Abstract
Due to the high mortality incident brought about by traumatic brain injury (TBI), methods that would enable one to better understand the underlying mechanisms involved in it are useful for treatment. There are both in vivo and in vitro methods available for this purpose. In vivo models can mimic actual head injury as it occurs during TBI. However, in vivo techniques may not be exploited for studies at the cell physiology level. Hence, in vitro methods are more advantageous for this purpose since they provide easier access to the cells and the extracellular environment for manipulation.
Our protocol presents an in vitro model of TBI using stretch injury in brain microvascular endothelial cells. It utilizes pressure applied to the cells cultured in flexible-bottomed wells. The pressure applied may easily be controlled and can produce injury that ranges from low to severe. The murine brain microvascular endothelial cells (cEND) generated in our laboratory is a well-suited model for the blood brain barrier (BBB) thus providing an advantage to other systems that employ a similar technique. In addition, due to the simplicity of the method, experimental set-ups are easily duplicated. Thus, this model can be used in studying the cellular and molecular mechanisms involved in TBI at the BBB.
Keywords: Medicine, Issue 80, stretch injury, traumatic brain injury, blood-brain barrier, brain microvascular endothelial cells (cEND)
Introduction
Traumatic brain injury (TBI) is one of the leading causes of death worldwide. About 10 million people are affected annually by TBI making it a major health and medical problem1. Due to this, various in vivo and in vitro models of TBI have been established and developed to study its mechanisms2,3,4. A better understanding of TBI can help improve patient treatment and decrease the associated mortality, morbidity, and cost.
Many models for brain injury which utilize both in vivo and in vitro methods exist. In vivo models could mimic the actual event of head injury. However, due to the complexity of the in vivo situation, accessibility to the tissue of interest becomes limited2. In understanding the physiological response of individual cells as a result of the injury inflicted, it is important that the cells are isolated from the systemic effects which may inhibit or alter their individual response5. For this reason, cellular models of trauma provide valuable advantages over animal models since the mechanical environment of the cells can be precisely controlled6.
In vitro systems that employ the use of mechanical load to cells or tissues to determine alterations induced by such method of injury have been developed. For instance, a method for studying the effect of mechanical injury to cells has been established for astrocytes, neurons, glial cells and aortic endothelial cells7,8,9. The in vitro trauma model established for the study of rodent and human astrocyte reactivity10 employed a pressure control device identical to what we use for our model. The same method was applied to induce injury through stretch in mouse brain microvessel endothelial cells (bEnd3)11 and cortical neurons12,13 as well as, cerebral endothelial cells from newborn piglets14.The device deforms the bottom of the culture well thereby producing mechanical stretch injury10. It inflicts injury upon cultured cells by the application of air pressure above the cells. This pressure can deflect the membrane upon which the cells are growing, thereby stretching the cells. Various degrees of stretch (i.e. "low, "moderate," or "severe") can be achieved by setting the air pressure pulse duration and intensity accordingly. This method of stretch-induced injury has been correlated with traumatic injury in vivo7. Moreover, this method of injury allows for the precise control of the extracellular environment and can easily be reproduced.
Although a similar approach has been used for many other brain cell types including bEnd3, our model is of an advantage in that it makes use of the murine brain microvascular endothelial cells (cEND) generated in our laboratory. This cell line is a well-suited model of the blood brain barrier (BBB). In vitro cell cultures used as BBB models should possess characteristics that would enable them to serve as permeability screen. One important criterion for an in vitro cell model to be a predictor of BBB permeability is that it should possess physiologically realistic cell architecture15. Even though bEnd3 cells display distinctive spindle-like squamous morphology in culture16, they exhibit irregular morphogenetic behavior in vitro whereby they form cyst-like cavities rather than the regular tubular structures in fibrin gels17. Moreover, when the cells were injected into embryonic and newborn mice, they induced rapidly growing tumors lethal to embryonic mice but not in newborn and young mice. It is thus suggested that one or more processes governing normal endothelial growth, migration, and differentiation have been altered or eliminated in this cell line18. On the other hand, morphological, immunocytochemical evaluation of endothelial and BBB marker expression, bioelectric, and paracellular flux measurements demonstrate that our BBB model cEND is indeed a suitable model of the BBB19.
