major adverse events had a mean delay in diagnosis of 3.8 wk (P = 0.02). Time-of-diagnosis LVEF for those without serious adverse events showed mean value of 0.31, while those with the same serious adverse events showed mean of 0.24 (P < 0.001).
HYPERTENSION IN PREGNANCY POSES HIGHER RISK FOR DEVELOPMENT OF PPCM
Up to one-half of PPCM patients have experienced some form of hypertension during their index PPCM pregnancy[4,5]. Recent clues about the importance of hypertension in pregnancy derive from studies of toxemia of pregnancy (eclampsia and preeclampsia), showing the importance of some biomarkers that assist in early identification of patients at high risk[18-20]. These same biomarkers appear to be also present in PPCM not only as markers, but strongly suspect as causal factors in the pathogenesis of PPCM[21]. The functional cardiac abnormalities in severe preeclampsia reflect a diastolic dysfunction, and some of these women also go on to classical systolic dysfunction heart failure that meet diagnostic criteria for PPCM[22,23].
A recent epidemiology report out of North Carolina[24] shows that out of 79 PPCM patients, 51 (65%) had some form of hypertension. Eleven, (13.9%) had preeclampsia, 18 (22.8%) had gestational hypertension, 10 (12.7%) had chronic hypertension, 10 (12.7%) had chronic hypertension and preeclampsia, 1 had eclampsia. Only one had hemolysis, elevated liver enzymes and low platelet count syndrome.
PREVENTING SERIOUS COMPLICATIONS OF PPCM
Most serious complications of PPCM can be either avoided or decreased (See Case Reports 1 through 5). The most serious complications of PPCM (ventricular tachyarrhythmias, thromboembolic events, chronic cardiomyopathy) are found when the diagnostic or baseline LVEF is below 0.30 to 0.35[3-5,9-17]: In the Investigations of Pregnancy Associated Cardiomyopathy (IPAC) study, 5/6 major adverse events (death or transplant or left ventricular assist device) occurred in those with baseline LVEF < 0.30, confirming that women with severe systolic dysfunction at presentation have the poorest outcomes[17]. As such, this group may represent a target for future interventional trials.
It is also important to be certain that the best treatment is being implemented for all; but particularly for those in this LVEF under 0.30 category so as to help prevent the major complications: Adequate anticoagulation to help prevent thromboembolic phenomena; heart rhythm monitoring and devices to recognize and treat dangerous arrhythmias; and full use of evidence-based AHA Guideline therapy to help achieve eventual recovery[25].
REMARKABLE RECOVERY POTENTIAL
Full recovery of heart function occurs more frequently in PPCM than with any other dilated cardiomyopathy. Even with the very limited resources in Haiti, an organized program to diagnose and manage PPCM, with the first population-based PPCM registry, demonstrated the ability to improve full recovery from less than 4% to over one-third of women over a period of 4 years[26]. The first United States prospective study of PPCM, the IPAC study showed that full recovery (LVEF ≥ 0.50) at 6 mo postpartum came to a remarkable over 65 % of patients[17]. It is important to note that this level of full recovery occurred without the use of bromocriptine inhibition of the lactating hormone, prolactin. This is discussed in greater detail later. Other studies, all retrospective in nature, have also confirmed high rates of recovery[11,12,27].
Table 2 confirms the importance of diagnostic levels of systolic heart function (LVEF) to recovery. Health care providers and women in the latter stages of pregnancy are becoming more aware of the importance of early identification of PPCM; and are becoming more alert about how to differentiate normal late pregnancy signs and symptoms from early heart failure symptoms[9].
IMMUNE SYSTEM CHANGES IN PATHOGENESIS OF PPCM
Immune system changes (autoimmunity or immune system dysfunction) are an important part of the pathogenesis of PPCM[28]. Alterations in cellular immunity have been observed in PPCM patients compared to normal postpartum women. An increase in the activation of regulatory T-cells and innate immunity is a necessary part of all pregnancies. However, there is an increase of T cells (CD3+ CD4- CD8- CD38) in PPCM patients compared to healthy postpartum patients. Natural killer (NK) cells (CD3- CD56+ CD16+) are significantly reduced in PPCM patients compared to healthy postpartum women. Furthermore, while the decrease in percent of NK cells is similar in both black and white PPCM patients at entry to the study, this decrease persisted 2 mo later only in blacks[29-31]. IPAC, with a prospective study of 100 North American PPCM patients, is currently investigating if this immune system activation correlates with recovery outcomes[17]. (IPAC available at http://www.peripartumcmnetwork.pitt.edu). The earlier Investigations of Myocarditis and Acute Cardiomyopathy studies identified comparable findings in their PPCM cohort[30]. This remarkable finding relating to differences between African heritage and Caucasian PPCM mothers with respect to NK cells is undergoing additional studies[31].
INFLAMMATORY CARDIOMYOPATHY IN PATHOGENESIS OF PPCM
A cardiomyopathy with inflammatory cytokines is common in PPCM. This inflammatory process may be either cellular or molecular non-cellular or both[27,32-34]. Mean serum levels of high sensitivity C-Reactive Protein (hsCRP), a simple and inexpensive laboratory estimate reflecting proinflammatory cytokines, were found to be significantly elevated in 22 Haitian PPCM patients compared to 14 non-PPCM Haitian mothers (144.3 mg/L, range 2.8-946 vs 5.2 mg/L, range 1.8-9.9, P < 0.001)[14]. In the same population, significantly higher mean serum hsCRP levels were found in recovered PPCM patients compared to non-recovered PPCM patients (417 mg/L compared with 27 mg/L, P = 0.004), suggesting that a vigorous inflammatory response favored chances of recovery[33,34]. Elevated mean serum hsCRP levels have also recently been reported in 52 Chinese PPCM patients compared to 52 non-PPCM controls (28.2 mg/L vs 6.2 mg/L, P < 0.05)[35]. In South African PPCM patients at diagnosis, higher levels of serum hsCRP correlated with left ventricular end diastolic diameter (P = 0.003) and inversely with LVEF (P = 0.015)[32]. A recent article describing a prospectively identified cohort of 46 PPCM patients in India also reports significantly elevated levels of serum hsCRP, Tumor Necrosis Factor-α, and Interleukin-6[36]. These inflammatory markers also helped to predict outcome.
The biomarker of serum hsCRP will only be elevated in the presence of an inflammatory cardiomyopathy, a frequent occurrence in PPCM. However, one would not expect an elevation of serum hsCRP if no inflammatory cardiomyopathy exists, such as in the presence of a familial dilated cardiomyopathy or in a relapse of heart failure in a previously unrecovered PPCM mother in a post-PPCM pregnancy.
Multiple proinflammatory cytokines involved in the pathogenesis of PPCM include Fas, hsCRP, Interferon-γ, Interleukin-6, Transforming Growth Factor-β, Tumor Necrosis Factor-α and others in the process of evaluation[28,34,37].
GENETIC FACTORS IN PPCM
An important proportion of PPCM patients, around 5%-10%, have either a genetically caused condition (which would make the correct diagnosis familial dilated cardiomyopathy) or a genetic predisposition to develop PPCM when linked with additional factors[5,38]. Higher incidence of PPCM in those of African origin can be attributed in part to genetic factors, although environmental factors may also play an important role[39,40]. A genome-wide association of PPCM with chromosome 12p11 locus has been reported by Horne et al[38]. There may also be a genetic predisposition to the development of PPCM, with another factor or factors, involving a complex interaction of pregnancy-associated immune system changes[41].