Brain endothelium in vivo is characterized by an extremely tight permeability with trans-endothelial electrical resistance (TEER) ranging from 2,000-5,000 Ωcm2. For studies of brain microvasculature barrier properties to pharmaceuticals, paracellular restrictiveness and tightness of the cells should be considered. In most brain capillary endothelial cells (BCEC), this is not preserved as the cells exhibit TEER ranging from 50-100 Ωcm2 20. The immortalized brain endothelial cell line bEnd3 generates TEER values of no greater than 60 Ωcm2 15. In contrast differentiation of cEND cells with medium containing reduced serum display TEER values ranging from 300-500 Ωcm2 19,21.
To date, in vitro models of stretch injury in cultured brain endothelial cells are scarce. Hence, an in vitro model for trauma through stretch injury using cultured brain endothelial cells that act as model of the BBB may prove to be useful. In this protocol, we present an in vitro model that could mimic the actual impact that brain cells, specifically brain microvascular endothelial cells of the BBB, receive during TBI. The main advantage of this model is that the amount of injury applied to the cells as well as the extracellular environment can be easily controlled in a precise manner enabling easy reproducibility of experimental set-up.
Protocol
1. Seeding of Endothelial Cells into Well Plates
Cultivate murine brain microvascular endothelial cells (cEND) in T75 culture flask, changing the medium (DMEM containing 10% FCS, 50 U/ml penicillin/streptomycin, 1% L-glutamine) twice a week, until confluence is reached. (For the generation and immortalization of brain microvascular endothelial cells, please see Burek et al., 2012; Förster et al., 200521,19).
Wash the cells with phosphate buffered saline (PBS). Remove the PBS and trypsinize the cells with 2 ml warm trypsin-EDTA solution.
Incubate the cells at 37 °C for 5 min or until the cell layer is dispersed.
Add 8 ml culture medium into the cells. Tap the flask several times to detach the cells.
View cells under the microscope to ensure complete detachment from flask.
Pipette medium with detached cells up and down. Swirl the flask to mix the cell suspension.
Take 20 µl of the cell suspension, put into a hemocytometer and count the number of cells.
Determine the cell density and seed 20,000 cells/cm2 into the well.
Transfer the cell suspension into collagen1 precoated 6-well flexible-bottomed culture plates (57.75 cm2) in a total volume of 3 ml/ well. Each well has an area of 9.62 cm2.
Grow the cells at 37 °C for one week until confluent. Change the culture medium twice per week.
2. Cell Differentiation Prior to Stretch-induced Injury
Change the culture medium of the cells with differentiation medium (DMEM containing 1% serum-stripped fetal calf serum (ssFCS), 50 U/ml penicillin/streptomycin).
Incubate the cells at 37 °C for 24 hr.
3. Stretch-induced Injury of Endothelial Cells
Turn on the cell injury controller device.
Set the delay to 50 msec.
Set the regulator pressure to 15 psi and press the trigger a couple of times until the registered peak pressure becomes stable.
Set the regulator pressure to the desired value. Use Table 1 as a guide for generating various degrees of stretch injury.
Place the 6-well flexible-bottomed culture plate (57.75 cm2) into the tray holder. Make sure that the well selector is set to the correct well size (i.e. large well).
Place the adapter plug firmly over the well. Hold the plug firmly into place with one hand while the other hand pushes the trigger.
Record the peak pressure generated.
Immediately put the plate back into the 37 °C incubator for the desired length of time or use immediately for succeeding experiments or evaluation.
4. Assessment of Stretch Injury by Dye Uptake Assay
Immediately after the cells were stretched (step 3.7), add 30 µl of a 1 mg/ml solution of the viability stain that acts as a cytotoxicity marker (please see supplemental table of materials and equipment) to the cell culture medium (Note: 10 µl of the dye solution is to be used for every ml of cell culture medium).
Upon addition of the dye to the cells, immediately view under a fluorescence microscope.
5. Assessment of Stretch Injury by Lactate Dehydrogenase (LDH) Release
Immediately after stretching the cells (step 3.7), remove 200 µl of cell culture medium from the well. Do the same for every time point post-injury you would like to include in your investigation of LDH release (i.e. e.g. 30 min, 1 hr, 2 hr, etc.)
Centrifuge the cell culture medium at the highest setting of a microcentrifuge for 5 min to remove any cell debris. Remove the supernatant and use this for the succeeding steps.
Once you have finished step 5.1 and have taken the necessary samples you would like to have from the various time points you would like to investigate, lyse the cells using the lysis solution included in the LDH assay kit (please see supplemental table of materials and equipment).
Put 100 µl of the assay medium included in the assay kitto every well of a 96-wells plate provided in the kit.
Put 100 µl of the cell-free cell culture medium into two parallel wells of a 96-wells plate included in the assay kit.
Incubate the plate at 37 °C for 30 min.
Read the absorbance at 492 nm.
Representative Results
Cells cultured on collagen I precoated 6-well flexible bottomed culture plates (57.75 cm2) were subjected to various degrees of stretch injury using the cell stretcher device. After subjecting the cells to injury, they were examined under the microscope for the effects of stretch-induced injury to cell morphology. It was observed that as greater degree of stretch was applied to the cells a greater degree of cell distortion could also be observed (Figure 1). As shown in Figure 1A, control cells which were not subjected to injury appear as regularly shaped cerebrovascular endothelial cells (cEND) without any indication of cell swelling or distortion. When stretch injury was applied (Figures 1B-D), deformation could be observed under the light microscope. After stretching the cells severely with a peak pressure between 3.5-4.5 psi, the cEND cells appeared markedly retracted, swollen and deformed with notable intercellular spaces. In addition, uptake of viability stain (100 nM final concentration) also increased as the degree of stretch injury was increased (Figure 2). The viability stain used is a dye impermeant to healthy cells that becomes permeant when the plasma membrane integrity of cells is compromised. The dye was excluded from most of the control cells, hence, only a few of the cells were stained (Figure 2A) as compared to stretched cells (Figures 2B-D). More cells fluoresced green with an increased degree of stretch injury.
As a biochemical marker of injury, release of lactate dehydrogenase (LDH) enzyme was also examined according to manufacturer's instructions. Figure 3 shows that an increasing cell stretch injury caused increasing LDH release.
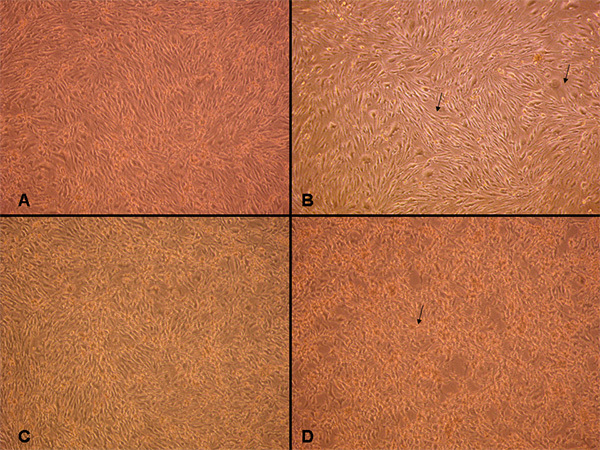 Figure 1. Light microscopy examination of normal vs. injured cells. (A) Normal unstretched confluent cell monolayer istightly packed and elongated. When cells were stretched by applying a peak pressure pulse of 1.8-2.0 psi (i.e. low stretch) they appear less compact, spaces indicated by arrows (B). When the cells were moderately injured with a 2.5-3.0 peak pressure pulse, some of them appeared swollen and deformed (C). The cells become retracted with severe stretch of 3.5-4.5 psi, as indicated by arrow (D). 100X magnification. Click here to view larger image.
Figure 1. Light microscopy examination of normal vs. injured cells. (A) Normal unstretched confluent cell monolayer istightly packed and elongated. When cells were stretched by applying a peak pressure pulse of 1.8-2.0 psi (i.e. low stretch) they appear less compact, spaces indicated by arrows (B). When the cells were moderately injured with a 2.5-3.0 peak pressure pulse, some of them appeared swollen and deformed (C). The cells become retracted with severe stretch of 3.5-4.5 psi, as indicated by arrow (D). 100X magnification. Click here to view larger image.
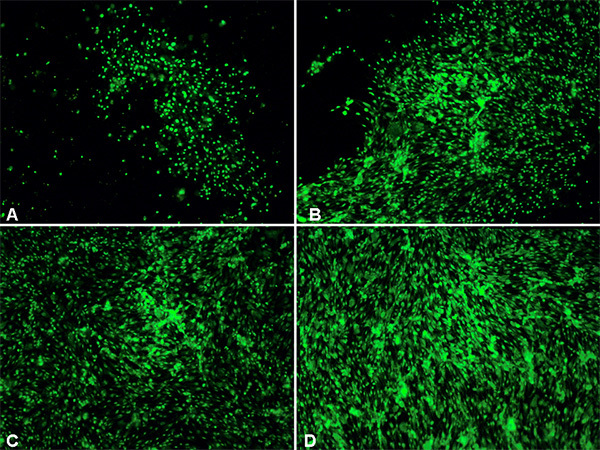 Figure 2. Fluorescence microscopic examination of normal vs. injured cells.Cells treated with viability stain 2hr after injury. A: control unstretched cells. B-D: stretched cells (B - low, C - moderate, D - severe). 100X magnification. Click here to view larger image.