It is important to explore further the relationship of PPCM with Idiopathic dilated cardiomyopathy (IDCM) since clinically there are many similarities. Up to one-quarter of familial dilated cardiomyopathy patients and 18% of sporadic IDCM have the presence of TTN, the protein encoding the sarcomere protein titin[42]. What proportion of PPCM patients also have this gene? Additional studies need to be carried out exploring the finding of a single nucleotide polymorphism, rs258415, to have genome-wide significance in PPCM versus control mothers[38]. Additional studies are ongoing and will certainly continue to add to our knowledge about inherited patterns and genetic influences in PPCM.
EVIDENCE-BASED TREATMENT OF PPCM
There is effective evidence-based treatment available with the combination of tolerable dosages of diuretics, Angiotensin Converting Enzyme Inhibitors (ACEI) and beta-blockers (BB) as outlined in published Guidelines. There need be no guess work in the application of effective treatment for PPCM since proved effective treatment of heart failure from PPCM is available and clearly defined in the American Heart Association and European Society of Cardiology Guidelines for treatment of heart failure with reduced LVEF[25,43]. This evidence-based treatment (categories of Class I: “Benefit exceeds risk, should use” and Level of Evidence A: “Data from multiple clinical trials and multiple populations”) for systolic heart failure with decreased LVEF consists in giving tolerable dosages of diuretics, ACEI (replaced by hydralazine with or without nitrates if still pregnant or breastfeeding) and BB. Angiotensin receptor blockers (ARB) may be used if there is ACEI intolerance; but just as with ACEI, they are not safe to take during pregnancy or conception. Otherwise, this Guideline-recommended treatment is the same as for heart failure in other non-ischemic cardiomyopathies.
Very severe systolic dysfunction at diagnosis with circulatory collapse will require other treatment for hemodynamic support; and prevent the initial use of BB. As mentioned in the section on thromboembolic events, appropriate anticoagulation until improvement of LVEF above 0.30-0.35 is indicated.
Work by Hilfiker-Kleiner et al[44] and Sliwa et al[45] with respect to potential cardiotoxic prolactin metabolites has stimulated interest in the use of prolactin inhibition by bromocriptine. In regards to the use of bromocriptine, the recent study out of Germany[16], found the greatest improvement (55 out of 57 or 96%) occurred in PPCM patients receiving combination treatment of BB, ACEI/ARB and bromocriptine (2.5-5 mg/d for 4 wk). These investigators reported “full recovery” (LVEF ≥ 0.55) for 45 out of 96 (47%) PPCM patients; but that there was no statistically significant difference in those who reached full recovery for the 64 who received bromocriptine compared with the 32 who did not receive bromocriptine. Out of 96 PPCM patients, 14 failed to improve. All of these had baseline LVEF ≤ 0.25.
These European investigators indicate that bromocriptine “may not be sufficiently effective in all patients, especially in PPCM patients with very low baseline EF”[16]. Their cohort of PPCM patients with very low baseline EF also frequently could not receive BB treatment due to low blood pressure and bradycardia. It is to be noted that the full recovery rates for these European patients were very similar to those reported by North American IPAC investigators, a study in which bromocriptine had not been a part of the treatment[17].
The best tolerated dosages of combination BB and ACEI treatment will be the most helpful in moving towards full recovery. A serious deficiency in treatment would be the use of only BB or only ACEI/ARB instead of a combination of the two at tolerated dosages. Very slow and small incremental increases in dosages as needed can circumvent the limiting factor of postural hypotension symptoms. This is the best way to successfully reach the more effective restorative effects with solid increases in LV systolic function.
Aside from hemodynamic benefits, the combination of BB + ACEI may be synergistic; and may depend upon their influence in helping to correct the immune system dysfunction that plays a pathogenic role in PPCM[46-48]. Anticoagulation to avoid thromboembolic events is extremely important for those who have LVEF < 0.35. In that lower cardiac function group it is important to monitor heart rhythm to detect and treat ventricular tachyarrhythmias.
Pentoxifylline, as an inhibitor of the proinflammatory cytokine, Tumor Necrosis Factor-α, appeared earlier in South Africa[49] to be helpful to improve left ventricular function. However, in our trials in Haiti, pentoxifylline failed to show any evidence for improved survival or improved clinical or echocardiographic left ventricular function[9,50].
Long-term follow-up is important as we continue to see late sudden death in some apparently recovered PPCM mothers; and do not know if this represents sudden cardiac death (SCD) and ventricular tachyarrhythmias as a consequence of PPCM-related scar tissue in the conduction system or from new onset disease, such as coronary artery disease[51,52].
POST-PPCM PREGNANCIES
The majority of PPCM mothers who experience apparent full recovery (LVEF ≥ 0.50) will not experience a relapse of heart failure with a subsequent pregnancy; or, if they unexpectedly experience a relapse, the treatment, when initiated early, is very effective[53,54]. In that case, the outcome is still good for mother and baby; and over 90% of those who begin the post-PPCM pregnancy with LVEF above 0.50 will recover to their pre-subsequent pregnancy cardiac function despite relapse[54]. Risk of relapse of heart failure in a post-PPCM pregnancy increases incrementally in proportion to the systolic dysfunction associated with LVEF < 0.55 (Figure 2)[54,55]. It is unclear what level of systolic dysfunction constitutes an absolute contraindication to a subsequent pregnancy; however, from extensive experience with post-PPCM pregnancies, it seems to me that the critical level is anything below LVEF 0.40[54].
Figure 2.
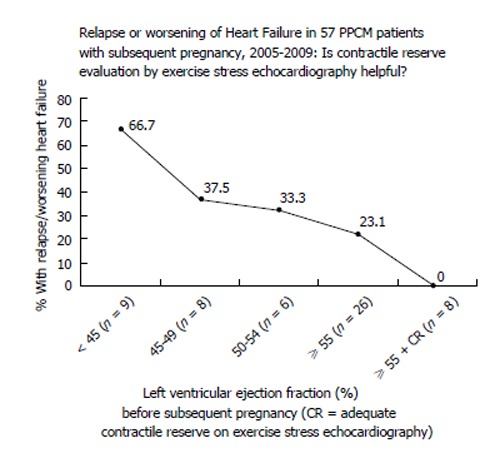
Risk for relapse of heart failure in a post-peripartum cardiomyopathy pregnancy[53,54]. PPCM: Peripartum cardiomyopathy.
The published monitoring strategies[53] are designed to help assure early detection of relapse of heart failure, when effective treatment can bring about stabilization and offer excellent potential for another recovery of heart function[51-56]. Although we may identify “full recovery” for PPCM as those with LVEF ≥ 0.50, some of these women still go on to a relapse of heart failure in a post-PPCM pregnancy[53-55] (Figure 2). That must mean that they really did not have a complete recovery or that they have a continuing reason for the development of pregnancy-associated heart failure; and we don’t yet know why. It is imperative to attempt to further identify the reasons for this, so that outcomes can still be satisfactory. Evidence supports the observation that even if these apparently completely recovered post-PPCM pregnancy mothers relapse, the treatment of their relapse of heart failure is very effective[53,54].
The outcome is not nearly so good for post-PPCM pregnancy in those who have not reached the threshold of apparent recovery from the index episode of PPCM[53,54]. We also do not know if prophylactic beta-blockade will prevent a relapse of heart failure with a post-PPCM pregnancy; or for that matter, if the BB might conceal early diagnosis of relapse, with delay of initiation of effective full treatment.