Figure 2. Fluorescence microscopic examination of normal vs. injured cells.Cells treated with viability stain 2hr after injury. A: control unstretched cells. B-D: stretched cells (B - low, C - moderate, D - severe). 100X magnification. Click here to view larger image.
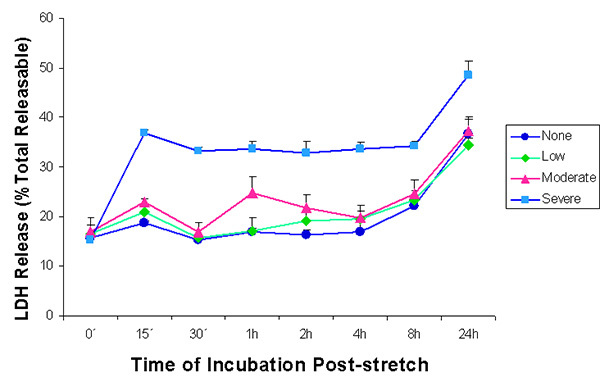 Figure 3. Lactate dehydrogenase (LDH) enzyme release into the supernatant after stretch injury. LDH released into the culture medium was measured at various time intervals after stretch induced injury. LDH was expressed as a percent of the total releasable LDH (LDH in media plus cells). Values are ± SEM. The n for every time point is 5, except for the 0 hr value subjected to severe stretch where n = 3. LDH release from cells that were subjected to low and moderate stretch did not differ significantly from that of unstretched controls and from each other. Cells that were severely stretched released a significantly greater amount of LDH as compared to all other samples, except for the moderately stretched sample at 1 hr. (p < 0.05, One factor ANOVA, Holm-Sidak method). Click here to view larger image.
Figure 3. Lactate dehydrogenase (LDH) enzyme release into the supernatant after stretch injury. LDH released into the culture medium was measured at various time intervals after stretch induced injury. LDH was expressed as a percent of the total releasable LDH (LDH in media plus cells). Values are ± SEM. The n for every time point is 5, except for the 0 hr value subjected to severe stretch where n = 3. LDH release from cells that were subjected to low and moderate stretch did not differ significantly from that of unstretched controls and from each other. Cells that were severely stretched released a significantly greater amount of LDH as compared to all other samples, except for the moderately stretched sample at 1 hr. (p < 0.05, One factor ANOVA, Holm-Sidak method). Click here to view larger image.
Table 1. Guide for generating various degrees of stretch injury.
| Regulator Pressure | Peak Pressure | Degree of Injury |
|---|---|---|
| 15 psi | 1.2-1.5 psi | < Low |
| 20-25 psi | 1.8-2.0 psi | Low |
| 30-35 psi | 2.5-3.0 psi | Moderate |
| 40-50 psi | 3.5-4.5 psi | Severe |
| 60 psi | 4.8-6.0 psi | > Severe |
Discussion
The effects of mechanical injury in vitro have been studied and methods have been established for astrocytes, neurons, glial cells and aortic endothelial cells8, 9, 22. There is, however, to date still no known in vitro model of stretch injury in cultured brain endothelial cells. Cellular models of trauma provide valuable advantages over animal models since the mechanical environment of the cells can be precisely controlled6. Hence, an in vitro model for trauma through stretch injury using cultured endothelial brain cells that act as model of the blood brain barrier (BBB) such as what our protocol presents may prove to be useful.
This protocol makes use of cEND cells, an established BBB model in our laboratory. Since BBB breakdown is often documented in TBI patients and TBI is often linked to the disruption of the BBB which can result to edema formation23, 24, the method presented here may specifically be used in conducting BBB studies in relation to TBI.