Even now, there are at least 3 observations that help us to distinguish “full recovery” from “apparent, but incomplete recovery”[53,54]. First, an LVEF before subsequent pregnancy of 0.55 is a better indicator than an LVEF of 0.50 that the recovery is more likely to be successful without relapse of heart failure in another pregnancy (Figure 2). Secondly, a deterioration of LVEF with the gradual withdrawal of either BB or ACEI treatment is a good indicator that solid recovery has not yet occurred. Thirdly, inadequate contractile reserve on exercise stress echocardiography can be a predictor of likely relapse of heart failure in a post-PPCM pregnancy. With inadequate contractile reserve, it is better to defer subsequent pregnancy and strive for further improvement[53,54]. It should be emphasized that a history of ventricular tachyarrhythmias warrants the continuation of BB treatment “for life”.
WORLD-WIDE PPCM
Pregnancy associated cardiomyopathy occurs globally, but with geographic variations for incidence, morbidity, mortality and unique characteristics. Cultural practices in Nigeria involving postpartum salt-loading and heated mud beds play an important role in the high incidence of heart failure, a variant of PPCM[57]. High incidence of PPCM in Haiti seems to reflect the genetic influence of African heritage as well as micronutrient deficiencies, perhaps zinc, involved in immune system dysfunction[26,58,59]. Overlap of PPCM and high incidence of HIV-disease appear to influence approach to PPCM in South Africa[60]. Larger proportions of population with African heritage result in greater incidence and prevalence of PPCM[40,61]. In China, common use of herbal remedies may influence outcome for PPCM patients, but valid research is limited[62,63].
WHAT INITIATES PPCM?
We do not yet know what is the actual “trigger” (there may be more than one) that initiates the process resulting in PPCM. This is perhaps the most difficult of all the quandaries about PPCM. We simply do not know. Some entertained the idea that fetal cells crossing into the maternal circulation may have targeted the mother’s heart (fetal microchimerism)[28]. If anything, we now realize that these fetal cells may actually be helpful rather than harmful[64]. Viral infection, as a trigger, has not been excluded; but neither has there been strong reinforcement of the likelihood. In personal files suggesting a possible link, I have identified 19 patients in whom the time framework of onset of new heart failure associated with pregnancy suggested a viral infection etiology (Table 3)[65-70]. The largest of these studies[66] showed similar incidence of the same viruses in endomyocardial biopsy tissue in both PPCM mothers and non-PPCM controls, making it unconvincing that virus played a role in those PPCM patients. It certainly seems likely that viral genomes in myocardial tissue may actually be “innocent bystanders” and not causal of disease, at least for some viruses. On the reverse side, it appears that for some cardiotropic viruses, once sensitization occurs, there may be an ongoing inflammatory process with or without viral genome persistence in the heart[71].
Table 3.
Role of viral infection in the etiology of peripartum cardiomyopathy: Pathogenesis or mere presence?
| ID | PPCM patient | Virus | Type of test | Comments |
| 1 | Author case file, Norway | Parvovirus B19 | IgM/IgG + | EMB = neg myocarditis |
| EMB + PCR | ||||
| 2 | Case report, Italy[65] | Coxsackievirus B | IgM + blood | EMB = lymphocytic myocarditis |
| PCR + blood | ||||
| 3 | Case report, Germany[66] | Parvovirus B19 | EMB + PCR | EMB = borderline myocarditis |
| 4 | Case report, Germany[66] | Parvovirus B19 | EMB + PCR | EMB = borderline myocarditis |
| 5 | Case report, Germany[66] | E-B Virus | EMB + PCR | EMB = borderline myocarditis |
| 6 | Case report, Germany[66] | Human Herpesvirus 6 | EMB + PCR | EMB = borderline myocarditis |
| 7 | Case report, Germany[66] | Human Herpesvirus 6 | EMB + PCR | EMB = borderline myocarditis |
| 8 | Case report, Germany[66] | Cytomegalovirus | EMB + PCR | EMB = borderline myocarditis |
| 9 | Case report, Germany[66] | Parvovirus B19 | EMB + PCR | EMB = inflammatory cardiomyopathy |
| 10 | Author case file, United States | Parvovirus B19 | IgM/IgG + blood | Exposure to PVB19 child during pregnancy |
| 11 | Author case file, United States | Parvovirus B19 cytomegalovirus | IgG + blood | Hydrops fetus, stillborn |
| 12 | Case report, Japan[67] | Influenza A/B | Paired sera antibody rise | EMB = neg. Treatment with IV immunoglobulin |
| 13 | Case report, Japan[67] | Influenza B | Paired sera antibody rise | EMB neg. Treatment with IV immunoglobulin |
| 14 | Author case file, United States | Parvovirus B19 | IgG + blood | Exposure to PVB19 child during pregnancy |
| 15 | Author case file, United States | Cytomegalovirus | IgM + blood | LVEF 15%, IgG + blood E-B virus |
| 16 | Case report, Taiwan[68] | PCR neg for all 4 tested | EMB/PCR neg, but myocarditis | 2 mo pp, RV/LV failure, patient died VF |
| 17 | Author case file, United States | H1N1 Influenza | Nasal swab, no Rx given | LVEF 40% at Dx, day 1 postpartum |
| 18 | Case report, United States[69] | Parvovirus B19 | EMB + PCR | HF 27 wk, g3p2 EMB neg myocarditis |
| 19 | Case report, Belgium[70] | E-B virus | Postpartum facial palsy full recovery 6 mo |
EMB: Endomyocardial biopsy; PCR: Polymerase chain reaction; LV: Left ventricular; LVEF: LV ejection fraction; PPCM: Peripartum cardiomyopathy; PVB19: Parvovirus B19; RV: Right ventricular; VF: Ventricular fibrillation; HF: Heart failure.
In any case it seems likely that multiple triggers exist; often in the form of foreign antigens, serving in the role of “molecular mimicry”[72,73], with epitope spreading, able to initiate an organ specific autoimmune disease[28,72-74]. It is important to continue to put the pieces of the PPCM puzzle together and eventually the exact trigger or triggers will fit into the overall scheme of things. In the meantime, outcome results continue to improve, despite our lack of knowledge about actual trigger(s) for the process.
PPCM IN THOSE OF AFRICAN HERITAGE
We do not yet know why PPCM has been documented to be both more frequent and a more severe disease in those of African heritage[13,17,31,75,76]. In the first North American prospective PPCM study, those with African heritage had a lower baseline LVEF and this poorer function persisted throughout the 12 mo study period[17].
Harper et al[24] identify the birth prevalence in North Carolina, United States, of PPCM for “black, non-Hispanics” as 1 case for every 1087 live births, four times the prevalence for “white, non-Hispanics” at 1 case for every 4266 live births. A California healthcare system reported the incidence of PPCM in blacks to be 1 case for every 1421 deliveries, 2.9 time higher compared with whites[40]. Amos et al[12], also identified a significant racial disparity in outcomes for PPCM in North Carolina, reporting that in their series of 55 PPCM patients, 51% of whom were “African American”, only 41% of African Americans recovered compared to 74% of “Whites”.
Goland et al[75] recently reported a comparison of 52 African American PPCM patients with 104 white PPCM patients, finding that the rate of left ventricular recovery to LVEF ≥ 0.50 was significantly lower in African Americans (40% vs 61%; P = 0.02). This negative comparative outcome for those of African heritage has been also documented in Georgia[75] and Louisiana[13]. Gentry, in Georgia, United States, indicated that African-American women had a 15.7-fold higher relative risk of PPCM compared to non-African Americans (OR = 15.7, 95%CI: 3.5-70.6)[76]. These outcomes in United States African American PPCM patients are more comparable to mortality and morbidity reports out of South Africa[32] and Haiti[26].
Significantly lower plasma levels of the proinflammatory cytokine, Transforming Growth Factor-β have been documented in both Haiti and South Africa[32,33]. While it is possible that this is due to genetic factors, we cannot exclude a non-genetic environmental biopathological process. In either case this could result in worse outcomes. This factor has not yet been evaluated in African-American PPCM mothers compared to Caucasian or Hispanic mothers. While zinc deficiency resulting in immune system dysfunction is suggested as a possible nutritional factor in Haiti, this possibility awaits additional study; and certainly plays no role in nations where severe poverty is not an issue[58,77].
It is important to promote further investigations of the previously mentioned differences in the postpartum rate of restoration of NK cells in African heritage compared to Caucasian mothers[31]. This may explain in part the lower diagnostic LVEF and the slower recovery rates found in these African-American mothers. It is possible that NK-T cells promote the expression of cardioprotective cytokines, such as Interleukin-10[78]. An extra benefit of BB treatment may also be an increase in the percentage of NK T-cells, possibly partially correcting the disparity observed in African-American mothers[30,79].
ROLE OF AUTOANTIBODIES IN PATHOGENESIS OF PPCM
We do not yet know how important a role cardiac autoantibodies play in PPCM. Are these autoantibodies, common in PPCM patients[28,80], not only biomarkers of a cardiomyopathy, but also pathogenic in the process (Figure 3)? Some cardiac autoantibodies, such as the antibody targeting the β1-adrenergic receptor, appear to be damaging to the heart[81]. One of the most recent reports[82] identifies autoantibodies against β1-adrenergic receptors and M2-muscarinic receptors to correlate with worse cardiac systolic function. The finding of these serum autoantibodies also in 6/36 (16.7%) normal pregnant women, however, is troubling; and reinforces the need to follow such patients because they may not be actually “normal”[83]. In our own studies, we found normal postpartum women to have none of the cardiac autoantibodies present in serum[80].
Figure 3.
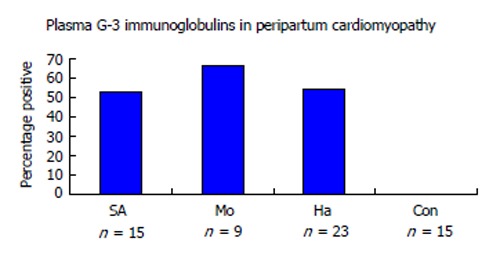
Autoantibodies in peripartum cardiomyopathy. Multiple types of cardiac antigen antibodies are common in PPCM. This Figure illustrates the presence of cardiac myosin heavy chain antibodies in PPCM patients from two African nations and Haiti. None were found in control normal postpartum patients from South Africa[81]. SA: South Africa; Mo: Mozambique; Ha: Haiti; Con: Controls; PPCM: Peripartum cardiomyopathy.
Preliminary studies suggest that removal of these antibodies results in improved cardiac function[84-86]. Perhaps the time has arrived for an interventional trial of immunoadsorption of these antibodies found in PPCM, particularly for those with low baseline LVEF, a group that is least likely to reach full recovery levels? This will not be easily accomplished because of the complicated procedure of apheresis and the precarious condition of the patients who could potentially be most helped by this process. An alternative that holds some promise of help is the use of peptides to neutralize putatively harmful cardiac autoantibodies, such as anti-β1-adrenoreceptor antibodies, a much simpler process[87,88].
ROLE OF PROLACTIN METABOLITES IN PATHOGENESIS OF PPCM
We do not yet know for sure that 14/16 kDa-prolactin metabolic products are cardiotoxic in humans; nor if inhibition of prolactin treatment produces better outcomes. As alluded to earlier, a strong foundation has been demonstrated for the cardiotoxic effects of “vasoinhibins”, the cleavage products of normal prolactin under situations of oxidative stress[44,45]. However, studies to-date testing the effectiveness of prolactin inhibition treatment have given mixed results[16]. Additional study with randomly-assigned PPCM patients to bromocriptine inhibition of prolactin cohort vs no bromocriptine inhibition treatment is underway and should help to clarify this potential treatment modality.
ROLE OF SOLUBLE FMS-LIKE TYROSIDE KINASE IN PATHOGENESIS OF PPCM
We do not yet know for sure that sFLT1 (also known as soluble vascular endothelial growth factor receptor-1) is cardiotoxic in humans; nor if inhibition of sFLT1 treatment will effectively promote the healing process. Soluble FLT1, a recently identified enzyme in the tyrosine kinase family, appears to be anti-angiogenic, cardiotoxic and particularly elevated in both PPCM and preeclampsia[18,21]. If confirmed in larger series of PPCM patients, such as currently being addressed in the IPAC study, this may lead to better treatments with promising anti-sFLT1 agents. With respect to preeclampsia, plasma sFLT1 has been found to be significantly elevated very early in some pregnancies, well before the clinical diagnosis of preeclampsia could be made[89]. Early detection of plasma sFLT1 may also assist in confirming an earlier diagnosis of both PPCM and preeclampsia.
In particular, multiple groups of investigators are defining the clinical importance of finding the higher serum sFLT1/placental growth factor (PLGF) ratios[19,89]. The highest ratios come about because of those with highest levels of serum sFLT1 (anti-angiogenic) and lowest levels of PLGF (pro-angiogenic) and it appears that this angiogenic imbalance can ultimately lead to heart failure[88]. In this process, placental hypoperfusion and maternal endothelial dysfunction play important roles[90]. This may turn out to be a very important development with respect to both diagnosis and management; but we are not yet certain. However, it is important to be alert to the possibility of peripartum heart failure from diastolic dysfunction, despite preserved systolic function with normal LVEF (would not meet current definition criteria for PPCM).
ROLE OF MICRONUTRIENTS IN PATHOGENESIS OF PPCM
Finally, we do not yet know if micronutrient and trace metal deficiencies play a role in the pathogenesis of PPCM in some unique situations. Earlier reports of endemic adolescent dilated cardiomyopathies due to selenium deficiency in China encouraged us to consider this possibility[91,92]. In the high-incidence PPCM country of Haiti, we searched diligently for this possibility, but could not confirm it[58]. However, further search led us to think that zinc deficiency could impact immune system functions and contribute to the process[93-95]. Efforts to facilitate recovery with nutritional supplements in certain situations have provided some support; but remain unconfirmed and need further investigation[96].
Please see Figure 4 with proposed multifactor hypotheses of the pathogenesis of PPCM. Case reports from the United States are included to illustrate some of the common serious complications that may accompany PPCM. These case reports come from the author’s personal case file: PPCM Case Reports With Adverse Events: Note that all cases had diagnostic LVEF < 0.30.
Figure 4.
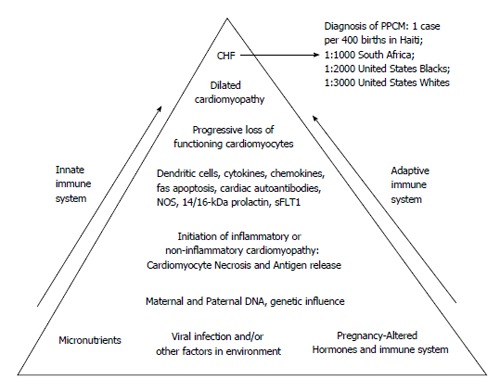
Schematic hypothesis for pathogenesis of peripartum cardiomyopathy. At the base of the pyramid are listed multiple potential contributing factors. Potential viruses include coxsackievirus B3, adenovirus, and parvovirus B19. Dendritic cells are activated by antigen(s) with initiation of a process leading to a cardiomyopathy that may be histologically either inflammatory or non-inflammatory. Cardiomyocyte damage results in the release of previously sequestered cardiac proteins with subsequent production of various autoantibodies, including but not limited to cardiac myosin heavy chain, cardiac Tropin-I, putative cardiac transaldolase), and cardiac beta 1-adrenergic receptor autoantibodies. Production of cytokines, chemokines, nitric oxide synthase (NOS) contribute to the negative inotropic effect. Fas-mediated apoptosis contributes to eventual cardiomyocyte loss. Ultimately, with the progressive loss of functioning cardiomyocytes, dilated cardiomyopathy and congestive heart failure (CHF) ensue, permitting a clinical diagnosis of PPCM. Both inate and adaptive immunity are involved, with participation of both cellular and humoral immune systems. Recently, other potential cardiotoxic substances have been identified, including 14/16 kDa-prolactin metabolites and kinase enzyme system, sFLT1[21,26,28,32,33,37,38,44,63,66,70,77,80,93]. PPCM: Peripartum cardiomyopathy.