In this model, it is important to take care of how much degree of stretch injury is applied to the cells. In as much as the cells are injured in any case, and with whatever amount of pressure is applied, the degree of injury that can impact endothelial cells differ much greatly from other cells types. Aortic endothelial cells are more resistant to stretch injury than astrocytes or mixed glial cells9. In addition, they repair more rapidly after injury as compared to the other cell types. Therefore, for brain endothelial cells, particularly cEND cells, greater amount of stretch injury is needed to produce a high degree of injury. One could attain the desired degree of injury by applying the corresponding pressure indicated in Table 1. For cEND cells, however, severe injury is preferred due to their resistance to strain. The LDH assays conducted showed that as the degree of stretch increases, more LDH is secreted into the supernatant. In contrast, the cells which were given a low amount of stretch injury produced LDH in an amount similar to control cells. As mentioned in the protocol, one must take care that the appropriate amount of medium is used since an increase or decrease in the amount of medium may result to differences in the peak pressure applied to the wells. For example, a well containing 5 ml of fluid registers a peak pressure in the average of 4.0 psi while an empty well registers an average of 3.8 psi when 45 psi pressure is applied. Therefore, it is best to push the trigger several times over a control well to ensure that the peak pressure which will be generated corresponds to the desired amount.
In our experiments we used a viability stain to determine the effect of stretch to the permeability of the cell membrane. The optics of the flexible-bottomed culture plates we used enables us to view the stained cells directly under the microscope. However, when one wants to conduct immunolabelling studies directly after stretch-injury, difficulties may arise. First, the size and thickness of the plate may pose a problem with some microscope viewing platforms. Second, the optics of the flexible membrane of the well may be a hindrance to clear viewing.
Despite the aforementioned limitations, however, the described procedure can be used as a model of in vitro mechanical injury of the BBB. Traumatic brain injury (TBI) involves two components, namely, ischemia and trauma. Ischemia can occur as a secondary injury following TBI in instances when there is serious blood loss resulting in low blood pressure or as a result of brain swelling restricting oxygen supply to the brain. It is considered as a delayed, nonmechanical damage representing consecutive pathological processes initiated at the moment of injury25. The occurrence of hypoxia after severe traumatic brain injury is common26. Oxygen glucose deprivation (OGD) is the method currently being used to model ischemia in vitro. Thus, subjecting cells to OGD as a secondary insult to the cells after stretch can mimic the incidence of TBI followed by ischemia. Hence, to improve our current in vitro model of TBI and pattern it as close as possible to an actual TBI as it occurs in vivo, in the future we will also employ OGD in combination with stretch injury.
Disclosures
No conflicts of interest declared.
Acknowledgments
This research was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under grant number FO 315/4- and the European Union Seventh Framework Programme (FP7/2007-2013) under Grant agreement No. HEALTH-F2-2009- 241778 to CF.
References
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22(5):341–353. [PubMed] [Google Scholar]
- Morrison B, III, Saatman KE, Meaney DF, McIntosh TK. In vitro centralnervoussystemmodels of mechanicallyinducedtrauma: a review. J. Neurotrauma. 1998;15(11):911–928. doi: 10.1089/neu.1998.15.911. [DOI] [PubMed] [Google Scholar]
- Albert-Weissenberger C, Sirén AL. Experimental traumatic brain injury. Exp. Transl. Stroke Med. 2010;2(16):1–8. doi: 10.1186/2040-7378-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison B, Elkin B, Dollé JP, Yarmush M. In vitro models of traumatic brain injury. Annu. Rev. Biomed. Eng. 2011;13:91–126. doi: 10.1146/annurev-bioeng-071910-124706. [DOI] [PubMed] [Google Scholar]
- Cargill RS, Thibault LE. Acute alterations in [Ca2+]i in NG108-15 cells subjected to high strain rate deformation and chemical hypoxia: an in vitro model for neural trauma. J. Neurotrauma. 1996;13(7):395–407. doi: 10.1089/neu.1996.13.395. [DOI] [PubMed] [Google Scholar]