Case 1 (United States): Onset with fetal distress and superior mesenteric artery thromboembolism
A 37 year-old gravida 4, para 2 patient presented in the 40th wk of pregnancy with swollen legs, mild dyspnea and fetal distress. She underwent emergency Cesarean section with rescued male infant. Post-operatively, she developed diffusely tender abdomen with absence of bowel sounds. Computed tomography scan of the abdomen suggested small bowel infarction. Chest X-ray revealed cardiomegaly, small right pleural effusion and increased pulmonary vascularity. An echocardiogram showed left ventricular enlargement, end-diastolic diameter of 6 cm and LVEF of 0.17. Exploratory abdominal surgery confirmed necrosis of the small bowel, which was inoperable. She experienced circulatory collapse, cardiac arrest, and unsuccessful resuscitation.
Case 2 (United States): Onset with ventricular tachyarrhythmia, SCD
A 26-year-old gravida 1 patient in her 36th wk of pregnancy collapsed in her garage. She was found by family member who started cardiac cardiopulmonary resuscitation and called emergency services. Her cardiac rhythm normalized and she was taken to the hospital. Her echocardiogram showed mildly dilated left ventricle, end-diastolic diameter 5.1 cm, LVEF at diagnosis 0.17. There was an absence of fetal heart tones, with eventual vaginal delivery of stillborn; but the mother’s heart function returned to normal over the next 6 mo.
Case 3 (United States): Onset with cerebrovascular thromboembolism
A 26 year-old gravida 2, para 1 patient in her 37th wk of pregnancy presented with paralysis of the right arm and leg (hemiplegia). Echocardiogram demonstrated thrombus in left ventricle, end-diastolic diameter left ventricle 5.4 cm, LVEF at diagnosis 0.15. Treatment included anticoagulation, hydralazine and metoprolol long-acting. With stabilization of cardiac function, a Cesarean section was performed with birth of a healthy male infant. Heart function gradually normalized and one year later her only neurological deficit was mild weakness in right leg.
Case 4 (United States): Late diagnosis, chronic severe cardiomyopathy
A 20-year-old primipara developed preeclampsia in her last month of pregnancy. With stabilization of her blood pressure, a Cesarean section was carried out with the birth of healthy twins. She experienced postpartum edema, dyspnea, and abdominal pain. Abdominal ultrasound revealed cholelithiasis and laparoscopic cholecystectomy was performed. Post-operatively, she experienced more edema, dyspnea, and cough. She went to the Emergency Room twice, where blood tests showed abnormal liver function tests; Chest X-ray showed cardiomegaly, An echocardiogram demonstrated LVEF of 0.10. Her hemodynamic instability required a left ventricular assist device. Her LVEF persisted in the range of < 0.20 and she was placed on the transplant list.
Case 5 (United States): Subsequent pregnancy before recovery with eventual chronic dilated cardiomyopathy
A 31-year-old gravida 2, para 2 patient was diagnosed with PPCM two weeks postpartum with echocardiographic LVEF at diagnosis of 0.24. She received treatment with lisinopril and carvedilol with improvement of her LVEF to 0.46. She phased out all medication and 3 years later became pregnant. She delivered a healthy female child; but subsequently experienced dyspnea on exertion and persistent pedal edema 3 d postpartum. An echocardiogram revealed reduction of echocardiographic LVEF to 0.34. She received treatment with lisinopril and carvedilol with gradual improvement of LVEF to 0.42, where it continued unchanged 3 years later.
Footnotes
P- Reviewers: Hung MJ, Lee TM, Teragawa H S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
References
- 1.Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, Ansari A, Baughman KL. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283:1183–1188. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 2.Hibbard JU, Lindheimer M, Lang RM. A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstet Gynecol. 1999;94:311–316. doi: 10.1016/s0029-7844(99)00293-8. [DOI] [PubMed] [Google Scholar]
- 3.Elkayam U, Akhter MW, Singh H, Khan S, Bitar F, Hameed A, Shotan A. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111:2050–2055. doi: 10.1161/01.CIR.0000162478.36652.7E. [DOI] [PubMed] [Google Scholar]
- 4.Cruz MO, Briller J, Hibbard JU. Update on peripartum cardiomyopathy. Obstet Gynecol Clin North Am. 2010;37:283–303. doi: 10.1016/j.ogc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2011;58:659–670. doi: 10.1016/j.jacc.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 6.Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet. 2006;368:687–693. doi: 10.1016/S0140-6736(06)69253-2. [DOI] [PubMed] [Google Scholar]
- 7.Brown CS, Bertolet BD. Peripartum cardiomyopathy: a comprehensive review. Am J Obstet Gynecol. 1998;178:409–414. doi: 10.1016/s0002-9378(98)80034-3. [DOI] [PubMed] [Google Scholar]
- 8.Bachelier-Walenta K, Hilfiker-Kleiner D, Sliwa K. Peripartum cardiomyopathy: update 2012. Curr Opin Crit Care. 2013;19:397–403. doi: 10.1097/MCC.0b013e328364d7db. [DOI] [PubMed] [Google Scholar]
- 9.Fett JD. Earlier detection can help avoid many serious complications of peripartum cardiomyopathy. Future Cardiol. 2013;9:809–816. doi: 10.2217/fca.13.63. [DOI] [PubMed] [Google Scholar]
- 10.Goland S, Modi K, Bitar F, Janmohamed M, Mirocha JM, Czer LS, Illum S, Hatamizadeh P, Elkayam U. Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail. 2009;15:645–650. doi: 10.1016/j.cardfail.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Goland S, Bitar F, Modi K, Safirstein J, Ro A, Mirocha J, Khatri N, Elkayam U. Evaluation of the clinical relevance of baseline left ventricular ejection fraction as a predictor of recovery or persistence of severe dysfunction in women in the United States with peripartum cardiomyopathy. J Card Fail. 2011;17:426–430. doi: 10.1016/j.cardfail.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Amos AM, Jaber WA, Russell SD. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J. 2006;152:509–513. doi: 10.1016/j.ahj.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Modi KA, Illum S, Jariatul K, Caldito G, Reddy PC. Poor outcome of indigent patients with peripartum cardiomyopathy in the United States. Am J Obstet Gynecol. 2009;201:171.e1–171.e5. doi: 10.1016/j.ajog.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 14.Fett JD, Sannon H, Thélisma E, Sprunger T, Suresh V. Recovery from severe heart failure following peripartum cardiomyopathy. Int J Gynaecol Obstet. 2009;104:125–127. doi: 10.1016/j.ijgo.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Safirstein JG, Ro AS, Grandhi S, Wang L, Fett JD, Staniloae C. Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the internet. Int J Cardiol. 2012;154:27–31. doi: 10.1016/j.ijcard.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 16.Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen P, Tsikas D, Jordan J, Lichtinghagen R, von Kaisenberg CS, et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108:366. doi: 10.1007/s00395-013-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNamara D, Damp J, Elkayam U, Hsich E, Ewald G, Cooper L, Modi K, Ramani G, Alexis J, Semigran M, Drazner M, Haythe J, Pisarcik J, Marek J, Gorcsan J, Fett J. Abstract 12898: Myocardial recovery at six months in peripartum cardiomyopathy: Results of the NHLBI multicenter IPAC study (Circulation 2013, Supplement) Issue 22, Nov 26. [Google Scholar]
- 18.Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, Karumanchi SA. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911–919. doi: 10.1161/CIRCULATIONAHA.111.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perni U, Sison C, Sharma V, Helseth G, Hawfield A, Suthanthiran M, August P. Angiogenic factors in superimposed preeclampsia: a longitudinal study of women with chronic hypertension during pregnancy. Hypertension. 2012;59:740–746. doi: 10.1161/HYPERTENSIONAHA.111.181735. [DOI] [PubMed] [Google Scholar]
- 20.Bello N, Rendon IS, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:1715–1723. doi: 10.1016/j.jacc.2013.08.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–338. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamiya CA, Kitakaze M, Ishibashi-Ueda H, Nakatani S, Murohara T, Tomoike H, Ikeda T. Different characteristics of peripartum cardiomyopathy between patients complicated with and without hypertensive disorders. -Results from the Japanese Nationwide survey of peripartum cardiomyopathy- Circ J. 2011;75:1975–1981. doi: 10.1253/circj.cj-10-1214. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto H, Takenaka T, Saitoh Y. Is hypertensive disorder a unique risk factor for peripartum cardiomyopathy and pregnancy-associated cardiomyopathy? Circ J. 2011;75:1827–1828. doi: 10.1253/circj.cj-11-0632. [DOI] [PubMed] [Google Scholar]
- 24.Harper MA, Meyer RE, Berg CJ. Peripartum cardiomyopathy: population-based birth prevalence and 7-year mortality. Obstet Gynecol. 2012;120:1013–1019. doi: 10.1097/aog.0b013e31826e46a1. [DOI] [PubMed] [Google Scholar]
- 25.American Heart Association . The AHA Guidelines and Scientific Statements Handbook. Fuster V (Ed.) Wiley- Blackwell: Oxford, UK; 2009. [Google Scholar]
- 26.Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. 2005;80:1602–1606. doi: 10.4065/80.12.1602. [DOI] [PubMed] [Google Scholar]
- 27.Felker GM, Jaeger CJ, Klodas E, Thiemann DR, Hare JM, Hruban RH, Kasper EK, Baughman KL. Myocarditis and long-term survival in peripartum cardiomyopathy. Am Heart J. 2000;140:785–791. doi: 10.1067/mhj.2000.110091. [DOI] [PubMed] [Google Scholar]
- 28.Ansari AA, Fett JD, Carraway RE, Mayne AE, Onlamoon N, Sundstrom JB. Autoimmune mechanisms as the basis for human peripartum cardiomyopathy. Clin Rev Allergy Immunol. 2002;23:301–324. doi: 10.1385/CRIAI:23:3:301. [DOI] [PubMed] [Google Scholar]
- 29.McTiernan C, Hanley-Yanez K, Pisarcik JE, Morel PA, Cooper LT, Elkayam E, Fett JD, McNamara DM. Activation of cellular immunity in peripartum cardiomyopathy: results of the multicenter IPAC Registry. Circulation. 2011;124:A14173, Supplement Vol 124. [Google Scholar]
- 30.Cooper LT, Mather PJ, Alexis JD, Pauly DF, Torre-Amione G, Wittstein IS, Dec GW, Zucker M, Narula J, Kip K, et al. Myocardial recovery in peripartum cardiomyopathy: prospective comparison with recent onset cardiomyopathy in men and nonperipartum women. J Card Fail. 2012;18:28–33. doi: 10.1016/j.cardfail.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McTiernan C, Hanley-Yanez K, Cooper LT, Rajagopalan N, Thohan V, Zucker M, Boehmer J, Bozkurt B, Mather P, Thornton J, et al. Racial differences in circulating Natural Killer cells in peripartum cardiomyopathy: Results of the NHLBI-sponsored IPAC investigation. Circulation. 2013;128:Supplement, Issue 22, Abstract 16587. [Google Scholar]
- 32.Sliwa K, Förster O, Libhaber E, Fett JD, Sundstrom JB, Hilfiker-Kleiner D, Ansari AA. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J. 2006;27:441–446. doi: 10.1093/eurheartj/ehi481. [DOI] [PubMed] [Google Scholar]
- 33.Ellis JE, Ansari AA, Fett JD, Carraway RD, Randall HW, Mosunjac MI, Sundstrom JB. Inhibition of progenitor dendritic cell maturation by plasma from patients with peripartum cardiomyopathy: role in pregnancy-associated heart disease. Clin Dev Immunol. 2005;12:265–273. doi: 10.1080/17402520500304352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fett JD, Sundstrom JB, Ansari , AA Abstract 1959: Evidence that plasma C-Reactive Protein may provide diagnostic help in peripartum cardiomyopathy. Circulation. 2007;116:II_422. [Google Scholar]
- 35.Huang GY, Zhang LY, Long-Le MA, Wang LX. Clinical characteristics and risk factors for peripartum cardiomyopathy. Afr Health Sci. 2012;12:26–31. [PMC free article] [PubMed] [Google Scholar]
- 36.Sarojini A, Sai Ravi Shanker A, Anitha M. Inflammatory Markers-Serum Level of C-Reactive Protein, Tumor Necrotic Factor-α, and Interleukin-6 as Predictors of Outcome for Peripartum Cardiomyopathy. J Obstet Gynaecol India. 2013;63:234–239. doi: 10.1007/s13224-013-0428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fett JD, Ansari AA. Inflammatory markers and cytokines in peripartum cardiomyopathy: a delicate balance. Expert Opin Ther Targets. 2010;14:895–898. doi: 10.1517/14728222.2010.511181. [DOI] [PubMed] [Google Scholar]
- 38.Horne BD, Rasmusson KD, Alharethi R, Budge D, Brunisholz KD, Metz T, Carlquist JF, Connolly JJ, Porter TF, Lappé DL, et al. Genome-wide significance and replication of the chromosome 12p11.22 locus near the PTHLH gene for peripartum cardiomyopathy. Circ Cardiovasc Genet. 2011;4:359–366. doi: 10.1161/CIRCGENETICS.110.959205. [DOI] [PubMed] [Google Scholar]
- 39.Fett JD, Sundstrom BJ, Etta King M, Ansari AA. Mother-daughter peripartum cardiomyopathy. Int J Cardiol. 2002;86:331–332. doi: 10.1016/s0167-5273(02)00357-1. [DOI] [PubMed] [Google Scholar]
- 40.Brar SS, Khan SS, Sandhu GK, Jorgensen MB, Parikh N, Hsu JW, Shen AY. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol. 2007;100:302–304. doi: 10.1016/j.amjcard.2007.02.092. [DOI] [PubMed] [Google Scholar]