- Geddes-Klein D, Schiffman K, Meaney D. Mechanisms and Consequences of neuronal stretch injury in vitro differ with the model of trauma. J. Neurotrauma. 2006;23(2):93–204. doi: 10.1089/neu.2006.23.193. [DOI] [PubMed] [Google Scholar]
- Ellis EF, McKinney JS, Willoughby KA, Liang S, Povlischock JT. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J. Neurotrauma. 1995;12(3):325–339. doi: 10.1089/neu.1995.12.325. [DOI] [PubMed] [Google Scholar]
- McKinney JS, Willoughby KA, Liang S, Ellis EF. Stretch-induced injury of cultured neuronal, glial and endothelia cells (Effect of polyethylene glycol-conjugated superoxide dismutase. Stroke. 1996;27(5):934–940. doi: 10.1161/01.str.27.5.934. [DOI] [PubMed] [Google Scholar]
- Wanner IB, Deik A, et al. A new in vitro model of the glialscarinhibitsaxongrowth. Glia. 2008;56(15):1691–16709. doi: 10.1002/glia.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner IB. An In Vitro Trauma Model to Study Rodent and Human Astrocyte Reactivity. Methods Mol. Biol. 2012;814:189–219. doi: 10.1007/978-1-61779-452-0_14. [DOI] [PubMed] [Google Scholar]
- Berrout J, Jin M, O'Neil RG. Critical role of TRPP2 and TRPC1 channels in stretch-induced injury of blood-brain barrier endothelial cells. Brain Res. 2011;1436:1–12. doi: 10.1016/j.brainres.2011.11.044. [DOI] [PubMed] [Google Scholar]
- Weber JT, Rzigalinski BA, Willoughby KA, Moore SF, Ellis EF. Alterations in calcium-mediated signal transduction after traumatic brain injury of cortical neurons. Cell Calcium. 1999;26:289–299. doi: 10.1054/ceca.1999.0082. [DOI] [PubMed] [Google Scholar]
- Zhang L, Rzigalinski BA, Ellis EF, Satin LS. Reduction of voltage-dependent Mg2+ blockade of NMDA current in mechanically injured neurons. Science. 1996;274:1921–1923. doi: 10.1126/science.274.5294.1921. [DOI] [PubMed] [Google Scholar]
- Gidday JM, Beetsch JW, Park TS. Endogenous glutathione protects cerebral endothelial cells from traumatic brain injury. J. Neurotrauma. 1999;16(1):27–36. doi: 10.1089/neu.1999.16.27. [DOI] [PubMed] [Google Scholar]
- Gumbleton M, Audus KL. Progress and limitations in the use of in vitro cell cultures to serve as permeability screen for the blood-brain barrier. J. Pharm. Sci. 2001;90:1681–1698. doi: 10.1002/jps.1119. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Campbell L, Barar J, Connell D, Akhtar S, Gumbleton M. Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood-brain barrier model for drug uptake and transport studies. Brain Res. 2003;990(1-2):95–112. doi: 10.1016/s0006-8993(03)03443-7. [DOI] [PubMed] [Google Scholar]
- Montesano R, Pepper MS, Mohle-Steinlein U, Risau W, Wangner EF, Orci L. Icreased proteolytic activity is responsible for the aberrant morphogenetic behaviour of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- RayChaudhury A, Frazier W, D'Amore P. Comparison of normal and tumorigenic endothelial cells: differences in thrombospondin production and responses to transforming growth factor-beta. J. Cell Sci. 1994;107:39–46. doi: 10.1242/jcs.107.1.39. [DOI] [PubMed] [Google Scholar]
- Förster C, Silwedel C, et al. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J. Physiol. 2005;565(Pt 2):475–486. doi: 10.1113/jphysiol.2005.084038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LL, Hall DE, et al. A cell culturemodel of the blood-brain barrier. J. Cell Biol. 1991;115(6):1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burek NM, Salvador E, Förster CY. Generation of an immortalized murine brain microvascular endothelial cell line as an in vitro blood brain barrier model. J. Vis. Exp. 2011. [DOI] [PMC free article] [PubMed]
- Weber JT, Rzigalinski BA, Gabler HC. Alterations in calcium-mediated signal transduction after traumatic injury of cortical neurons. Cell Calcium. 1999;26:289–299. doi: 10.1054/ceca.1999.0082. [DOI] [PubMed] [Google Scholar]
- Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal SC, Schaible EV, et al. Inhibition of proteasomal glucocorticoid receptor degradation restores dexamethasone-mediated stabilization of the blood-brain barrier after traumatic brain injury. Crit. Care Med. 2013. [DOI] [PubMed]
- Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- Tanno H, Nockels RP, Pitts LH, Noble LJ. Breakdown of the blood-brain barrier after fluid percussion brain injury in the rat: Part 2: Effect of hypoxia on permeability to plasma proteins. J. Neurotrauma. 1992;9(4):335–347. doi: 10.1089/neu.1992.9.335. [DOI] [PubMed] [Google Scholar]