- 41.Cemin R, Janardhanan R, Donazzan L, Daves M. Peripartum cardiomyopathy: moving towards a more central role of genetics. Curr Cardiol Rev. 2013;9:179–184. doi: 10.2174/1573403X113099990029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366:619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 44.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 45.Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema JP, Becker A, McMurray J, Yamac H, Labidi S, Struman I, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121:1465–1473. doi: 10.1161/CIRCULATIONAHA.109.901496. [DOI] [PubMed] [Google Scholar]
- 46.Platten M, Youssef S, Hur EM, Ho PP, Han MH, Lanz TV, Phillips LK, Goldstein MJ, Bhat R, Raine CS, et al. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci USA. 2009;106:14948–14953. doi: 10.1073/pnas.0903958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Godsel LM, Leon JS, Wang K, Fornek JL, Molteni A, Engman DM. Captopril prevents experimental autoimmune myocarditis. J Immunol. 2003;171:346–352. doi: 10.4049/jimmunol.171.1.346. [DOI] [PubMed] [Google Scholar]
- 48.Yuan Z, Shioji K, Kihara Y, Takenaka H, Onozawa Y, Kishimoto C. Cardioprotective effects of carvedilol on acute autoimmune myocarditis: anti-inflammatory effects associated with antioxidant property. Am J Physiol Heart Circ Physiol. 2004;286:H83–H90. doi: 10.1152/ajpheart.00536.2003. [DOI] [PubMed] [Google Scholar]
- 49.Sliwa K, Skudicky D, Candy G, Bergemann A, Hopley M, Sareli P. The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur J Heart Fail. 2002;4:305–309. doi: 10.1016/s1388-9842(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 50.Fett JD, Sanon H, Carraway RD, Markham DW, Ernst S. Abstract 189: Pentoxifylline treatment for peripartum cardiomyopathy? J Cardiac Fail. 2013;19:S65–S66. [Google Scholar]
- 51.Tokuda M, Stevenson WG, Nagashima K, Rubin DA. Electrophysiological mapping and radiofrequency catheter ablation for ventricular tachycardia in a patient with peripartum cardiomyopathy. J Cardiovasc Electrophysiol. 2013;24:1299–1301. doi: 10.1111/jce.12250. [DOI] [PubMed] [Google Scholar]
- 52.Biteker M, Ilhan E, Biteker G, Duman D, Bozkurt B. Delayed recovery in peripartum cardiomyopathy: an indication for long-term follow-up and sustained therapy. Eur J Heart Fail. 2012;14:895–901. doi: 10.1093/eurjhf/hfs070. [DOI] [PubMed] [Google Scholar]
- 53.Fett JD. Personal commentary: monitoring subsequent pregnancy in recovered peripartum cardiomyopathy mothers. Crit Pathw Cardiol. 2009;8:172–174. doi: 10.1097/HPC.0b013e3181c42faa. [DOI] [PubMed] [Google Scholar]
- 54.Fett JD, Fristoe KL, Welsh SN. Risk of heart failure relapse in subsequent pregnancy among peripartum cardiomyopathy mothers. Int J Gynaecol Obstet. 2010;109:34–36. doi: 10.1016/j.ijgo.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, Wani OR, Hameed A, Gviazda I, Shotan A. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344:1567–1571. doi: 10.1056/NEJM200105243442101. [DOI] [PubMed] [Google Scholar]
- 56.Fett JD. Validation of a self-test for early diagnosis of heart failure in peripartum cardiomyopathy. Crit Pathw Cardiol. 2011;10:44–45. doi: 10.1097/HPC.0b013e31820b887b. [DOI] [PubMed] [Google Scholar]
- 57.Okeke T, Ezenyeaku C, Ikeako L. Peripartum cardiomyopathy. Ann Med Health Sci Res. 2013;3:313–319. doi: 10.4103/2141-9248.117925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fett JD, Ansari AA, Sundstrom JB, Combs GF Jr. Peripartum cardiomyopathy: a selenium disconnection and an autoimmune connection. Int J Cardiol. 2002;86:311–316. doi: 10.1016/s0167-5273(02)00359-5. [DOI] [PubMed] [Google Scholar]
- 59.The International Bank for Reconstruction and Development/The World Bank . Promoting Nutrition Security in Haiti: An Assessment of Pre- and Post-Earthquake Conditions and Recommendations for the Way Forward. Washington: DC 20433, USA; 2010. [Google Scholar]
- 60.Sliwa K, Forster O, Tibazarwa K, Libhaber E, Becker A, Yip A, Hilfiker-Kleiner D. Long-term outcome of peripartum cardiomyopathy in a population with high seropositivity for human immunodeficiency virus. Int J Cardiol. 2011;147:202–208. doi: 10.1016/j.ijcard.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 61.Kao DP, Hsich E, Lindenfeld J. Characteristics, Adverse Events, and Racial Differences Among Delivering Mothers with Peripartum Cardiomyopathy. JACC Heart Fail. 2013;1:409–416. doi: 10.1016/j.jchf.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai H, Li Y, Han K, Gong M, Ma A. Effectiveness of Chinese herbal medicine as an adjunctive treatment for dilated cardiomyopathy in patients with heart failure. J Altern Complement Med. 2013;19:811–819. doi: 10.1089/acm.2012.0361. [DOI] [PubMed] [Google Scholar]
- 63.Liu ZL, Liu ZJ, Liu JP, Kwong JS. Herbal medicines for viral myocarditis. Cochrane Database Sys Rev. 2013;8:CD003711. doi: 10.1002/14651858.CD003711.pub5. [DOI] [PubMed] [Google Scholar]
- 64.Fett JD. Fetal and maternal microchimerism: a boost for mom and baby? Int J Cardiol. 2011;147:347–348. doi: 10.1016/j.ijcard.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 65.Ambrosini G, Nanhorngue K, Pascoli I, Cester M, Cosmi E. Mirror syndrome due to coxackie B virus associated to maternal peripartum cardiomyopathy. J Perinat Med. 2008;36:453–454. doi: 10.1515/JPM.2008.075. [DOI] [PubMed] [Google Scholar]
- 66.Bültmann BD, Klingel K, Näbauer M, Wallwiener D, Kandolf R. High prevalence of viral genomes and inflammation in peripartum cardiomyopathy. Am J Obstet Gynecol. 2005;193:363–365. doi: 10.1016/j.ajog.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 67.Muroya T, Ikeda S, Yamasa T, Koga S, Kawahara E, Togami K, Mizuta Y, Kohno S. High dose immune globulin therapy ameliorates peripartum cardiomyopathy with elevated serum antibody titer to influenza virus: case report of two patients. Med Sci Monit. 2010;16:CS11–CS14. [PubMed] [Google Scholar]
- 68.Ho CH, Wu YC, Lin YY, Hsu CW, Tsai SH. Postural hypotension as the initial presentation of fulminant right ventricular myocarditis. Am J Emerg Med. 2010;28:708–710. doi: 10.1016/j.ajem.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 69.Stewart GC, Lopez-Molina J, Gottumukkala RV, Rosner GF, Anello MS, Hecht JL, Winters GL, Padera RF, Baughman KL, Lipes MA. Myocardial parvovirus B19 persistence: lack of association with clinicopathologic phenotype in adults with heart failure. Circ Heart Fail. 2011;4:71–78. doi: 10.1161/CIRCHEARTFAILURE.110.958249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salame Y, Tsepelidis S, Roelants F, Leblicq P, Flamant M, Gosseries C. [Bell’s palsy and cardiomyopathy in the postpartum: case report and review of the literature] Rev Med Brux. 2011;32:39–42. [PubMed] [Google Scholar]
- 71.Kishimoto C, Takamatsu N, Ochiai H, Kuribayashi K. Nucleotide differences of coxsackievirus B3 and chronic myocarditis. Heart Vessels. 2014:Epub ahead of print. doi: 10.1007/s00380-014-0478-7. [DOI] [PubMed] [Google Scholar]
- 72.Reddy J, Massilamany C, Buskiewicz I, Huber SA. Autoimmunity in viral myocarditis. Curr Opin Rheumatol. 2013;25:502–508. doi: 10.1097/BOR.0b013e3283620036. [DOI] [PubMed] [Google Scholar]
- 73.Chastain EM, Miller SD. Molecular mimicry as an inducing trigger for CNS autoimmune demyelinating disease. Immunol Rev. 2012;245:227–238. doi: 10.1111/j.1600-065X.2011.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sundstrom JB, Fett JD, Carraway RD, Ansari AA. Is peripartum cardiomyopathy an organ-specific autoimmune disease? Autoimmun Rev. 2002;1:73–77. doi: 10.1016/s1568-9972(01)00009-x. [DOI] [PubMed] [Google Scholar]
- 75.Goland S, Modi K, Hatamizadeh P, Elkayam U. Differences in clinical profile of African-American women with peripartum cardiomyopathy in the United States. J Card Fail. 2013;19:214–218. doi: 10.1016/j.cardfail.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 76.Gentry MB, Dias JK, Luis A, Patel R, Thornton J, Reed GL. African-American women have a higher risk for developing peripartum cardiomyopathy. J Am Coll Cardiol. 2010;55:654–659. doi: 10.1016/j.jacc.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB. Nutrition. 2011;27:816–823. doi: 10.1016/j.nut.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 78.Sobirin MA, Kinugawa S, Takahashi M, Fukushima A, Homma T, Ono T, Hirabayashi K, Suga T, Azalia P, Takada S, et al. Activation of natural killer T cells ameliorates postinfarct cardiac remodeling and failure in mice. Circ Res. 2012;111:1037–1047. doi: 10.1161/CIRCRESAHA.112.270132. [DOI] [PubMed] [Google Scholar]
- 79.Maisel AS. Beneficial effects of metoprolol treatment in congestive heart failure. Reversal of sympathetic-induced alterations of immunologic function. Circulation. 1994;90:1774–1780. doi: 10.1161/01.cir.90.4.1774. [DOI] [PubMed] [Google Scholar]
- 80.Warraich RS, Sliwa K, Damasceno A, Carraway R, Sundrom B, Arif G, Essop R, Ansari A, Fett J, Yacoub M. Impact of pregnancy-related heart failure on humoral immunity: clinical relevance of G3-subclass immunoglobulins in peripartum cardiomyopathy. Am Heart J. 2005;150:263–269. doi: 10.1016/j.ahj.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 81.Yoshikawa T, Baba A, Nagatomo Y. Autoimmune mechanisms underlying dilated cardiomyopathy. Circ J. 2009;73:602–607. doi: 10.1253/circj.cj-08-1151. [DOI] [PubMed] [Google Scholar]
- 82.Liu J, Wang Y, Chen M, Zhao W, Wang X, Wang H, Zhang Z, Zhang J, Xu L, Chen J, et al. The correlation between peripartum cardiomyopathy and autoantibodies against cardiovascular receptors. PLoS One. 2014;9:e86770. doi: 10.1371/journal.pone.0086770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fett JD, Christie LG, Carraway RD, Ansari AA, Sundstrom JB, Murphy JG. Unrecognized peripartum cardiomyopathy in Haitian women. Int J Gynaecol Obstet. 2005;90:161–166. doi: 10.1016/j.ijgo.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 84.Deubner N, Berliner D, Schlipp A, Gelbrich G, Caforio AL, Felix SB, Fu M, Katus H, Angermann CE, Lohse MJ, et al. Cardiac beta1-adrenoceptor autoantibodies in human heart disease: rationale and design of the Etiology, Titre-Course, and Survival (ETiCS) Study. Eur J Heart Fail. 2010;12:753–762. doi: 10.1093/eurjhf/hfq072. [DOI] [PubMed] [Google Scholar]
- 85.Caforio AL, Tona F, Bottaro S, Vinci A, Dequal G, Daliento L, Thiene G, Iliceto S. Clinical implications of anti-heart autoantibodies in myocarditis and dilated cardiomyopathy. Autoimmunity. 2008;41:35–45. doi: 10.1080/08916930701619235. [DOI] [PubMed] [Google Scholar]
- 86.Haberland A, Wallukat G, Dahmen C, Kage A, Schimke I. Aptamer neutralization of beta1-adrenoceptor autoantibodies isolated from patients with cardiomyopathies. Circ Res. 2011;109:986–992. doi: 10.1161/CIRCRESAHA.111.253849. [DOI] [PubMed] [Google Scholar]
- 87.Münch G, Boivin-Jahns V, Holthoff HP, Adler K, Lappo M, Truöl S, Degen H, Steiger N, Lohse MJ, Jahns R, et al. Administration of the cyclic peptide COR-1 in humans (phase I study): ex vivo measurements of anti-β1-adrenergic receptor antibody neutralization and of immune parameters. Eur J Heart Fail. 2012;14:1230–1239. doi: 10.1093/eurjhf/hfs118. [DOI] [PubMed] [Google Scholar]
- 88.Patel PA, Hernandez AF. Targeting anti-beta-1-adrenergic receptor antibodies for dilated cardiomyopathy. Eur J Heart Fail. 2013;15:724–729. doi: 10.1093/eurjhf/hft065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verlohren S, Herraiz I, Lapaire O, Schlembach D, Zeisler H, Calda P, Sabria J, Markfeld-Erol F, Galindo A, Schoofs K, et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346–352. doi: 10.1161/HYPERTENSIONAHA.113.01787. [DOI] [PubMed] [Google Scholar]
- 90.Vest AR, Cho LS. Hypertension in pregnancy. Curr Atheroscler Rep. 2014;16:395. doi: 10.1007/s11883-013-0395-8. [DOI] [PubMed] [Google Scholar]
- 91.Xu GL, Wang SC, Gu BQ, Yang YX, Song HB, Xue WL, Liang WS, Zhang PY. Further investigation on the role of selenium deficiency in the aetiology and pathogenesis of Keshan disease. Biomed Environ Sci. 1997;10:316–326. [PubMed] [Google Scholar]
- 92.Levander OA, Beck MA. Interacting nutritional and infectious etiologies of Keshan disease. Insights from coxsackie virus B-induced myocarditis in mice deficient in selenium or vitamin E. Biol Trace Elem Res. 1997;56:5–21. doi: 10.1007/BF02778980. [DOI] [PubMed] [Google Scholar]
- 93.Ruel MT, Menon P, Loechl C, Pelto G. Donated fortified cereal blends improve the nutrient density of traditional complementary foods in Haiti, but iron and zinc gaps remain for infants. Food Nutr Bull. 2004;25:361–376. doi: 10.1177/156482650402500406. [DOI] [PubMed] [Google Scholar]
- 94.Stoye D, Schubert C, Goihl A, Guttek K, Reinhold A, Brocke S, Grüngreiff K, Reinhold D. Zinc aspartate suppresses T cell activation in vitro and relapsing experimental autoimmune encephalomyelitis in SJL/J mice. Biometals. 2012;25:529–539. doi: 10.1007/s10534-012-9532-z. [DOI] [PubMed] [Google Scholar]
- 95.Prasad AS. Impact of the discovery of human zinc deficiency on health. J Am Coll Nutr. 2009;28:257–265. doi: 10.1080/07315724.2009.10719780. [DOI] [PubMed] [Google Scholar]
- 96.Jeejeebhoy F, Keith M, Freeman M, Barr A, McCall M, Kurian R, Mazer D, Errett L. Nutritional supplementation with MyoVive repletes essential cardiac myocyte nutrients and reduces left ventricular size in patients with left ventricular dysfunction. Am Heart J. 2002;143:1092–1100. doi: 10.1067/mhj.2002.121927. [DOI] [PubMed] [Google Scholar]


